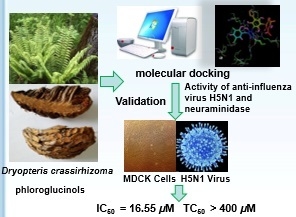
Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma
Abstract
:1. Introduction
2. Results and Discussion
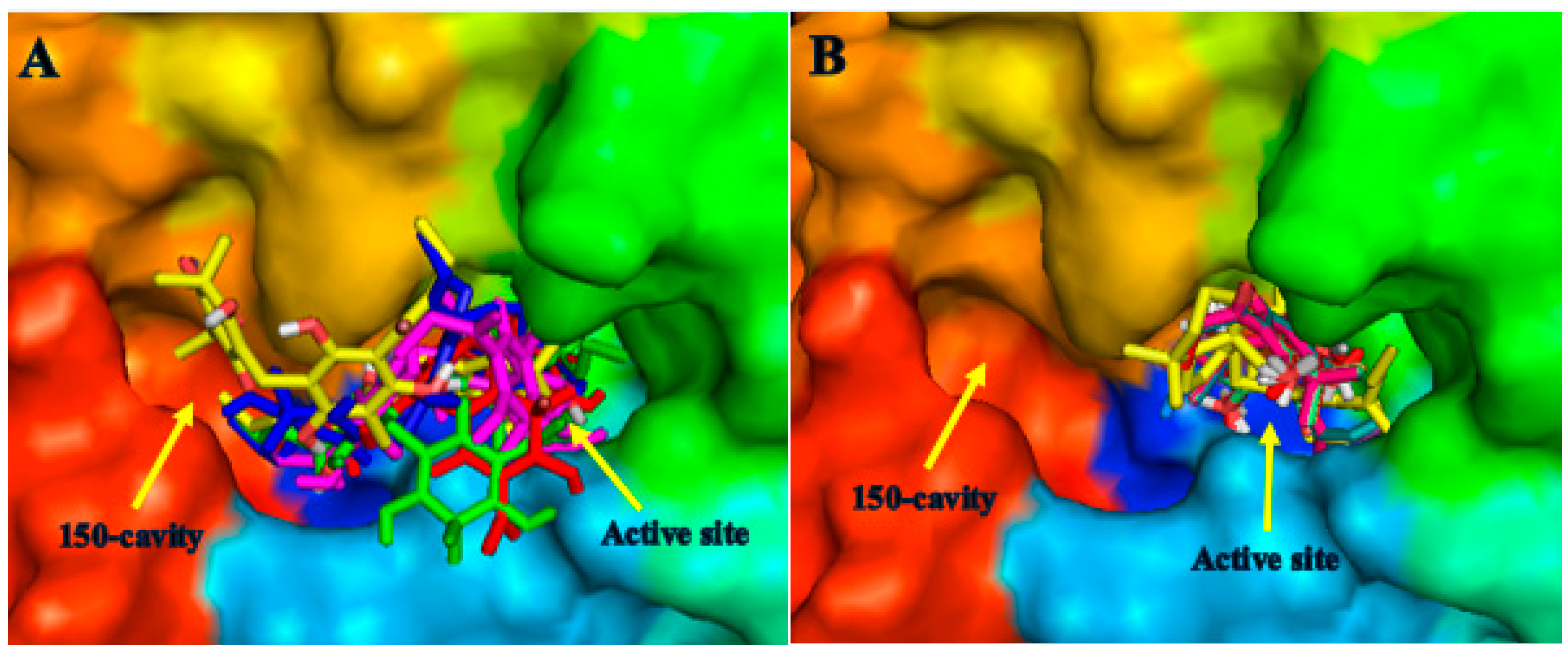
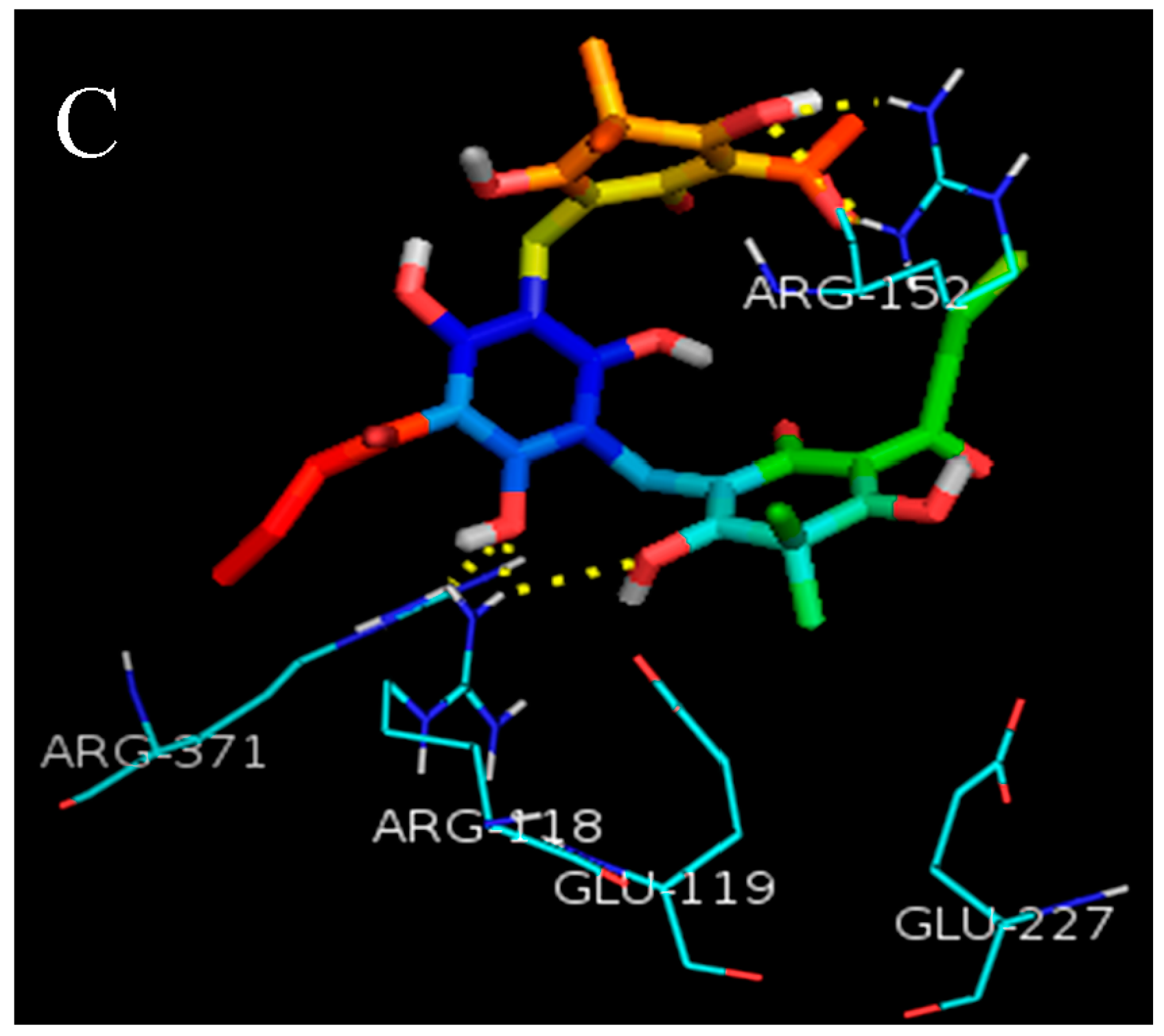
2.1. Molecular Docking
2.2. Isolation and Structure Characterization
2.3. Validation of Molecular Docking by NA Inhibition Assay
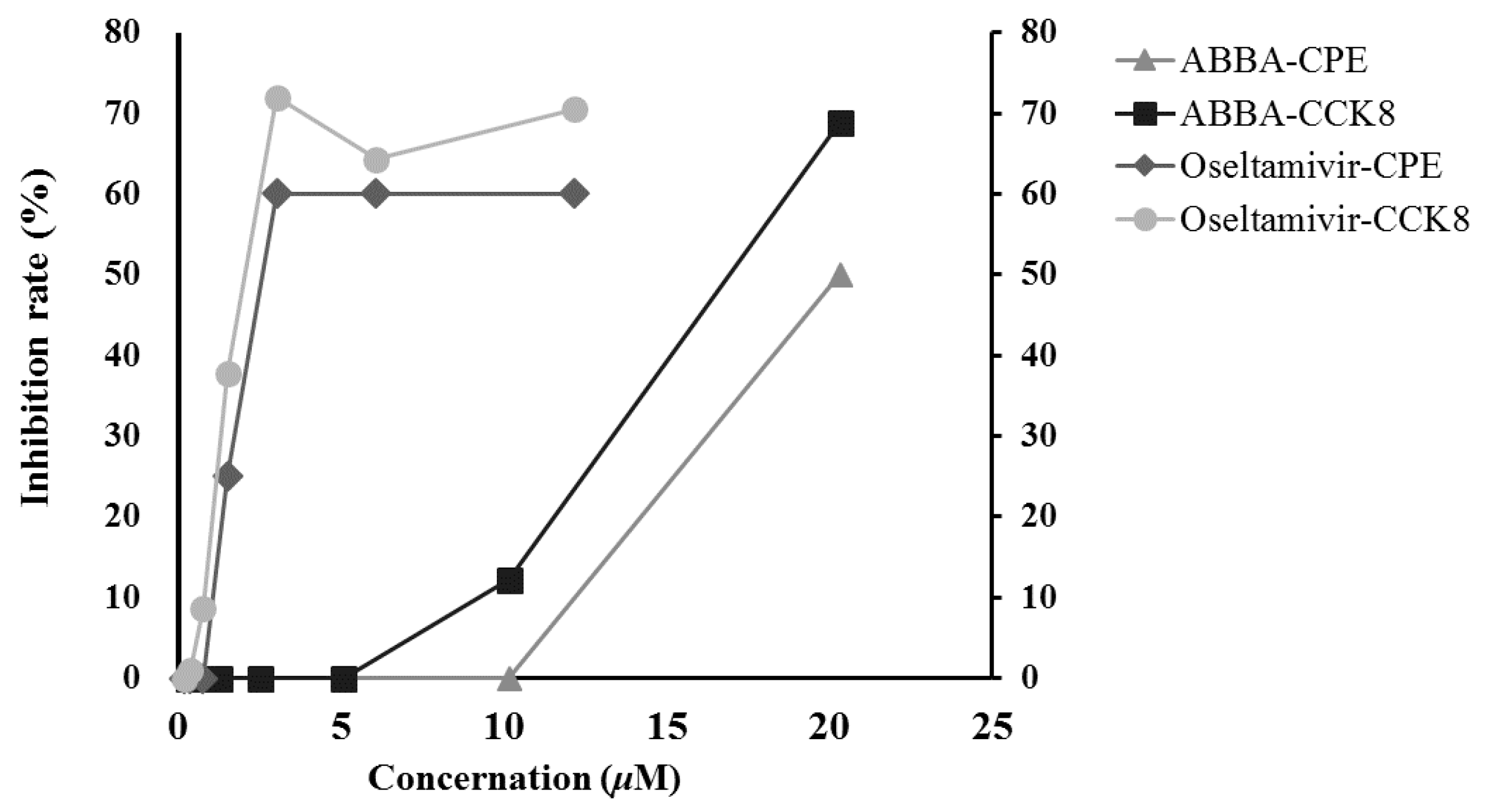
2.4. Anti-Influenza Virus H5N1 Activity In Vitro
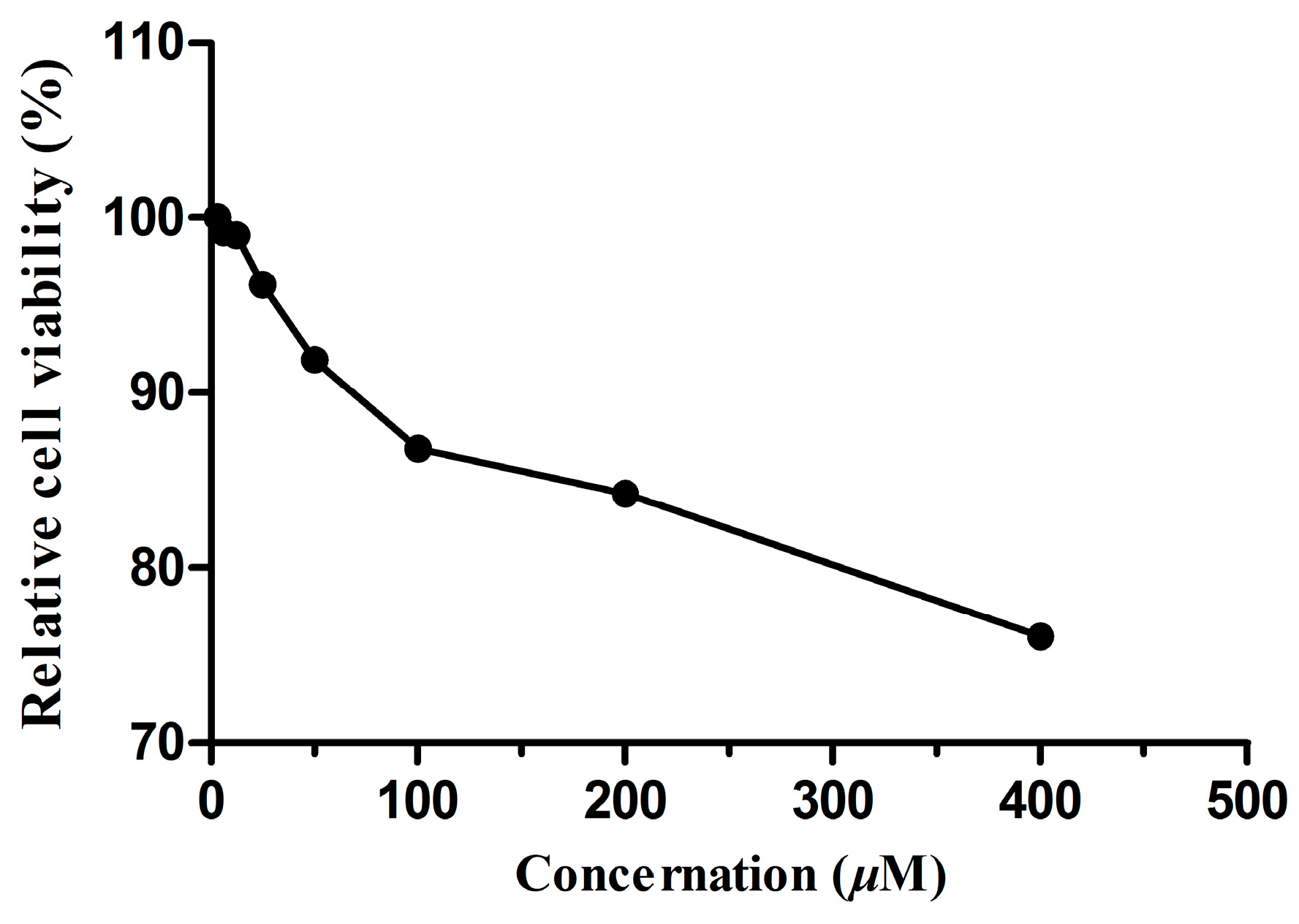
2.5. Cellular Toxicity of Dryocrassin ABBA
3. Materials and Methods
3.1. Plant Material
3.2. General Apparatus and Chemicals
3.3. Viruses, Cells and Reagents
3.4. Molecular Docking
3.5. Extraction and Isolation
3.6. NA Inhibition Assay
3.7. Anti-Influenza Virus H5N1 Activity in Vitro
3.8. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwon, H.I.; Song, M.S.; Pascua, P.N.Q.; Baek, Y.H.; Lee, J.H.; Hong, S.-P.; Rho, J.-B.; Kim, J.-K.; Poo, H.; Kim, C.-J.; et al. Genetic characterization and pathogenicity assessment of highly pathogenic H5N1 avian influenza viruses isolated from migratory wild birds in 2011, South Korea. Virus Res. 2011, 160, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mounts, A.W.; Kwong, H.; Izurieta, H.S.; Ho, Y.; Au, T.; Lee, M.; Buxton Bridges, C.; Williams, S.W.; Mak, K.H.; Katz, J.M.; et al. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong. 1997. J. Infect. Dis. 1999, 180, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Klimov, A.; Katz, J.; Regnery, H.; Lim, W.; Hall, H.; Perdue, M.; Swayne, D.; Bender, C.; Huang, J.; et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998, 279, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Prabhu, R.M.; Smith, T.F.; Baddour, L.M. Avian influenza: A new pandemic threat? Mayo Clin. Proc. 2004, 79, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.M.; Kiso, M.; Someya, K. Avian flu-Isolation of drug-resistant H5N1 virus. Nature 2005, 437, 1108. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, H.Y.; Ji, J.; Wang, G.X. In vivo anthelmintic activity of Dryopteris crassirhizoma, Kochia scoparia, and Polygala tenuifolia against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol. Res. 2012, 110, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Chi, C.; Fu, Y.W.; Zhang, Q.Z.; Wang, G.X. In vivo anthelmintic effect of flavonol rhamnosides from Dryopteris crassirhizoma against Dactylogyrus intermedius in goldfish (Carassius auratus). Parasitol. Res. 2013, 112, 4097–4104. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kang, O.H.; Choi, J.G.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Lee, G.H.; Park, P.S.; Kim, Y.C.; Sohn, D.H.; et al. Antibacterial effect of Dryopteris crassirhizoma against methicillin-resistant Staphylococcus aureus. Fitoterapia 2007, 78, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, G.J.; Yoon, D.H.; Yu, T.; Oh, J.; Jeong, D.; Lee, J.; Kim, S.H.; Kim, T.W.; Cho, J.Y. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J. Ethnopharmacol. 2013, 145, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Miyashiro, H.; Nakamura, N.; Hattori, M. Two new triterpenes from the rhizome of Dryopteris crassirhizoma, and inhibitory activities of its constituents on human immunodeficiency virus-1 protease. Chem. Pharm. Bull. 2008, 56, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Tomiyama, M.; Ma, C.M.; Nakamura, N.; Hattori, M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem. Pharm. Bull. 2001, 49, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Kim, J.C.; Lee, S.M. Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB from Dryopteris crassirhizoma. Arch. Pharm. Res. 2009, 32, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Zeng, Z.L.; Fang, B.H.; Yang, Y.Y.; Mou, D.H.; Ye, W.C. Analysis of chemical constituents of the anti-avian influenza virus site of Dryopteris crassirhizoma by HPCL-ESI/APCI-MS. J. Instrum. Anal. 2008, 27, 623–626. [Google Scholar]
- Kapadia, G.J.; Tokuda, H.; Konoshima, T.; Takasaki, M.; Takayasu, J.; Nishino, H. Anti-tumor promoting activity of Dryopteris phlorophenone derivatives. Cancer Lett. 1996, 105, 161–165. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, F.S.; Li, C.Y.; Wang, W.; Luo, M.; Fu, Y.J.; Zu, Y.G. Aspidin BB, a phloroglucinol derivative, induces cell cycle arrest and apoptosis in human ovarian HO-8910 cells. Chem. Biol. Interact. 2013, 204, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Nakane, H.; Arisawa, M.; Fujita, A.; Koshimura, S.; Ono, K. Inhibition of HIV-reverse transcriptase activity by some phloroglucinol derivatives. FEBS Lett. 1991, 286, 83–85. [Google Scholar] [CrossRef]
- Lee, S.M.; Na, M.K.; An, R.B.; Min, B.S.; Lee, H.K. Antioxidant activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol. Pharm. Bull. 2003, 26, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, L.; Si, J.Y.; Sun, Y.P.; Liu, J.H.; Cao, L.; Feng, W.H. Inhibition of porcine reproductive and respiratory syndrome virus replication by flavaspidic acid AB. Antivir. Res. 2013, 97, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.B.; Zhang, Q.; Wang, J.; Wu, G.J.; Shi, N.N.; He, C.; Gao, Z.P. Dryocrassin ABBA, a novel active substance for use against amantadine-resistant H5N1 avian influenza virus. Front. Microbiol. 2016, 6, 592. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Russell, R.J.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Spyrakis, F.; Amadasi, A.; Fornabaio, M.; Abraham, D.J.; Mozzarelli, A.; Kellogg, G.E.; Cozzini, P. The consequences of scoring docked ligand conformations using free energy correlations. Eur. J. Med. Chem. 2007, 42, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Haire, L.F.; Stevens, D.J.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.J.; Skehel, J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Josef, E.; Tadeus, R.; Widen, C.J. The Phloroglucinols of Dryopteris aitoniana PICHI SERM. (Dryopteridaceae, Pteridophyta). Helv. Chim. Acta 1985, 68, 1251–1275. [Google Scholar]
- Ito, H.; Muranaka, T.; Mori, K.; Jin, Z.X.; Tokuda, H.; Nishino, H.; Yoshida, T. Ichthyotoxic phloroglucinol derivatives from Dryopteris fragrans and their anti-tumor promoting activity. Chem. Pharm. Bull. 2000, 48, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Noro, Y.; Okuda, K.; Shimada, H.; Hisada, S.; Inagaki, I.; Tanaka, T.; Yokohashi, H. Dryocrassin: A new acylphloroglucinol from Dryopteris crassirhizoma. Phytochemistry 1973, 12, 1491–1493. [Google Scholar] [CrossRef]
- Widen, C.J.; Pyysalo, H.; Salovaara, P. Separation of naturally occurring acylphloroglucinols by high performance liquid chromatography. J. Chromatogr. 1980, 188, 213. [Google Scholar] [CrossRef]
- Gao, Z.P.; Zulfiqar, A.; Zhao, J.P.; Qiao, L.; Lei, H.M.; Lu, Y.R.; Ikhlas, A.K. Phytochemical investigation of the rhizomes of Dryopteris crassirhizoma. Phytochem. Lett. 2008, 1, 188–190. [Google Scholar] [CrossRef]
- Wollenweber, E.; Stevens, J.F.; Ivanic, M.; Deinzer, M.L. Acylphloroglucinols and flavonoid aglycones produced by external glands on the leaves of two Dryopteris ferns and Currania robertiana. Phytochemistry 1998, 48, 931–939. [Google Scholar] [CrossRef]
- Na, M.; Jang, J.; Min, B.S.; Lee, S.J.; Lee, M.S.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg. Med. Chem. Lett. 2006, 16, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Socolsky, C.; Domínguez, L.; Asakawa, Y.; Bardón, A. Unusual terpenylated acylphloroglucinols from Dryopteris wallichiana. Phytochemistry 2012, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Widen, C.J.; Pyysalo, H.; Reichstein, T. Fast-atom-bombardment mass spectra of phloroglucinols from Dryopteris Ferns. Helv. Chim. Acta 1994, 77, 1985–1998. [Google Scholar] [CrossRef]
- Takahashi, R.; Tanaka, O.; Shibata, S. Ergosterol peroxide from Peltigera aphthosa and P. dolichorrhiza. Phytochemistry 1972, 11, 1850. [Google Scholar] [CrossRef]
- Hisada, S.; Shiraishi, K.; Inagaki, I. A new acylphloroglucinol from Dryopteris dickinsii. Phytochemistry 1972, 11, 1850–1851. [Google Scholar] [CrossRef]
- Patama, T.T.; Widen, C.J. Phloroglueinol derivatives from Dryopteris fusco-atra and D. Hawaiiensis. Phytochemistry 1991, 30, 3305–3310. [Google Scholar] [CrossRef]
- Tryon, R.; Widén, C.J.; Huhtikangas, A.; Lounasmaa, M. Phloroglucinol derivatives in Dryopteris parallelogramma and D. patula. Phytochemistry 1973, 12, 683–687. [Google Scholar] [CrossRef]
- Li, J. The Isolation and Purification of the Polysaccharides from Radix Isatidis, and Their Effects on the Inhibition of Influenza Virus Neuraminidase. Master’s Thesis, Northeast Normal University, Changchun, China, 2013. [Google Scholar]
- Mulakala, C.; Nerinckx, W.; Reilly, P.J. Docking studies on glycoside hydrolase family 47 endoplasmic reticulum α-(1→2)-mannosidase I to elucidate the pathway to the substrate transition state. Carbohydr. Res. 2006, 341, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Alia, H.I.; Ashidab, N.; Nagamatsua, T. Antitumor studies. Part 4: Design, synthesis, antitumor activity, and molecular docking study of novel 2-substituted 2-deoxoflavin-5-oxides, 2-deoxoalloxazine-5-oxides, and their 5-deaza analogs. Bioorg. Med. Chem. Lett. 2008, 16, 922–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.P.; Ma, T.W.; Fu, W.C.; Peng, X.C.; Zhang, A.H.; Zhu, H.L. The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2010, 45, 5064–5070. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Takei, N.; Matsuo, Y. Fluorometric measurement of neuraminidase activity of influenza viruses. Hiroshima J. Med. Sci. 1984, 33, 287–292. [Google Scholar] [PubMed]
- Kwon, H.J.; Kim, H.H.; Yoon, S.Y.; Ryu, Y.B.; Chang, J.S.; Cho, K.O.; Rho, M.C.; Park, S.J.; Lee, W.S. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol. J. 2010, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–13 are available from the authors.


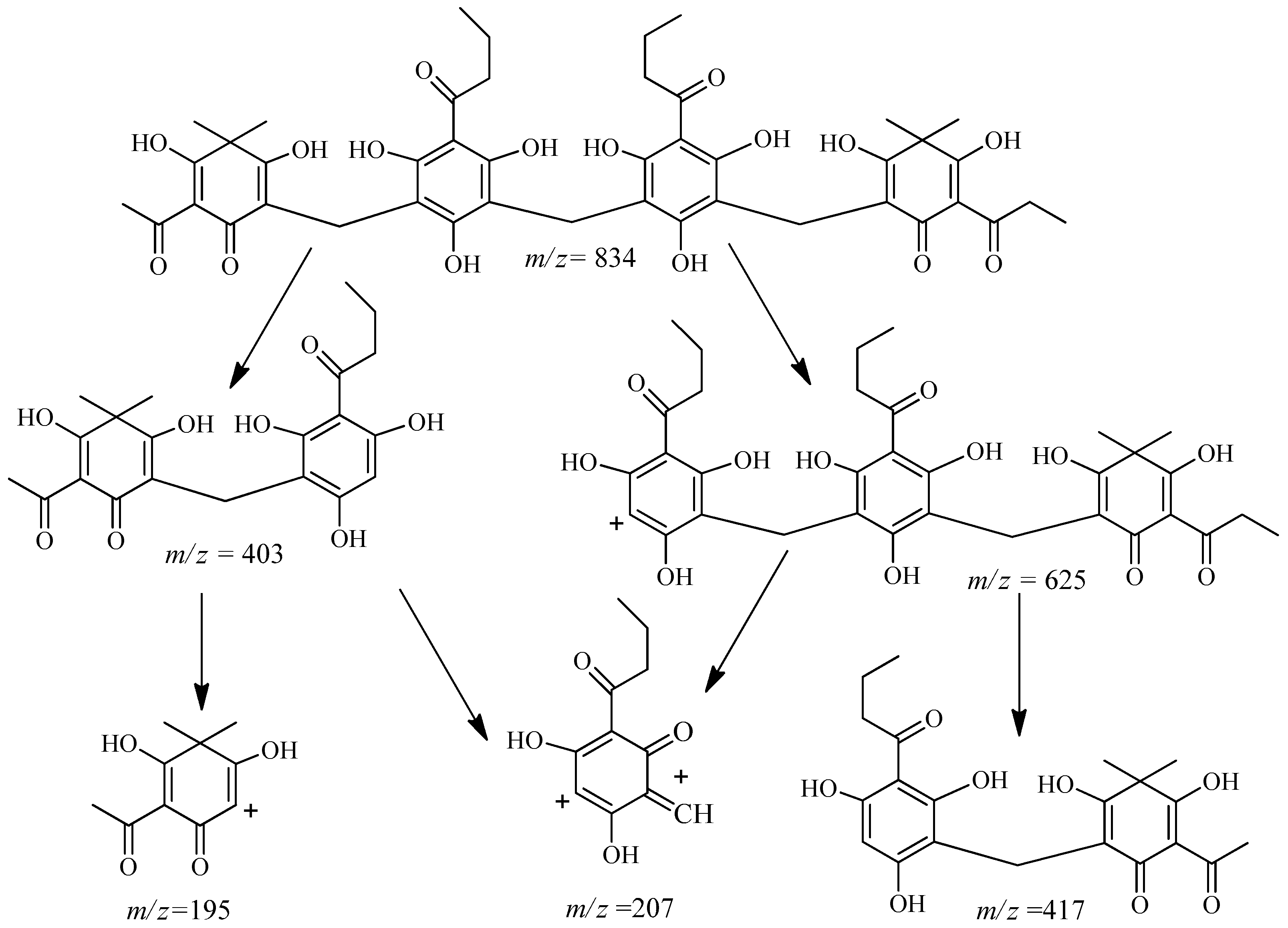
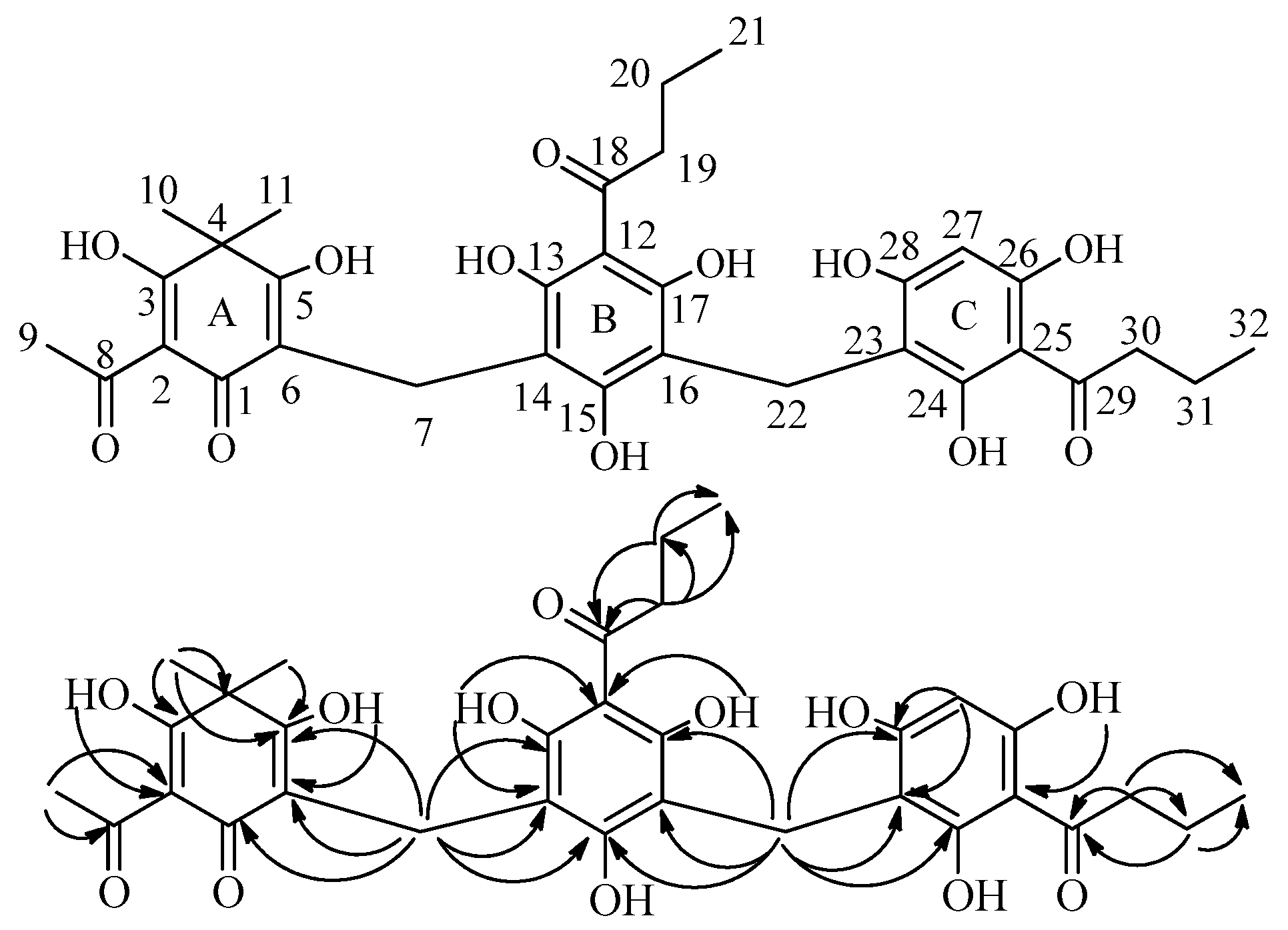

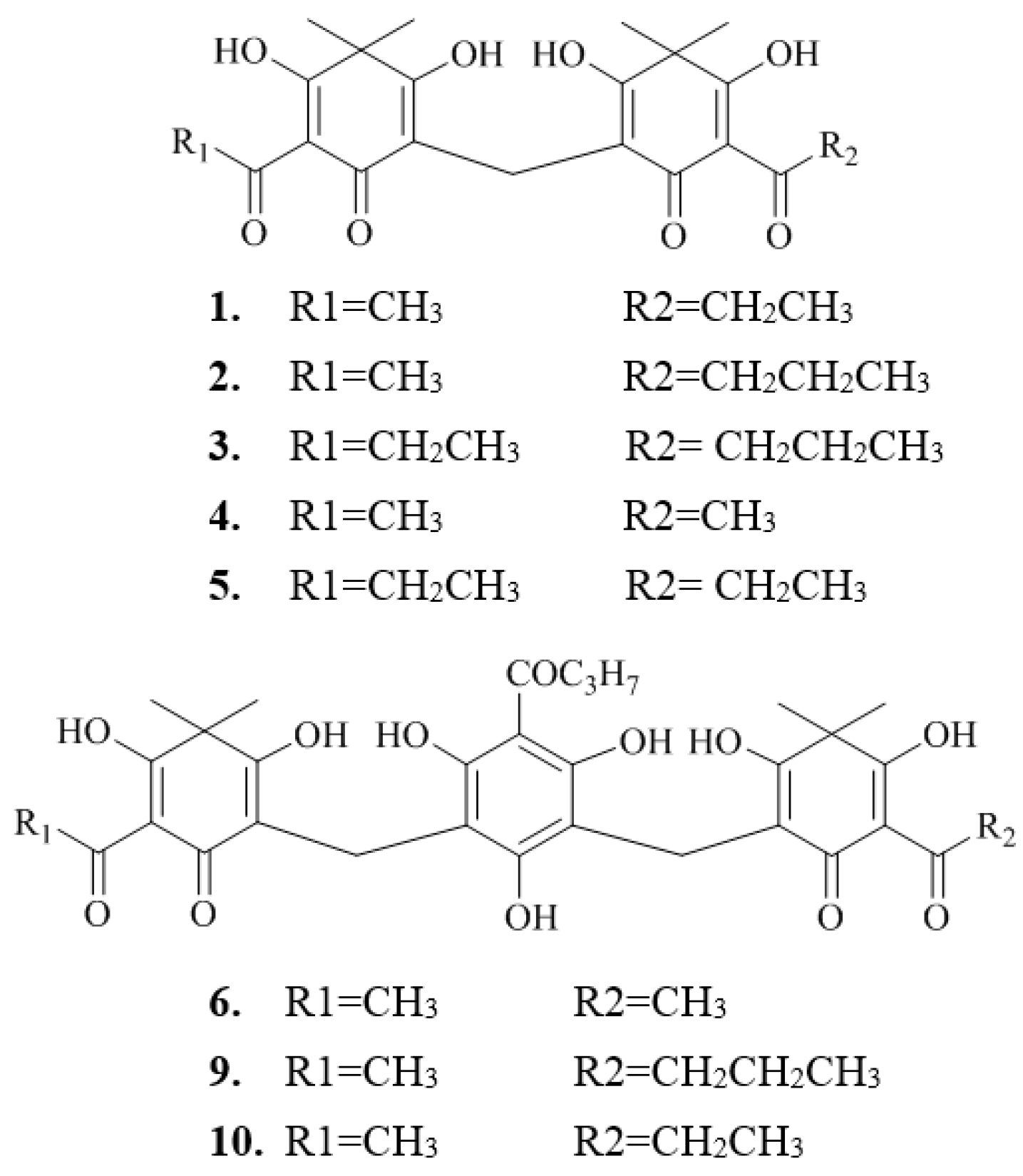
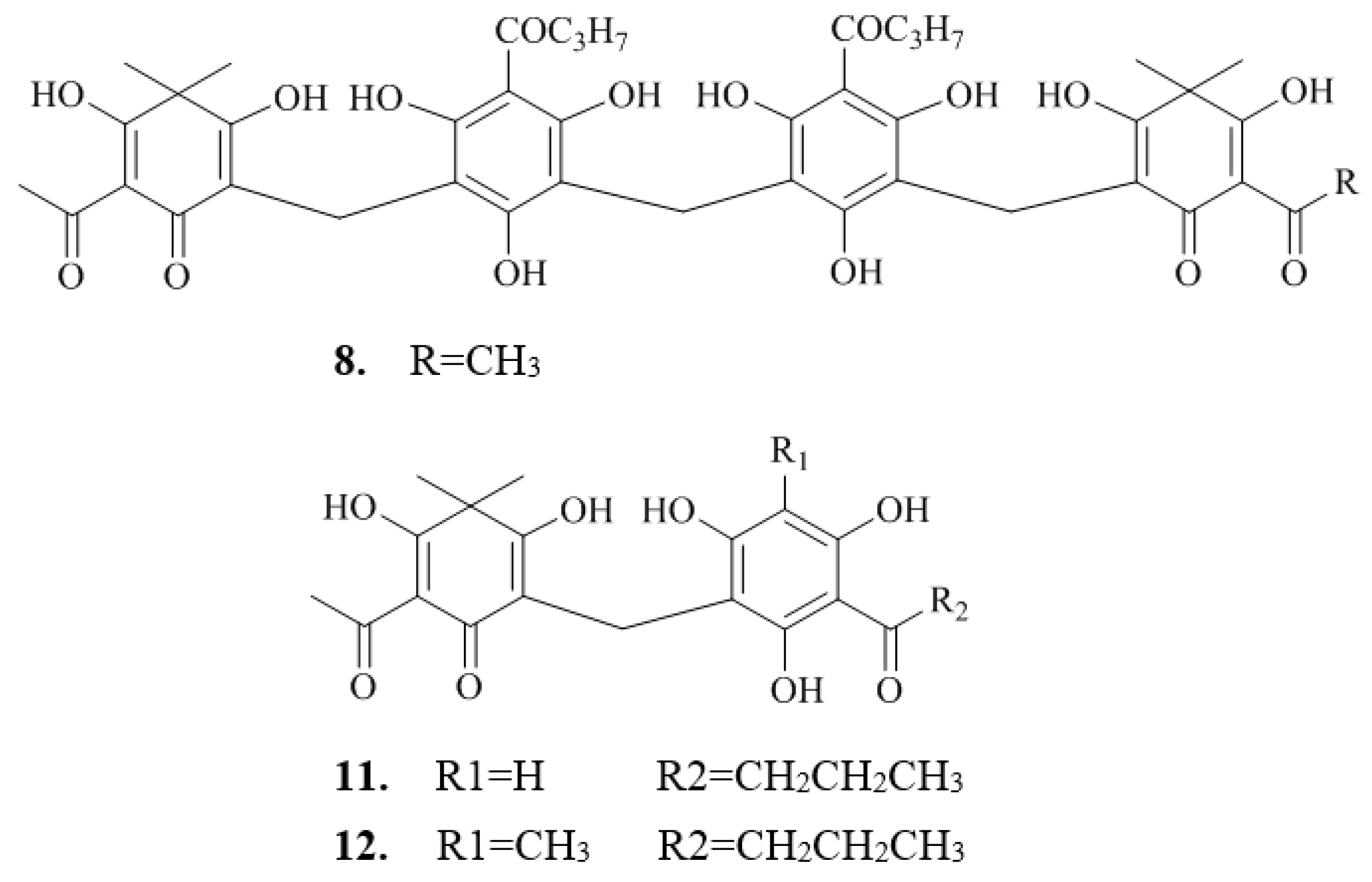
| Compound | MF | MW | Affinity (Kcal/mol) |
|---|---|---|---|
| Dryofragin | C28H38O5 | 454 | −8.0 |
| Albaspidin AA | C21H24O8 | 404 | −8.0 |
| Albaspidin AB | C23H28O8 | 432 | −8.1 |
| Albaspidin AP | C22H26O8 | 418 | −8.0 |
| Flavaspidic acid AB | C22H26O8 | 418 | −8.2 |
| Flavaspidic acid PB | C23H28O8 | 432 | −8.1 |
| Norflavaspidic acid AB | C21H24O8 | 404 | −8.0 |
| Filixic acid ABA | C32H36O12 | 612 | −8.0 |
| Filixic acid ABB | C32H36O12 | 640 | −8.1 |
| Dryocrassin ABBA | C43H48O16 | 820 | −8.3 |
| Position | Compound 7 | Compound 8 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 (1′) | 188.1 (188.0) | 188.0 | ||
| 2 (2′) | 108.2 (108.1) | 108.2 | ||
| 3 (3′) | 18.54 (OH) | 199.2 (198.5) | 18.52 (OH) | 199.3 |
| 4 (4′) | 44.5 (44.3) | 44.5 | ||
| 5 (5′) | 9.71 (OH) | 172.8 (172.5) | 9.80 (OH) | 172.8 |
| 6 (6′) | 111.2 (111.1) | 111.2 | ||
| 7 (7′) | 3.57 s | 17.4 | 3.57 s | 17.4 |
| 8 (8′) | 105.6 (105.3) | 105.7 | ||
| 9 (9′) | 16.09 (OH) | 160.5 | 16.10 (OH) | 160.6 |
| 10 (10′) | 158.9 | 158.9 | ||
| 11 (11′) | 11.75 (OH) | 159.7 | 11.73 (OH) | 159.7 |
| 12 (12′) | 106.7 | 106.7 | ||
| 13 (13′) | 10.14 (OH) | 158.7 | 10.12 (OH) | 158.7 |
| 14 | 3.85 s | 17.1 | 3.87 s | 17.1 |
| 15 | 203.6 | 203.5 | ||
| 15′ | 207.5 | 203.5 | ||
| 16 | 2.75 s | 29.7 | 2.77 s | 29.4 |
| 16′ | 3.21 m | 34.9 | 2.77 s | 29.4 |
| 17 (17′) | 1.43 s | 25.3 | 1.46 s | 25.3 |
| 18 (18′) | 1.53 s | 24.1 | 1.57s | 24.1 |
| 19 (19′) | 208.2 (208.1) | 208.3 | ||
| 20 (20′) | 3.21 m | 46.0 | 3.22 m | 46.1 |
| 21 (21′) | 1.73 m | 18.0 (17.8) | 1.76 m | 17.9 |
| 22 (22′) | 1.01 t | 14.0 | 1.03 t | 14.0 |
| 23′ | 1.19 t | 8.4 | ||
| Position | Compound 13 | Trisflavaspidic Acid ABB | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 188.0 | 188.2 | ||
| 2 | 108.2 | 108.5 | ||
| 3 | 18.45 (OH) | 199.1 | 18.34 (OH) | 199.3 |
| 4 | 44.4 | 44.7 | ||
| 5 | 18.46 (OH) | 172.6 | 18.34 (OH) | 172.7 |
| 6 | 111.1 | 111.4 | ||
| 7 | 3.52 s | 17.9 | 3.46 s | 17.4 |
| 8 | 203.5 | 203.7 | ||
| 9 | 2.74 s | 29.7 | 2.68 s | 29.6 |
| 10 | 1.43 s | 25.2 | 1.37 s | 25.5 |
| 11 | 1.55 s | 24.1 | 1.47 s | 24.4 |
| 12 | 106.8 | 106.4 | ||
| 13 | 16.01 (OH) | 159.6 | 16.01 (OH) | 159.8 |
| 14 | 106.7 | 107.0 | ||
| 15 | 9.55 s | 160.5 | 9.60 s | 160.7 |
| 16 | 108.5 | 108.6 | ||
| 17 | 16.00 (OH) | 160.7 | 15.97 (OH) | 160.2 |
| 18 | 208.0 | 208.2 | ||
| 19 | 3.05 t | 46.0 | 3.08 t | 46.2 |
| 20 | 1.73 m | 18.0 | 1.66 m | 18.4 |
| 21 | 1.01 m | 14.0 | 0.94 m | 14.2 |
| 22 | 3.80 s | 17.1 | 3.76 s | 17.0 |
| 23 | 105.7 | 105.9 | ||
| 24 | 13.01 s | 158.9 | 12.94 s | 159.0 |
| 25 | 105.3 | 105.6 | ||
| 26 | 11.65 s | 159.5 | 11.59 s | 159.1 |
| 27 | 5.90 s | 96.6 | 102.0 | |
| 28 | 10.09 (OH) | 158.6 | 10.05 (OH) | 158.9 |
| 29 | 206.7 | 207.1 | ||
| 30 | 3.15 t | 45.6 | 3.08 t | 46.0 |
| 31 | 1.73 m | 18.0 | 1.66 m | 18.1 |
| 32 | 1.01 m | 14.0 | 0.94 m | 14.1 |
| 33 | 2.03 | 7.7 | ||
| Compounds | H5N1 (A/Anhui/1/2005) |
|---|---|
| IC50 (μM) | |
| Dryocrassin ABBA | 18.59 ± 4.53 |
| Filixic acid ABA | 29.57 ± 2.48 |
| Filixic acid ABB | 96.58 ± 7.67 |
| Dryocrassin ABBP | 48.90 ± 2.91 |
| Nortrisflavaspidic acid ABB | 51.49 ± 6.84 |
| Oseltamivir (nM) | 4.62 ± 0.77 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yan, Y.-T.; Fu, S.-Z.; Peng, B.; Bao, L.-L.; Zhang, Y.-L.; Hu, J.-H.; Zeng, Z.-P.; Geng, D.-H.; Gao, Z.-P. Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma. Molecules 2017, 22, 431. https://doi.org/10.3390/molecules22030431
Wang J, Yan Y-T, Fu S-Z, Peng B, Bao L-L, Zhang Y-L, Hu J-H, Zeng Z-P, Geng D-H, Gao Z-P. Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma. Molecules. 2017; 22(3):431. https://doi.org/10.3390/molecules22030431
Chicago/Turabian StyleWang, Juan, Yan-Tao Yan, Shen-Zhen Fu, Bing Peng, Lin-Lin Bao, Yan-Ling Zhang, Jing-Hong Hu, Zu-Ping Zeng, Dong-Hao Geng, and Zeng-Ping Gao. 2017. "Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma" Molecules 22, no. 3: 431. https://doi.org/10.3390/molecules22030431