Design of New Benzo[h]chromene Derivatives: Antitumor Activities and Structure-Activity Relationships of the 2,3-Positions and Fused Rings at the 2,3-Positions
Abstract
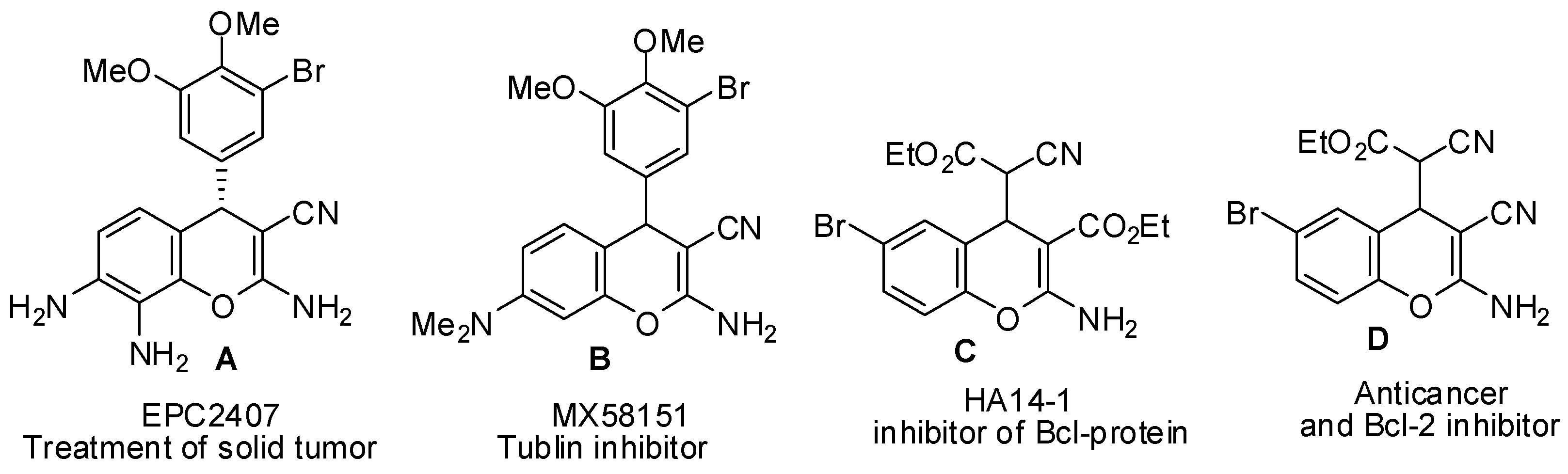
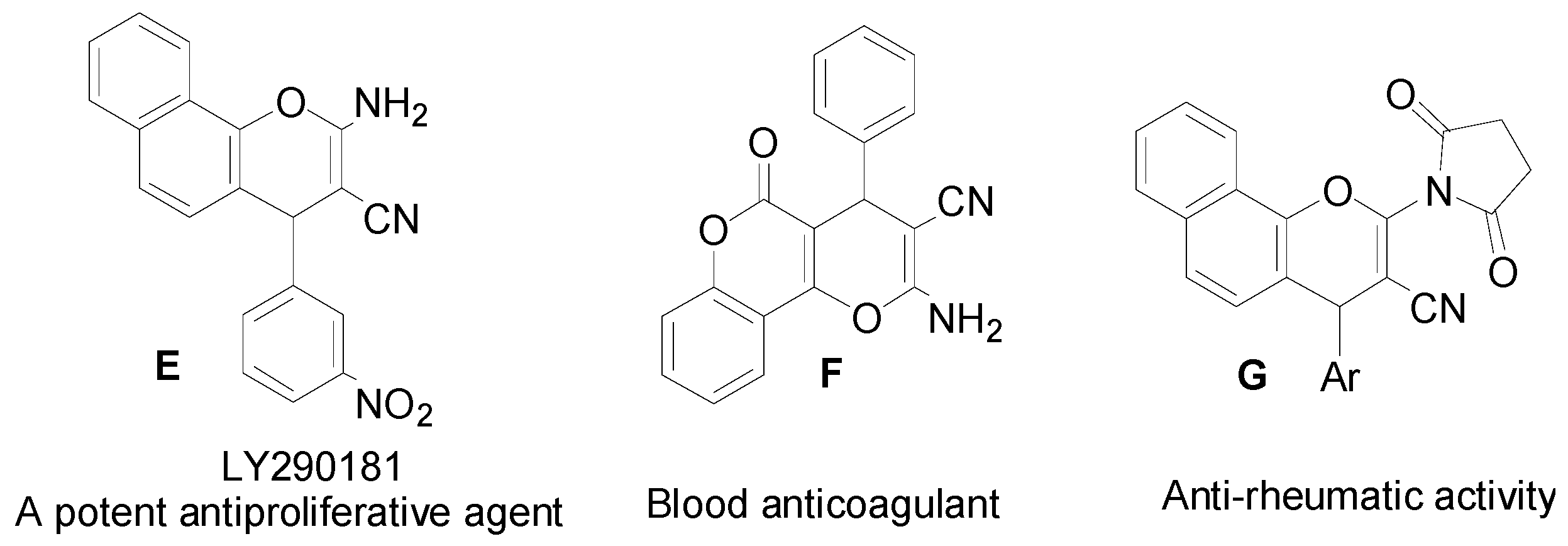
:1. Introduction
2. Results and Discussion
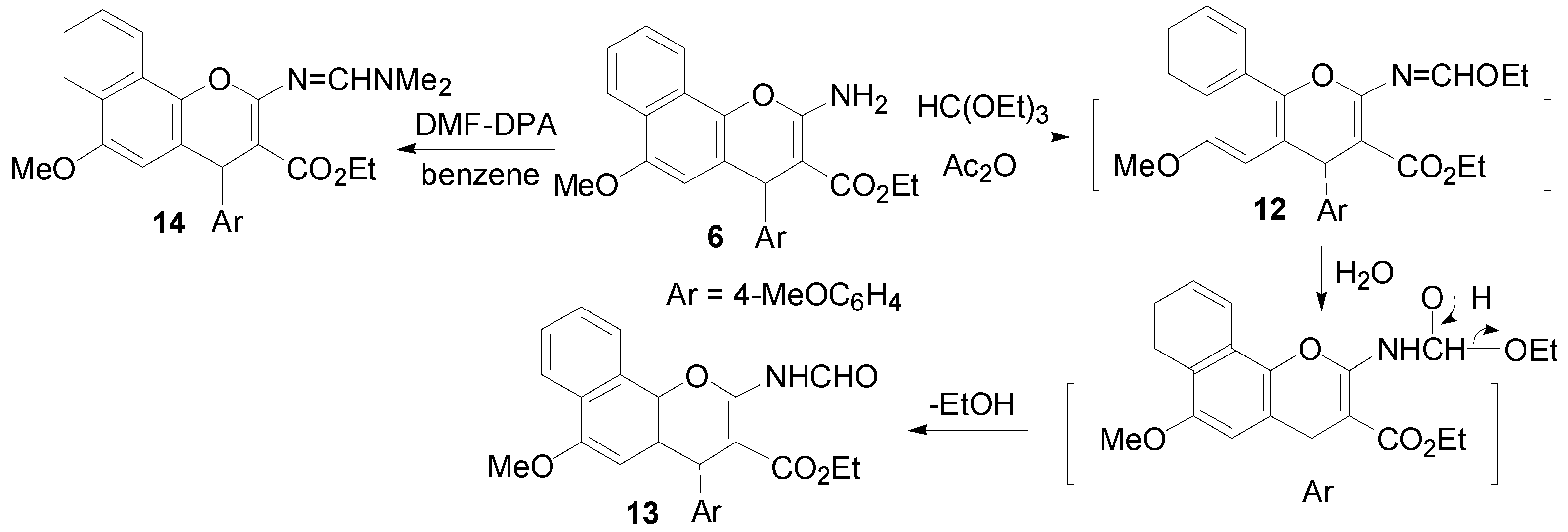
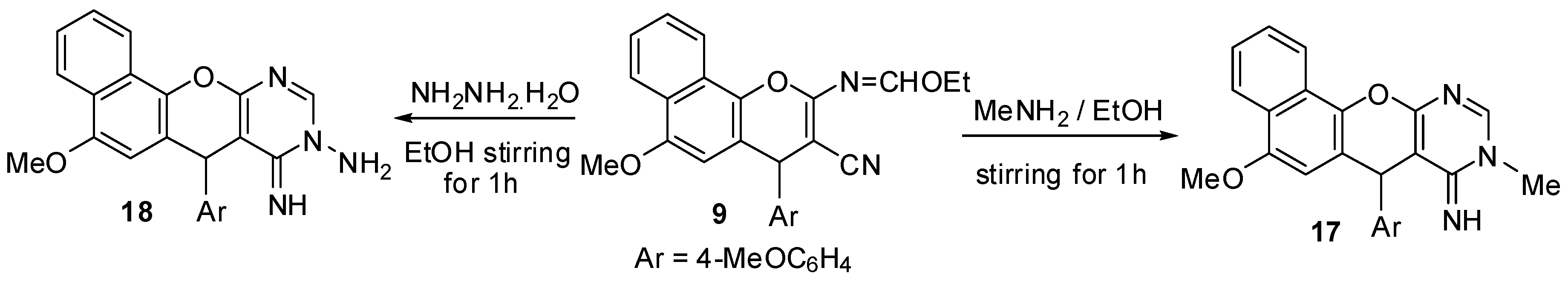
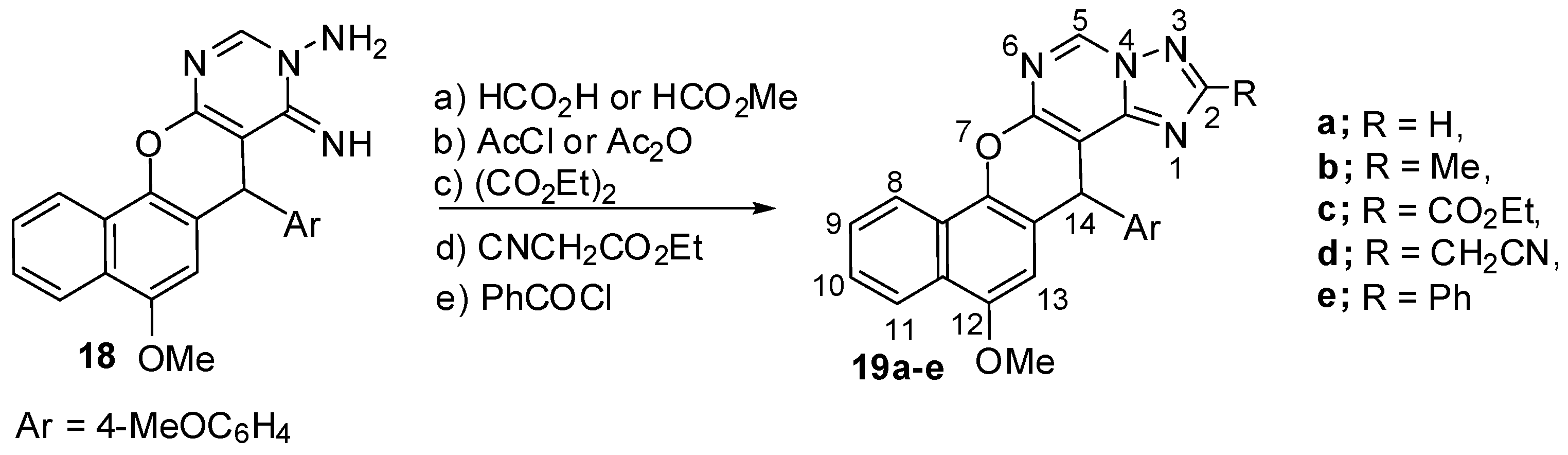
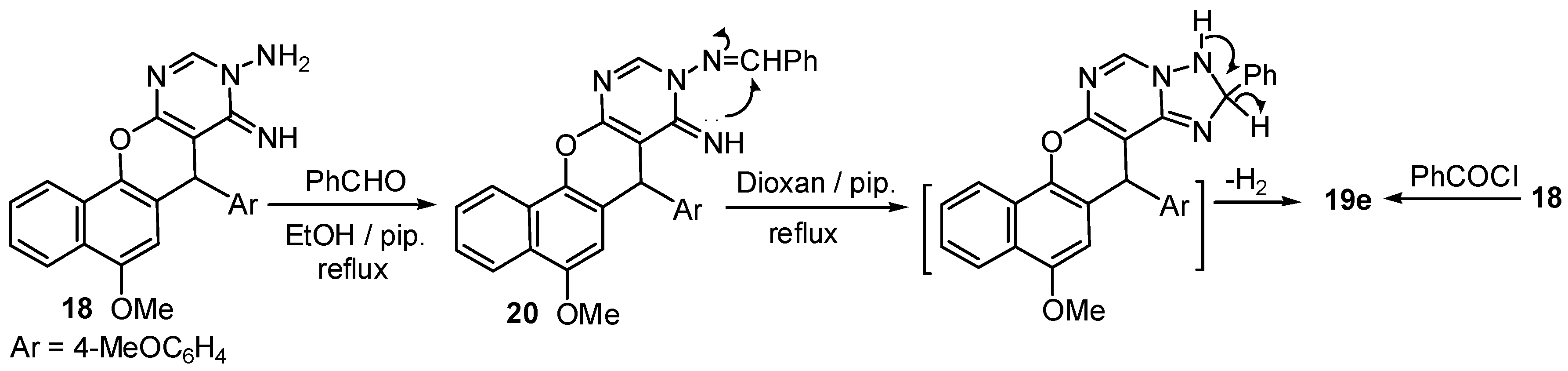
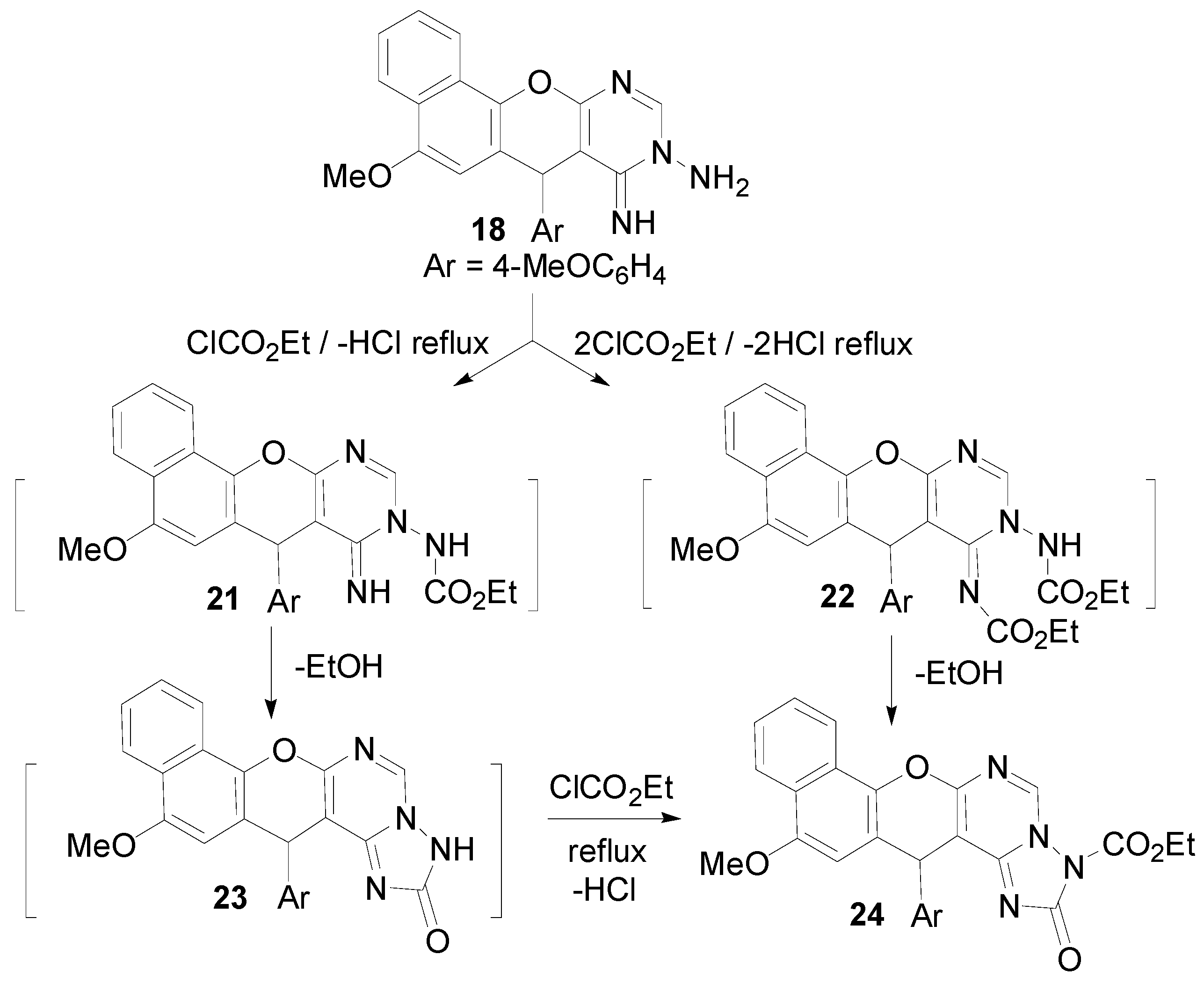
2.1. Chemistry
2.2. Antitumor Evaluation
2.3. SAR Studies
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.3. Antitumor Activity Assay
3.3.1. Cell Culture
3.3.2. Cytotoxicity Evaluation Using Viability Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, G.; Sharma, A.; Kaur, H.; Ishar, M. Chromanyl-isoxazolidines as Antibacterial agents: Synthesis, Biological Evaluation, Quantitative Structure Activity Relationship, and Molecular Docking Studies. Chem. Biol. Drug. Des. 2016, 87, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Vala, N.D.; Jardosh, H.H.; Patel, M.P. 5-PS-TBD triggered general potocol for the synthesis of 4H-chromenes, pyrano[4,3-b]pyran and pyrano[3,2-c]chromene derivatives of 1H-pyrazole and their biological activities. Chin. Chem. Lett. 2016, 27, 168–172. [Google Scholar] [CrossRef]
- Bingi, C.; Emmadi, N.R.; Chennapuram, M.; Poornachandra, Y.; Kumar, C.G.; Nanubolu, J.B.; Atmakur, K. One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm. Bioorg. Med. Chem. Lett. 2015, 25, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Killander, D.; Sterner, O. Synthesis of the Bioactive Benzochromenes Pulchrol and Pulchral, Metabolites. Eur. J. Org. Chem. 2014, 8, 1594–1596. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Divya, B.; Swaina, M.; Rao, T.P.; Yadav, J.S.; Vishnu Vardhan, M.V. A domino Knoevenagel hetero-Diels-Alder reaction for the synthesis of polycyclic chromene derivatives and evaluation of their cytotoxicity. Bioorg. Med. Chem. Lett. 2012, 22, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Kumar, M.; Modukuri, R.K.; Srivastava, A.; Puri, A. Discovery and synthesis of novel substituted benzocoumarins as orally active lipid modulating agents. Bioorg. Med. Chem. Lett. 2011, 21, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; Berghot, M.A.; Amer, F.A.; Badawy, D.S.; Bayoumy, N.M. Synthesis and Antioxidant and Antitumor Activity of Novel Pyridine, Chromene, Thiophene and Thiazole Derivatives. Arch. Pharm. Chem. 2012, 345, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Nareshkumar, J.; Jiayi, X.; Ramesh, M.K.; Fuyong, D.; Guo, J.Z.; Emmanuel, P. Identification and Structure-Activity Relationships of Chromene-Derived Selective Estrogen Receptor Modulators for Treatment of Postmenopausal Symptoms. J. Med. Chem. 2009, 52, 7544–7569. [Google Scholar]
- El-Sayed, A.T.; Ibrahim, M.A. Synthesis and Antimicrobial Activity of Chromone-linked-2-Pyridone Fused with 1,2,4-Triazoles, 1,2,4-Triazines and 1,2,4-Triazepines Ring Systems. J. Braz. Chem. 2010, 21, 1007–1016. [Google Scholar]
- Foroumadi, A.; Emami, S.; Sorkhi, M.; Nakhjiri, M.; Nazarian, Z.; Heydar, S.; Ardestani, S.; Poorrajab, F.; Shafiee, A. Chromene-Based Synthetic Chalcones as Potent Antileishmani-al Agents: Synthesis and Biological Activity. Chem. Biol. Drug. Des. 2010, 75, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.C.A.; da Silva, C.C.; Ferreira, I.C.P.; Machado, G.M.C.; Leon, L.L.; de Oliveira, A.J.B. Antileishmanial activity of indole alkaloids from Aspidospermaramiflorum. Phytomedicine 2007, 14, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther. 2004, 3, 1365–1373. [Google Scholar] [PubMed]
- Lee, K.-S.; Khil, L.-Y.; Chae, S.-H.; Kim, D.; Lee, B.-H.; Hwang, G.-S.; Moon, C.-H.; Chang, T.-S.; Moon, C.-K. Effects of DK-002, a synthesized (6aS,cis)-9,10-Dimethoxy-7,11b-dihydro indeno[2,1-c]-chromene-3,6a-diol, on platelet activity. Life Sci. 2006, 78, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Kirilmis, C.; Ahmedzade, M.; Servi, S.; Koca, M.; Kizirgil, A.; Kazaz, C. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem. 2008, 43, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Elinson, M.N.; Ilovaisky, A.I.; Merkulova, V.M.; Belyakov, P.A.; Chizhov, A.O.; Nikishin, G.I. Solvent-free cascade reaction: Direct multicomponent assembling of 2-amino-4H-chromene scaffold from salicylaldehyde, malononitrile or cyanoacetate and nitroalkanes. Tetrahedron 2010, 66, 4043–4048. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Shafiei Haghighi, S. Multi-component synthesis of 2-amino-4H-chromenes catalyzed by nano ZnO in water. Collect. Czechoslov. Chem. Commun. 2011, 76, 1285–1298. [Google Scholar] [CrossRef]
- Boominathan, M.; Nagaraj, M.; Muthusubramanian, S.; Krishnakumar, R.V. Efficient atom economical one-pot multicomponent synthesis of densely functionalized 4H-chromene derivatives. Tetrahedron 2011, 67, 6057–6064. [Google Scholar] [CrossRef]
- Mehrabi, H.; Kazemi-Mireki, M. CuO nanoparticles: An efficient and recyclable nanocatalyst for the rapid and green synthesis of 3,4-dihydropyrano[c]chromenes. Chin. Chem. Lett. 2011, 22, 1419–1422. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Gupta, M.K.; Prathap, I.; Pandey, S.K. Amberlyst A-21®: An efficient, cost-effective and recyclable catalyst for the synthesis of substituted 4H-chromenes. Catal. Commun. 2007, 8, 2208–2211. [Google Scholar] [CrossRef]
- Makarem, S.; Mohammadi, A.A.; Fakhari, A.R. A multi-component electro-organic synthesis of 2-amino-4H-chromenes. Tetrahedron Lett. 2008, 49, 7194–7196. [Google Scholar] [CrossRef]
- Hamelin, J.; Bazureau, J.P.; Texier-Boullet, F. Microwaves in Organic Synthesis; Loupy, A., Ed.; Wiley-VCH: Weinheim, Germany, 2002; p. 253. [Google Scholar]
- Zbancioc, G.; Mangalagiu, I.I. Microwave-Assisted Synthesis of Highly Fluorescent Pyrrolopyridazine Derivatives. Synlett 2006, 5, 804–806. [Google Scholar] [CrossRef]
- Bejan, V.; Mantu, D.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of isoindolo-1,2-diazine: A comparative study. Ultrason. Sonochem. 2012, 19, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1,2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Safari, J.; Javadian, L. Ultrasound assisted the green synthesis of 2-amino-4H-chromene derivatives catalyzed by Fe3O4-functionalized nanoparticles with chitosan as a novel and reusable magnetic catalyst. Ultrason. Sonochem. 2015, 22, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, M.; Fan, C.A.; Zhang, F.M.; Tu, Y.Q. Rapid and Efficient Microwave Assisted Amination of Electron-Rich Aryl Halides without a Transition-Metal Catalyst. Org. Lett. 2003, 5, 3515–3517. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, M.; Fan, C.A.; Zhang, F.M.; Tu, Y.Q. Microwave-Promoted Three-Component Coupling of Aldehyde, Alkyne, and Amine via C–H Activation Catalyzed by Copper in Water. Org. Lett. 2004, 6, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Larhed, M. Microwave-Enhanced Aminocarbonylations in Water. Org. Lett. 2005, 7, 3327–3329. [Google Scholar]
- Kidwai, M.; Saxena, S.; Khan, M.K.R.; Thukra, S.S. Aqua mediated synthesis of substituted 2-amino-4H-chromenes and in vitro study as antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 4295–4298. [Google Scholar] [CrossRef] [PubMed]
- Surpur, M.P.; Kshirsagar, S.; Samant, S.D. Exploitation of the catalytic efficacy of Mg/Al hydrotalcite for the rapid synthesis of 2-aminochromene derivatives via a multicomponent strategy in the presence of microwaves. Tetrahedron Lett. 2009, 50, 719–722. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Sadek, K.U. Microwave-assisted reactions: Three-component process for the synthesis of 2-amino-2-chromenes under microwave heating. Chin. Chem. Lett. 2009, 20, 271–274. [Google Scholar] [CrossRef]
- Patil, S.; Patil, R.; Pfeffer, L.; Miller, D. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H.; Zhao, J.; Jia, S.; Xu, L.; Crogan-Grundy, C.; Denis, R.; Barriault, N.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Liu, D.; Zhang, Z.-J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.M.; Tian, D.; Xing, C. Structure-activity relationship studies of ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer. J. Med. Chem. 2006, 49, 7731–7739. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Singh, J.P.; Wilson, L. Suppression of Microtubule Dynamics by LY290181 a potential mechanism for its antiproliferative action. J. Biol. Chem. 1997, 272, 7681–7687. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Panda, D.; Wiernicki, T.R.; Wilson, L.; Jordan, M.A.; Singh, J.P. Inhibition of Mitosis and Microtubule Function through Direct Tubulin Binding by a Novel Antiproliferative Naphthopyran LY290181. Mol. Pharmacol. 1997, 52, 437–444. [Google Scholar] [PubMed]
- Wiener, C.; Schroeder, C.H.; West, B.D.; Link, K.P. Studies on the 4-hydroxycoumarins. XVIII. 3-[α-(acetamidomethyl)benzyl]-4-hydroxycoumarin and related products. J. Org. Chem. 1962, 27, 3086–3088. [Google Scholar] [CrossRef]
- Smith, C.W.; Bailey, J.M.; Billingham, M.E.J.; Chandrasekhar, S.; Dell, C.P.; Harvey, A.K.; Hicks, C.A.; Kingston, A.E.; Wishart, G.N. The anti-rheumatic potential of a series of 2,4-disubstituted-4H-naphtho[1,2-b]pyran-3-carbonitriles. Bioorg. Med. Chem. Lett. 1995, 5, 2783–2788. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Khattab, E.S.A.E.H.; Fouda, A.M. Synthesis, Structure-Activity Relation-ship (SAR) Studies on some 4-Aryl-4H-chromenes and Relationship between Lipophilicity and Antitumor Activity. Lett. Drug. Des. Discov. 2014, 11, 1167–1176. [Google Scholar] [CrossRef]
- Khafagy, M.M.; Abd El-Wahab, A.H.F.; Eid, F.A.; El-Agrody, A.M. Synthesis of Halogen Derivatives of Benzo[h]cheromene and Benzo[a]anthracene with Promising Antimicrobial Activities. Il Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- Bedair, A.H.; Emam, H.A.; El-Hady, N.A.; Ahmed, K.A.R.; El-Agrody, A.M. Synthesis and antimicrobial activities of novel naphtho[2,1-b]pyran, pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]-pyrimidine derivatives. Il Farmaco 2001, 56, 965–973. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rahman, A.U.; Choudhary, M.I.; Thomsen, W.J. Bioassay Technique for Drug Development; Harwood Academic Publishers: Newark, NJ, USA, 2001. [Google Scholar]
- Sample Availability: Samples of the compounds 4, 6–11 and 13–18 are available from the authors.



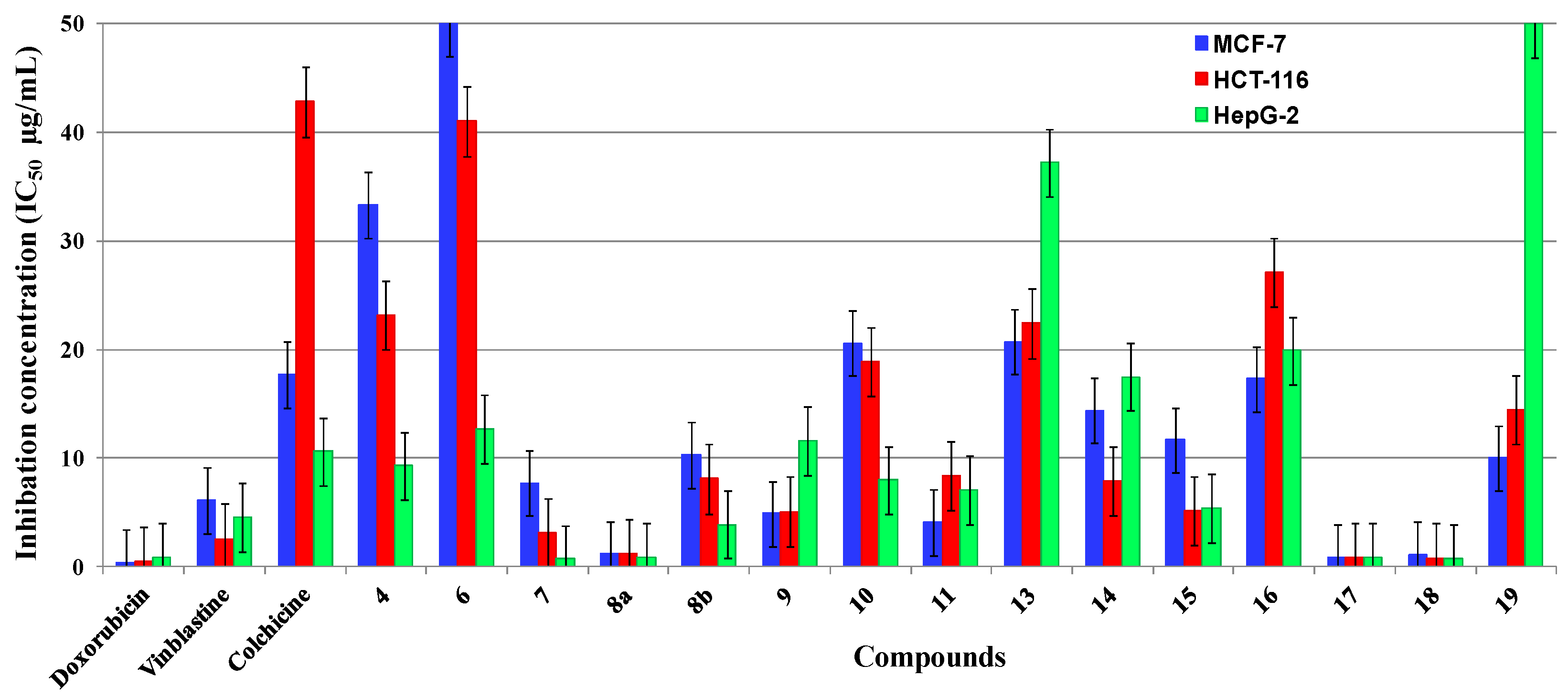
| Compound | R | IC50 (µg/mL) a | log P | ||
|---|---|---|---|---|---|
| MCF-7 | HCT-116 | HepG-2 | |||
| 4 | NH2 | 33.3 ± 0.2 | 23.2 ± 0.29 | 9.3 ± 0.4 | 4.20 ± 0.50 |
| 6 | NH2 | w | 41.0 ± 0.13 | 12.7 ± 0.15 | 4.58 ± 0.45 |
| 7 | N=CHPh | 7.7 ± 0.14 | 3.1 ± 0.23 | 0.7 ± 0.11 | 6.89 ± 0.63 |
| 8a | NHAc | 1.2 ± 0.35 | 1.2 ± 0.2 | 0.9 ± 0.1 | 4.36 ± 0.50 |
| 8b | NAc2 | 10.3 ± 0.14 | 8.1 ± 0.18 | 3.9 ± 0.3 | 3.81 ± 0.67 |
| 9 | N=CHOEt | 4.9 ± 0.14 | 5.1 ± 0.36 | 11.6 ± 0.04 | 6.12 ± 0.63 |
| 10 | N=CHNMe2 | 20.6 ± 0.01 | 18.9 ± 0.15 | 8.0 ± 0.17 | 4.92 ± 0.64 |
| 11 | N=CHNH2 | 4.1 ± 0.16 | 8.4 ± 0.02 | 7.1 ± 0.04 | 4.62 ± 0.63 |
| 13 | NHCHO | 20.7 ± 0.23 | 22.4 ± 0.18 | 37.2 ± 0.2 | 4.57 ± 0.46 |
| 14 | N=CHNMe2 | 14.4 ± 0.01 | 7.9 ± 0.05 | 17.5 ± 0.01 | 5.31 ± 0.63 |
| 15 | - | 11.7 ± 0.97 | 5.2 ± 0.29 | 5.4 ± 0.07 | 3.27 ± 0.56 |
| 16 | - | 17.3 ± 0.58 | 27.1 ± 0.13 | 19.9 ± 0.25 | 3.72 ± 0.69 |
| 17 | Me | 0.9 ± 0.06 | 0.9 ± 0.05 | 0.9 ± 0.11 | 3.52 ± 0.75 |
| 18 | NH2 | 1.1 ± 0.14 | 0.8 ± 0.12 | 0.8 ± 0.08 | 3.00 ± 0.76 |
| 20 | N=CHPh | 10.0 ± 0.9 | 14.5 ± 0.5 | w | 5.61 ± 0.78 |
| Vinblastine | - | 6.1 ± 0.03 | 2.6 ± 0.08 | 4.6 ± 0.01 | 4.58 ± 0.90 |
| Colchicine | - | 17.7 ± 0.01 | 42.8 ± 0.02 | 10.6 ± 0.04 | 0.92 ± 0.92 |
| Doxorubicin | - | 0.4 ± 0.01 | 0.5 ± 0.02 | 0.9 ± 0.04 | 2.82 ± 1.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okasha, R.M.; Alblewi, F.F.; Afifi, T.H.; Naqvi, A.; Fouda, A.M.; Al-Dies, A.-A.M.; El-Agrody, A.M. Design of New Benzo[h]chromene Derivatives: Antitumor Activities and Structure-Activity Relationships of the 2,3-Positions and Fused Rings at the 2,3-Positions. Molecules 2017, 22, 479. https://doi.org/10.3390/molecules22030479
Okasha RM, Alblewi FF, Afifi TH, Naqvi A, Fouda AM, Al-Dies A-AM, El-Agrody AM. Design of New Benzo[h]chromene Derivatives: Antitumor Activities and Structure-Activity Relationships of the 2,3-Positions and Fused Rings at the 2,3-Positions. Molecules. 2017; 22(3):479. https://doi.org/10.3390/molecules22030479
Chicago/Turabian StyleOkasha, Rawda M., Fawzia F. Alblewi, Tarek H. Afifi, Arshi Naqvi, Ahmed M. Fouda, Al-Anood M. Al-Dies, and Ahmed M. El-Agrody. 2017. "Design of New Benzo[h]chromene Derivatives: Antitumor Activities and Structure-Activity Relationships of the 2,3-Positions and Fused Rings at the 2,3-Positions" Molecules 22, no. 3: 479. https://doi.org/10.3390/molecules22030479






