Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Flavonols and Anthocyanin and Total Phenolic Content of the Two Onion Species
2.2. Antioxidant Activity
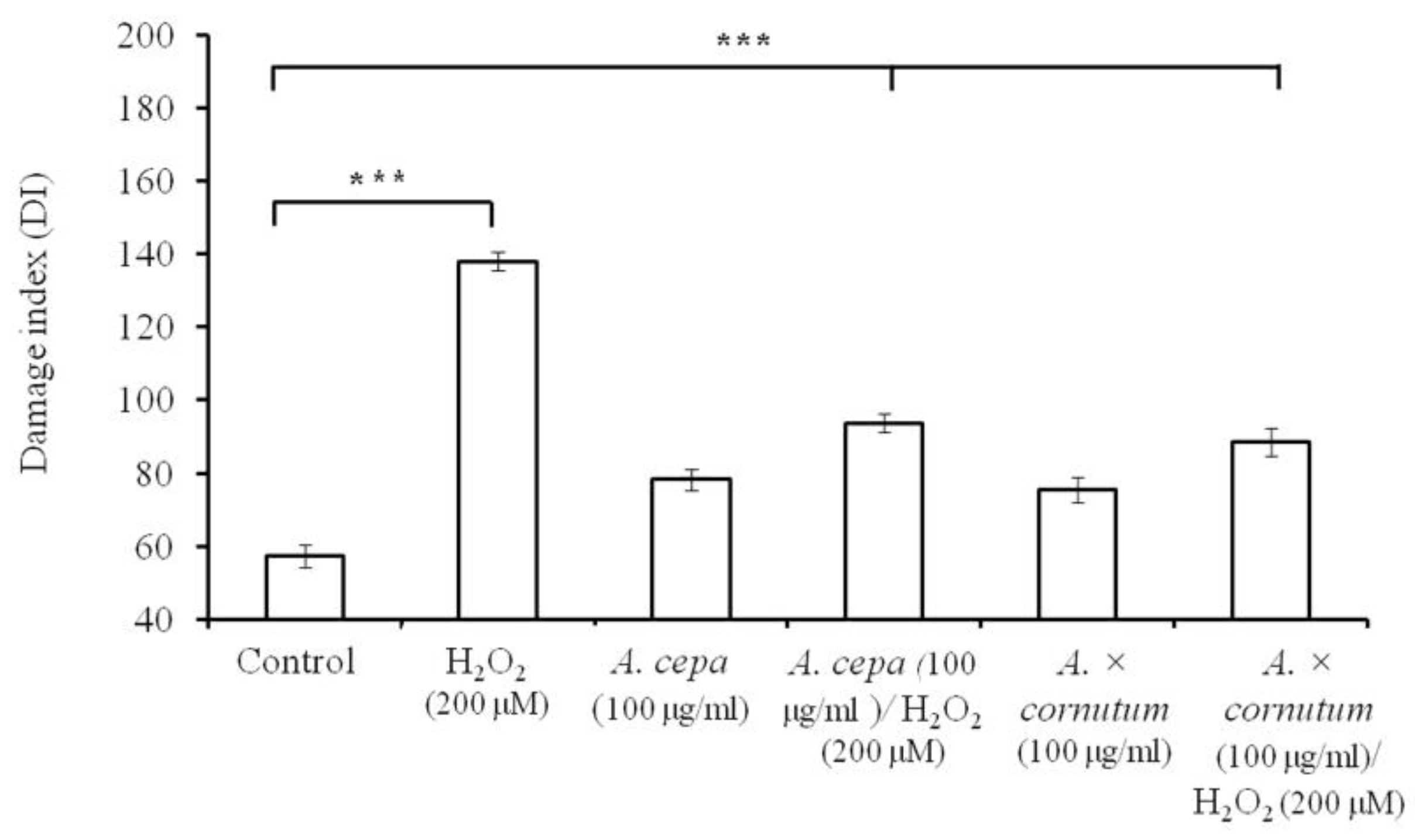
2.3. DNA Nicking Assay
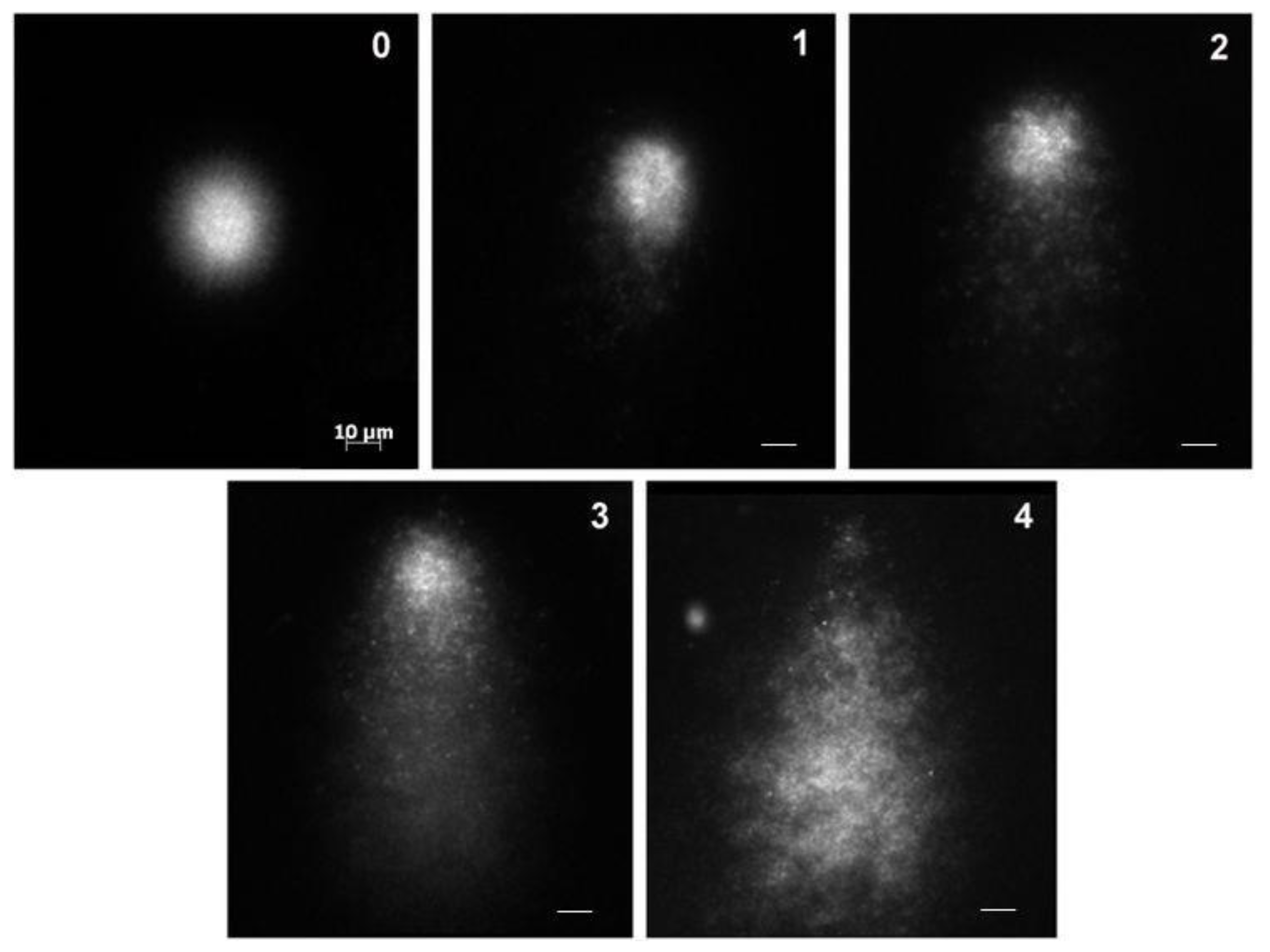
2.4. Comet Assay
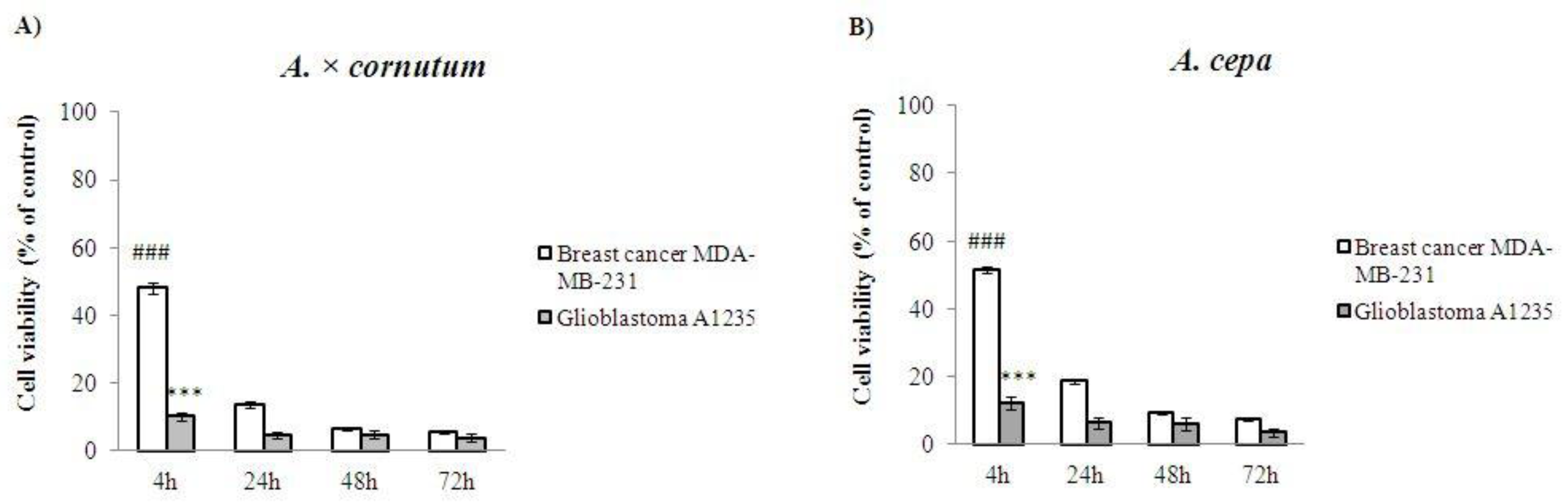
2.5. Cell Proliferation Assay
3. Materials and Methods
3.1. Chemical Reagents
3.2. Plant Material
3.3. Preparation of Onion Extracts
3.4. Analysis of the Phytochemicals
3.4.1. HPLC Analysis of Flavonols
3.4.2. HPLC Analysis of Anthocyanins
3.4.3. Determination of Total Phenolic Content
3.5. Antioxidant Activity
3.5.1. Measurement of the DPPH Radical Scavenging Activity
3.5.2. Oxygen Radical Absorbance Capacity Assay
3.6. Evaluation of Biological Activity
3.6.1. Blood Sampling and Treatment of Human Leukocytes
3.6.2. Comet Assay (Single-Cell Gel Electrophoresis)
3.6.3. DNA Nicking Assay
3.6.4. Cell Culture
3.6.5. Cell Proliferation Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxiodant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Razavi-Azarkhavi, K.; Behravan, J.; Mosaffa, F.; Sehatbakhsh, S.; Shirani, K.; Karimi, G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J. Complement. Integr. Med. 2014, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Bener, M.; Güçlü, K.; Apak, R. Antioxidant/antiradical properties of microwave-assisted extracts of three wild edible mushrooms. Food Chem. 2014, 157, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Puizina, J.; Javornik, B.; Bohanec, B.; Schweizer, D.; Maluszynska, J.; Papeš, D.; Schweizer, D. Random amplified polymorphic DNA analysis, genome size, and genomic in situ hybridization of triploid viviparous onions. Genome 1999, 42, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurushizde, M.; Mashayekhi, S.; Blattner, F.R.; Friesen, N.; Fritsch, R.M. Phylogenetic relationship of wild and cultivated species of Allium section Cepa inferred by nuclear rDNA ITS sequence analysis. Plant Syst. Evol. 2007, 269, 259–269. [Google Scholar] [CrossRef]
- Kendler, B.S. Garlic (Allium sativum) and onion (Allium cepa): A review of their relationship to cardiovascular disease. Prev. Med. 1987, 16, 670–685. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Kim, G.-S.; Kim, J.A.; Song, K.-S. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013, 9, 302–308. [Google Scholar] [PubMed]
- Goldman, I.L.; Kopelberg, M.; Debaene, J.E.; Schwartz, B.S. Antiplatelet activity in onion (Allium cepa) is sulfur dependent. Throm. Haemost. 1996, 76, 450–452. [Google Scholar]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species: A comparative study by means of HPLC-DAD-ESI-MS-MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, L.; Leonardi, C.; Esposito, F.; Salucci, M.; Maiani, G.; Quaglia, G.; Fogliano, V. Flavonoid and carbohydrate contents in Tropea red onions: Effects of homelike peeling and storage. J. Agric. Food Chem. 2002, 50, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Fredotović, Ž.; Šamanić, I.; Schneeweiss-Weiss, H.; Kamenjarin, J.; Jang, T.-S.; Puizina, J. Triparental origin of triploid onion, Allium × cornutum (Clementi ex Visiani, 1842), as evidenced by molecular, phylogenetic and cytogenetic analysis. BMC Plant Biol. 2014, 14, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Caridi, D.; Trenerry, V.C.; Rochfort, S.; Duong, S.; Laugher, D.; Jones, R. Profiling and quantifying quercetin glucosides in onion (Allium cepa L.) varieties using capillary zone electrophoresis and high performance liquid chromatography. Food Chem. 2007, 105, 691–699. [Google Scholar] [CrossRef]
- Lombard, K.A.; Geoffriau, E.; Peffley, E. Flavonoid quantification in onion by spectrophotometric and high performance liquid chromatography analysis. Hortic. Sci. 2002, 37, 682–685. [Google Scholar]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Sinal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Comp. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C. Analysis of the major flavonol glycosidespresent in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J. Sci. Food Agric. 1997, 74, 331–335. [Google Scholar] [CrossRef]
- Rhodes, M.; Price, K. Analytic problems in the study of flavonoid compounds in onion. Food Chem. 1996, 57, 113–117. [Google Scholar] [CrossRef]
- Santas, J.; Carbo, R.; Gordon, M.H.; Almajano, M.P. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front. Life Sci. 2014, 7, 224–228. [Google Scholar] [CrossRef]
- Sharma, K.; Assefa, A.D.; Kim, S.; Young Ko, E.; Lee, E.T.; Park, S.W. Evaluation of total phenolics, flavonoids and antioxidant activity of 18 Korean onion cultivars: A comparative study. J. Sci. Food Agric. 2014, 94, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Zill, H.; Fabiano-Tixier, A.-S.; Elmaataoui, M.; Dangles, O.; Chemat, F. A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties. Food Chem. 2011, 127, 1472–1480. [Google Scholar] [CrossRef]
- Benekeblia, N. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Braz. Arch. Biol. Technol. 2007, 48, 753–759. [Google Scholar] [CrossRef]
- Boivin, D.; Lamy, S.; Lord-Dufour, S.; Jackson, J.; Beaulieu, E.; Côté, M.; Moghrabi, A.; Barrete, S.; Gingras, D.; Béliveau, R. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chem. 2009, 112, 374–380. [Google Scholar] [CrossRef]
- Miller, H.E.; Rogelhof, F.; Marguart, L.; Prakash, A.; Kanter, M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J. Am. Coll. Nutr. 2008, 19, 1–8. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Oksman-Caldentey, K.-M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Yin, M.; Cheng, W. Antioxidant activity of several Allium members. J. Agric. Food Chem. 1998, 46, 4097–4101. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Singh, D.P.; Sharma, B.K.; Upadhyay, G.; Singh, H.B. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Duthie, S.J.; Collins, A.R.; Duthie, G.G.; Dobson, V.L. Quercetin and myricetin protect against hydrogen peroxide- induced DNA damage (strand breaks and oxidized pyrimidines) in human lymphocytes. Mutat. Res. 1997, 393, 223–231. [Google Scholar] [CrossRef]
- Noroozi, M.; Angerson, W.J.; Lean, M.E. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutr. 1998, 67, 1210–1218. [Google Scholar] [PubMed]
- Johnson, M.K.; Loo, G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000, 459, 211–218. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Lamy, E.; Jonas, D.; Stintzing, F.; Mersch-Sundermann, V.; Merfort, I. Fermentation enhances the biological activity of Allium cepa bulb extracts. J. Agric. Food Chem. 2012, 60, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Terdoslavich, M.; Brandoni, A.; Torres, A.M.; Vrhovsek, U.; Passamonti, S. Uptake of grape anthocyanins into the rat kidney and the involvement of bilitranslocase. Mol. Nutr. Food Res. 2008, 52, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 12, 99–101. [Google Scholar]
- Kulišić, T.; Dragović-Uzelac, V.; Miloš, M. Antioxidant activity of aqueous tea infusions prepared from oregano, thyme and wild thyme. Food Technol. Biotechnol. 2006, 44, 485–492. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active oxygen species. J. Agric. Food Chem. 1994, 43, 629–632. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantification of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
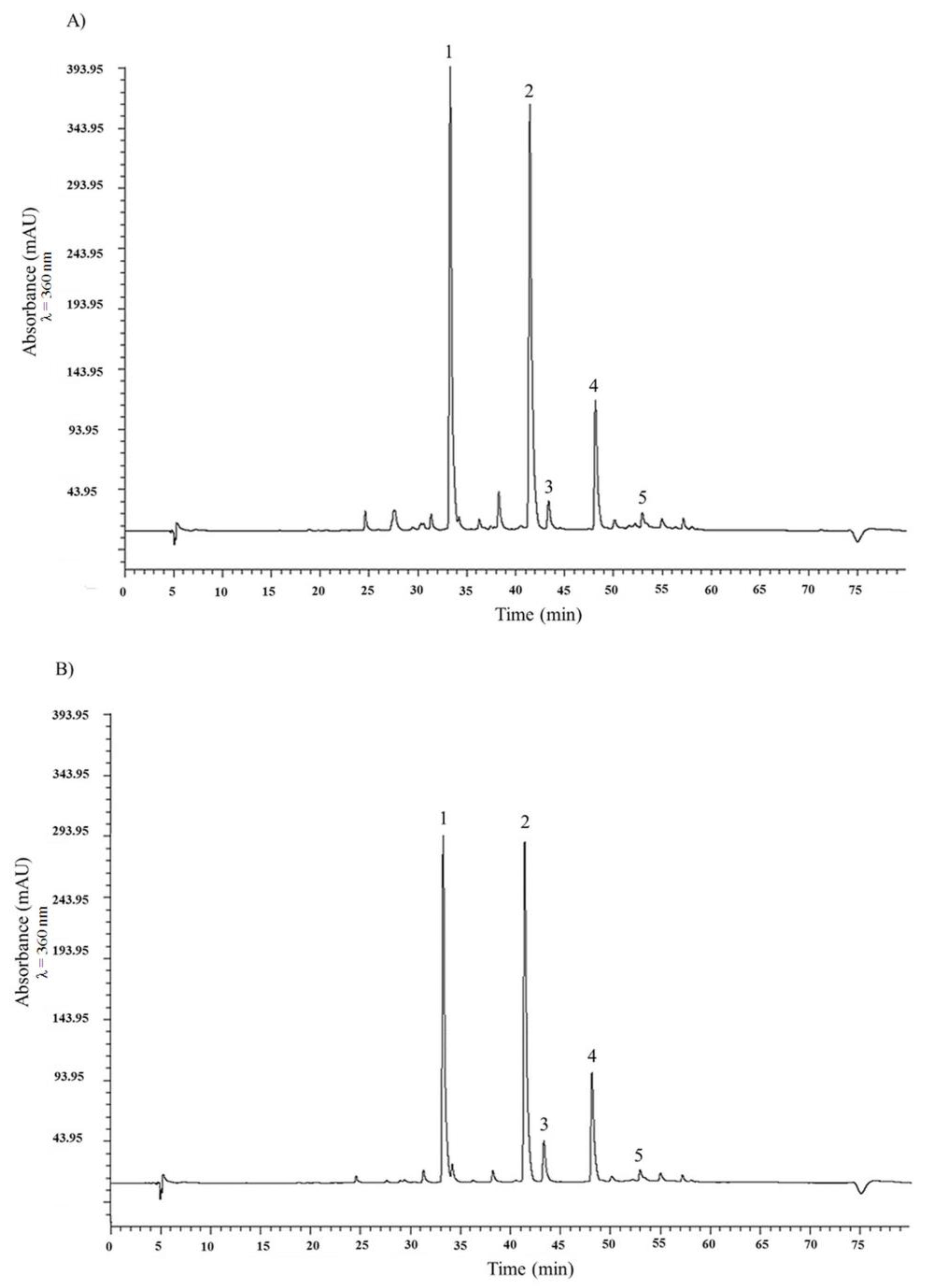

| A. × cornutum (tR, min) | A. cepa (tR, min) | |
|---|---|---|
| Flavonols a | ||
| Quercetin 3,4′-diglucoside (1) | 240.01 ± 0.39 (33.30) | 171.34 ± 0.13 (33.26) |
| Quercetin 4′-monoglucoside (2) | 159.86 ± 0.09 (42.45) | 117.38 ± 0.17 (41.41) |
| Myricetin (3) | 6.22 ± 0.09 (43.37) | 8.02 ± 0.02 (43.34) |
| Quercetin aglycone (4) | 24.13 ± 0.08 (48.17) | 19.85 ± 0.03 (48.13) |
| Isorhamnetin (5) | 7.43 ± 0.05 (52.97) | 4.74 ± 0.01 (52.94) |
| Anthocyanins a | ||
| Peonidin 3′-glucoside (6) | 0.54 ± 0.00 (11.35) | 0.19 ± 0.00 (12.11) |
| Petunidin 3′-glucoside acetate (7) | 0.52 ± 0.00 (21.72) | 0.13 ± 0.01 (22.58) |
| Delphinidin 3′-glucoside (9) | 0.15 ± 0.00 (31.37) | Nd |
| Malvidin 3′-glucoside (8) | 0.01 ± 0.00 (39.27) | 0.03 ± 0.00 (38.87) |
| Antioxidant Assay | A. × cornutum (100 μg/mL) | A. cepa (100 μg/mL) |
|---|---|---|
| DPPH (% DPPH inhibition) | 60.50 ± 3.84 | 64.82 ± 5.31 |
| ORAC (µmol TE/mL) | 19.38 ± 2.21 | 17.62 ± 0.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules 2017, 22, 448. https://doi.org/10.3390/molecules22030448
Fredotović Ž, Šprung M, Soldo B, Ljubenkov I, Budić-Leto I, Bilušić T, Čikeš-Čulić V, Puizina J. Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules. 2017; 22(3):448. https://doi.org/10.3390/molecules22030448
Chicago/Turabian StyleFredotović, Željana, Matilda Šprung, Barbara Soldo, Ivica Ljubenkov, Irena Budić-Leto, Tea Bilušić, Vedrana Čikeš-Čulić, and Jasna Puizina. 2017. "Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts" Molecules 22, no. 3: 448. https://doi.org/10.3390/molecules22030448






