Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria
Abstract
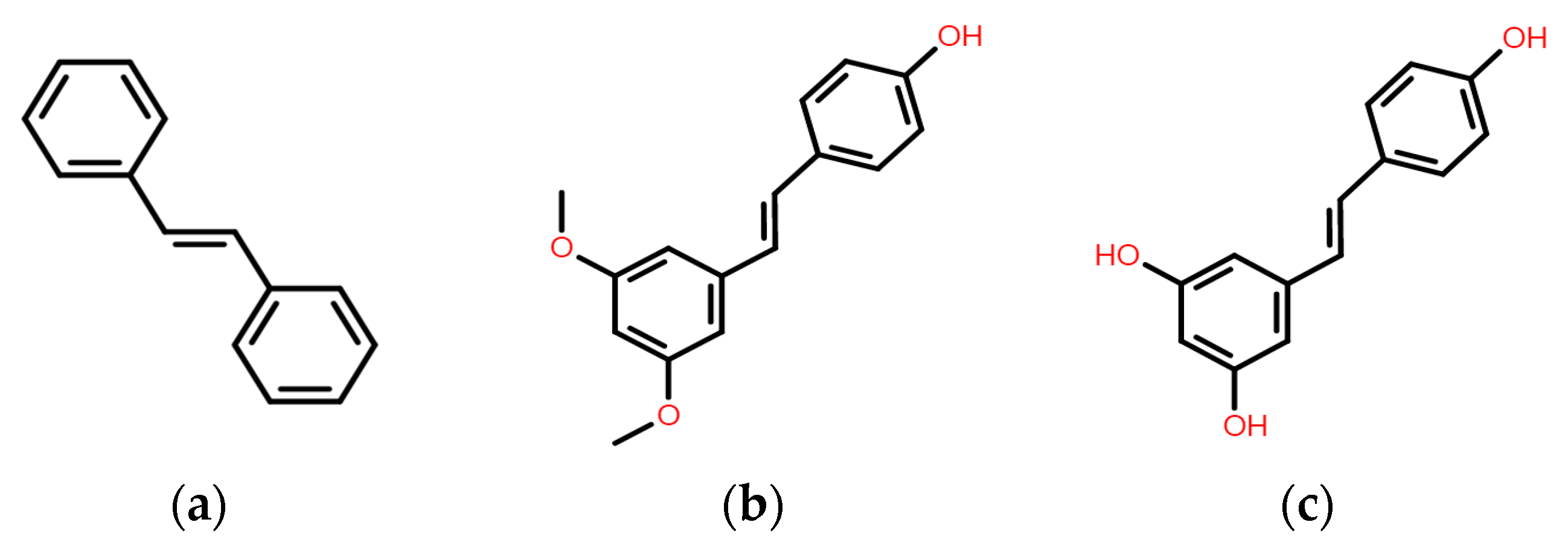
:1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) Values of Pterostilbene and Gentamicin against Gram-Positive Bacteria
2.2. The Minimum Bactericidal Concentration (MBC) Pterostilbene against Gram-Positive and Gram-Negative Bacteria
2.3. FICI Values and Interaction Effects of Pterostilbene and Gentamicin Combinations against Gram-Positive and Gram-Negative Bacteria
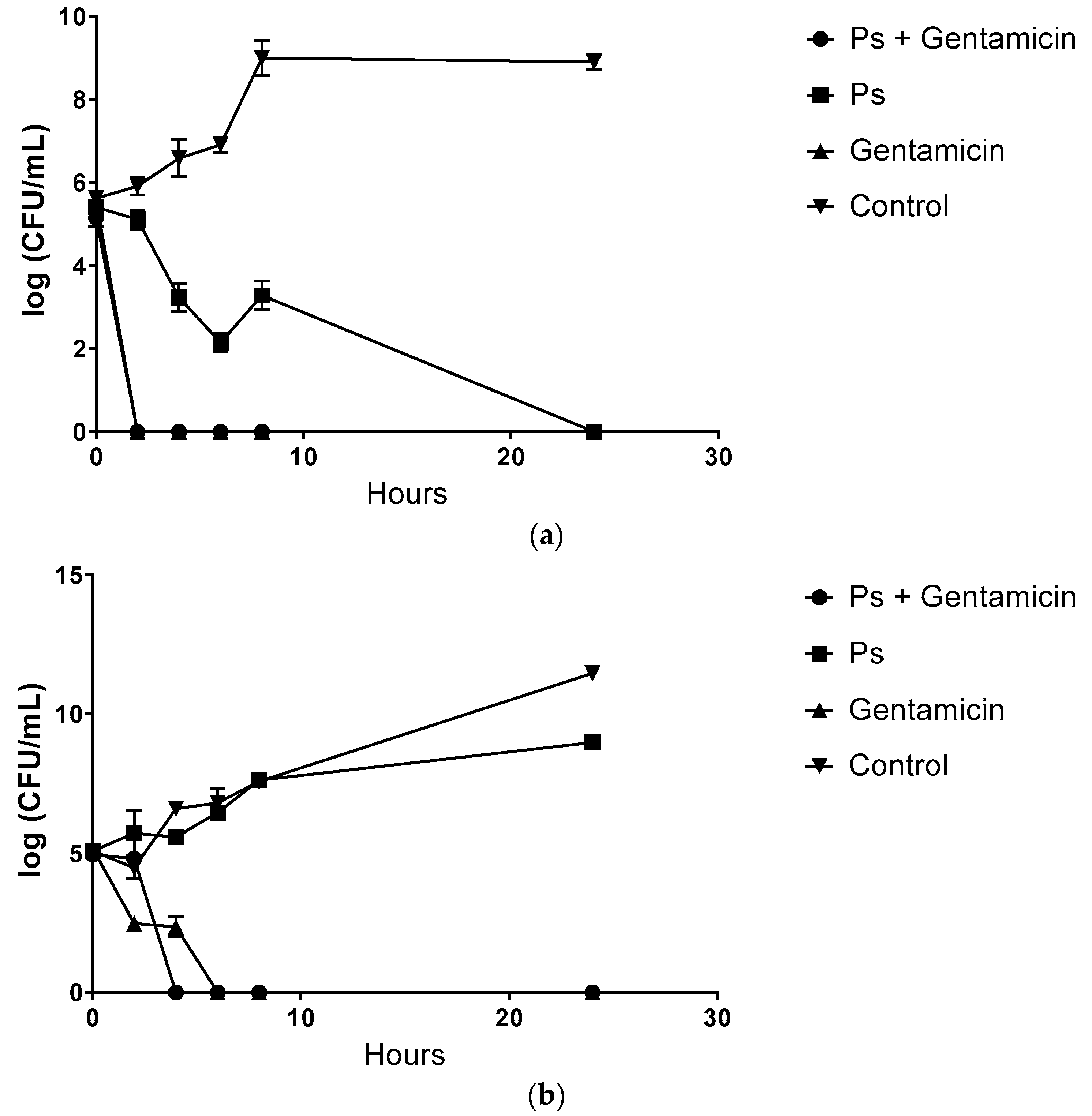
2.4. Time Kill Kinetic of Pterostilbene and Gentamicin Combinations against Gram-Positive and Gram-Negative Bacteria
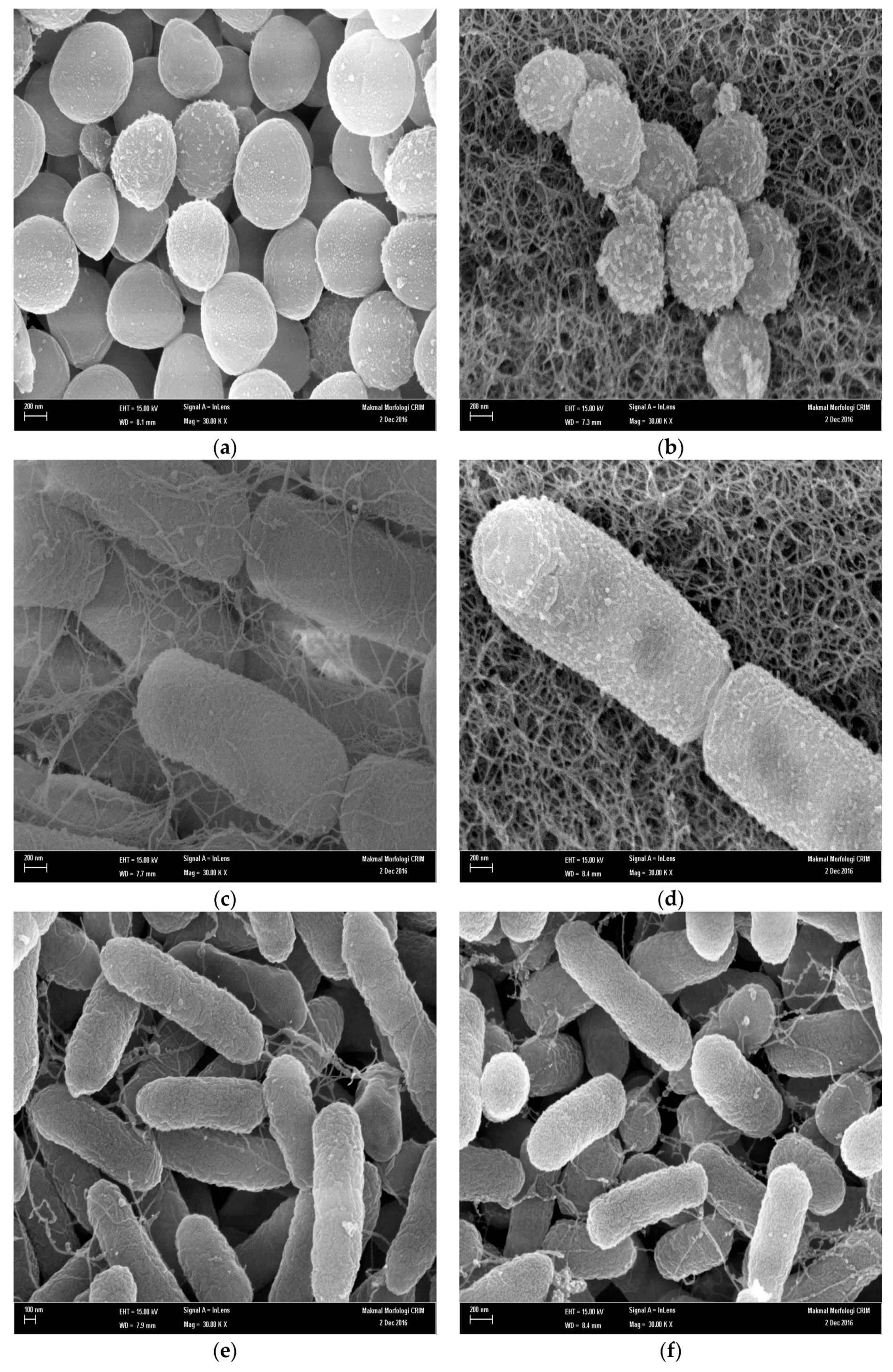
2.5. Scanning Electron Microscope (SEM) Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of Pterostilbene Compound
4.2. Preparation of Bacterial Inoculum
4.3. Determination of Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.4. Determination of Fractional Inhibitory Concentration (FIC)
4.5. Time-Kill Study
4.6. Scanning Electron Microscopic (SEM) Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sköld, O. Antibiotics: The greatest triumph of scientific medicine. In Antibiotics and Antibiotic Resistance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–3. [Google Scholar]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 8, 1533–1538. [Google Scholar] [CrossRef]
- Basri, D.F.; Chan, K.L.; Azmi, A.M.; Jalifah, L. Evaluation of the combined effects of stilbenoid from Shorea gibbosa and vancomycin against methicillin-resistant Staphylococcus aureus (MRSA). Pharmaceuticals 2012, 5, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Lee, W.X.; Shukor, N.I.A.; Jalifah, L. Bacteriostatic antimicrobial combination: Antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. BioMed Res. Int. 2014, 2014, 461756. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Mahady, G.B. Resveratrol as an antibacterial agent. In Resveratrol in Health and Disease; Aggarwal, B., Shishodia, S., Eds.; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006; pp. 465–474. [Google Scholar]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Roupe, K.A.; Remsberg, C.M.; Ya´nez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Yue, B.D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 12, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, AC.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 12, 6103–6105. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 15, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 12, 3453–3457. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Ya´nez, J.A.; Ohgami, Y.K.; Vega-Villa, R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 2, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry 1979, 6, 1025–1027. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Schilling, A.N.; Nikolaou, M. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J. Antimicrob. Chemother. 2005, 55, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: Some recent research. Food Chem. Toxicol. 2003, 41, 1447–1452. [Google Scholar] [CrossRef]
- Malik, C.; Agnès, K.; Abdelwahad, E.; Philippe, M.; Dominique, V.F.; Marielle, A. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar]
- Roberti, M.; Pizzirani, D.; Recanatini, M.; Simoni, D.; Grimaudo, S.; Cristina, A.D.; Abbadessa, V.; Gebbia, N.; Tolomeo, M.J. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P. Mechanisms of action of biocides. Int. Biodeterior. 1990, 26, 89–100. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effects of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Gonçalves, M.I.V.Z.; Di Gaspero, G. Phenolics in grape berry and key antioxidants. In The Biochemistry of the Grape Berry; Gerós, H., Chaves, M.M., Delrot, S., Eds.; Bentham Science Publishers: Bussum, The Netherlands, 2012; pp. 90–91. [Google Scholar]
- Ishak, S.F.; Ghazali, A.R.; Zin, N.M.; Basri, D.F. Pterostilbene enhanced anti-methicillin resistant Staphylococcus aureus (MRSA) activity of oxacillin. Am. J. Infect. Dis. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.N.; Siji, J.V.; Nambisan, B.; Mohandas, C. Activity and synergistic interactions of stilbenes and antibiotic combinations against bacteria in vitro. World J. Microbiol. Biotechnol. 2012, 28, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Cockeril, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferrado, M.J.; Hardy, D.J.; Hecht, D.W. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 2, p. 18. [Google Scholar]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms. Clin. Infect. Dis. 2004, 6, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Khairon, R. Pharmacodynamic interaction of Quercus infectoria galls extract in combination with vancomycin against MRSA using microdilution checkerboard and time-kill assay. Evid. Based Complement. Altern. Med. 2012, 2012, 493156. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C.; Gootz, T.D. Antimicrobial chemotherapy. In Medical Microbiology, 4th ed.; Boron, S., Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Lee, G.C.; Burgess, D.S. Polymyxins and doripenem combination against KPC-producing Klebsiella pneumonia. J. Clin. Med. Res. 2003, 2, 97–100. [Google Scholar]
- Basri, D.F.; Jaffar, N.; Zin, N.M.; Raj, S. Electron microscope study of gall extract from Quercus infectoria in combination with vancomycin against MRSA using post-antibiotic effect determination. Int. J. Pharm. 2013, 2, 150–156. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.

| Concentrations (mg/mL) | Staphylococcus aureus ATCC 25923 | Bacillus cereus ATCC 11778 | ||
|---|---|---|---|---|
| Pterostilbene | Gentamicin | Pterostilbene | Gentamicin | |
| 0.20000 | − | − | + | − |
| 0.10000 | − | − | + | − |
| 0.05000 | − | − | + | − |
| 0.02500 | − | − | + | − |
| 0.01250 | + | − | + | − |
| 0.00625 | + | − | + | − |
| 0.00313 | + | − | + | + |
| 0.00156 | + | − | + | + |
| 0.00078 | + | + | + | + |
| Concentrations (mg/mL) | Escherichia coli ATCC 35150 | Pseudomonas aeruginosa ATCC 15442 | Acinetobacter baumannii ATCC 19606 | |||
|---|---|---|---|---|---|---|
| Pterostilbene | Gentamicin | Pterostilbene | Gentamicin | Pterostilbene | Gentamicin | |
| 0.20000 | − | − | − | − | + | + |
| 0.10000 | − | − | − | − | + | + |
| 0.05000 | − | − | − | − | + | + |
| 0.02500 | + | − | − | − | + | + |
| 0.01250 | + | − | + | − | + | + |
| 0.00625 | + | − | + | − | + | + |
| 0.00313 | + | − | + | + | + | + |
| 0.00156 | + | + | + | + | + | + |
| 0.00078 | + | + | + | + | + | + |
| MIC (mg/mL) | Gram-Positive Bacteria | Gram-Negative Bacteria | |
|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Escherichia coli O157 | P. aeruginosa ATCC 15442 | |
| 0.20000 | − | + | + |
| 0.10000 | − | ND | + |
| 0.05000 | − | ND | + |
| 0.02500 | − | ND | + |
| 0.00125 | ND | ND | ND |
| Species | Antibacterial Agents | MIC (mg/mL) | FIC | ||
|---|---|---|---|---|---|
| Alone | Combination | FIC Individual | FIC Index | ||
| Staphylococcus aureus ATCC 25923 | Pterostilbene | 0.025 | 0.00157 | 0.0625 | 0.125 (S) |
| Gentamicin | 0.00157 | 0.0000981 | 0.0625 | ||
| Escherichia coli O157 | Pterostilbene | 0.2 | 0.0125 | 0.0625 | 0.3185 (S) |
| Gentamicin | 0.00313 | 0.0008 | 0.256 | ||
| Pseudomonas aeruginosa ATCC 15442 | Pterostilbene | 0.2 | 0.025 | 0.125 | 0.25 (S) |
| Gentamicin | 0.00625 | 0.00078 | 0.125 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.X.; Basri, D.F.; Ghazali, A.R. Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria. Molecules 2017, 22, 463. https://doi.org/10.3390/molecules22030463
Lee WX, Basri DF, Ghazali AR. Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria. Molecules. 2017; 22(3):463. https://doi.org/10.3390/molecules22030463
Chicago/Turabian StyleLee, Wee Xian, Dayang Fredalina Basri, and Ahmad Rohi Ghazali. 2017. "Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria" Molecules 22, no. 3: 463. https://doi.org/10.3390/molecules22030463





