1. Introduction
Allergic rhinitis (AR) is a common chronic inflammatory disease, which affects the health of nearly 30% of the world population [
1]. It is characterized by inflammation of nasal mucosa with hypersensitivity resulting from all kinds of allergens. Typical symptoms of AR including sneezing, rhinorrhea, nasal rubbing, nasal congestion, and obstruction, seriously affecting patients’ quality of life and work [
2,
3].
An allergic reaction is an immunological disorder and caused by genetic and environmental factors, among others [
4]. Among immune cells, T helper type 2 (Th2) cells and mast cells play crucial roles in the pathogenesis of allergic responses in AR. Activation of Th2 cells by antigens induces a production of Th2 cytokines, such as IL-4, which promotes a special production of immunoglobulin E (IgE) from B lymphocytes [
5,
6]. Once IgE binds to the high-affinity immunoglobulin Fc epsilon receptor I (FcεR I), a mast cell surface receptor, special IgE responses to antigens are triggered. Subsequently, mast cells are activated by the complex of IgE-FcεR I on the surface of mast cells, resulting in a degranulation response and secreting allergic mediators including inflammatory cytokines, histamine, β-hexosaminidase, chemokines, and arachidonic derivatives, which results in infiltrating inflammatory cells followed by acute or chronic inflammation in nasal and swollen nasal mucosa [
4,
7].
Current therapeutic drugs towards AR are limited to antihistamines, anti-allergen, anti-leukotriene, and intranasal corticosteroids that can only alleviate allergic symptoms but fail to regulate the allergic reaction and bring adverse effects such as headache, throat irritation, and nasal dryness. Consequently, researching and developing safe and effective therapeutic agents is necessary, and traditional Chinese medicine is an attractive area for its multi-target combination and regulation of allergic reaction pathogenesis [
8,
9]. Recently, polyherbal medicine exhibits good potential in the treatment of allergic diseases because of its efficacy and safety [
10,
11]. Shenqi, a traditional Chinese polyherbal medicine, has been successfully prescribed for the clinical treatment of allergic rhinitis by some traditional healers. The main active compounds of the Shenqi formula include
Codonopsis pilosula polysaccharide, Astragaloside, Baicalein, Imperatorin, Chlorgenic acid, Indirubin, Luteolin, and Methyleugenol. However, no research on Shenqi has been conducted to reveal possible mechanisms of treatment of AR.
Hence, we hypothesized that the anti-allergic rhinitis action of Shenqi might be associated with inhibition, inflammation, and immunoregulation. In the present study, we aimed to evaluate the effect of Shenqi on allergic inflammatory responses in OVA-induced allergic rhinitis rat models and on IgE-induced mast cell degranulation.
3. Discussion
In the present study, we evaluated the anti-allergic rhinitis effect and possible modulating mechanism of Shenqi for the first time, which is an ancient Chinese herbal formula widely used for treating AR. The results demonstrated that Shenqi exhibited remarkable anti-allergic effects by suppressing sneezing, nose scratching, and permeability of nasal mucosa in OVA-induced AR rats. Further investigation implied that the anti-allergic activity of Shenqi is achieved by inhibiting the degranulation in RBL-2H3 mast cells and the production of allergic or inflammatory mediators, such as IgE, histamine, IL-4, and IFN-γ in vivo or in vitro. We also found that the anti-allergic effect of Shenqi was related to improving the imbalance of Th1/Th2 (IFN-γ/IL-4) by modulating the expression of transcription factor GATA3 related to Th2- and T-bet-related to Th1 from primary spleen lymphocytes.
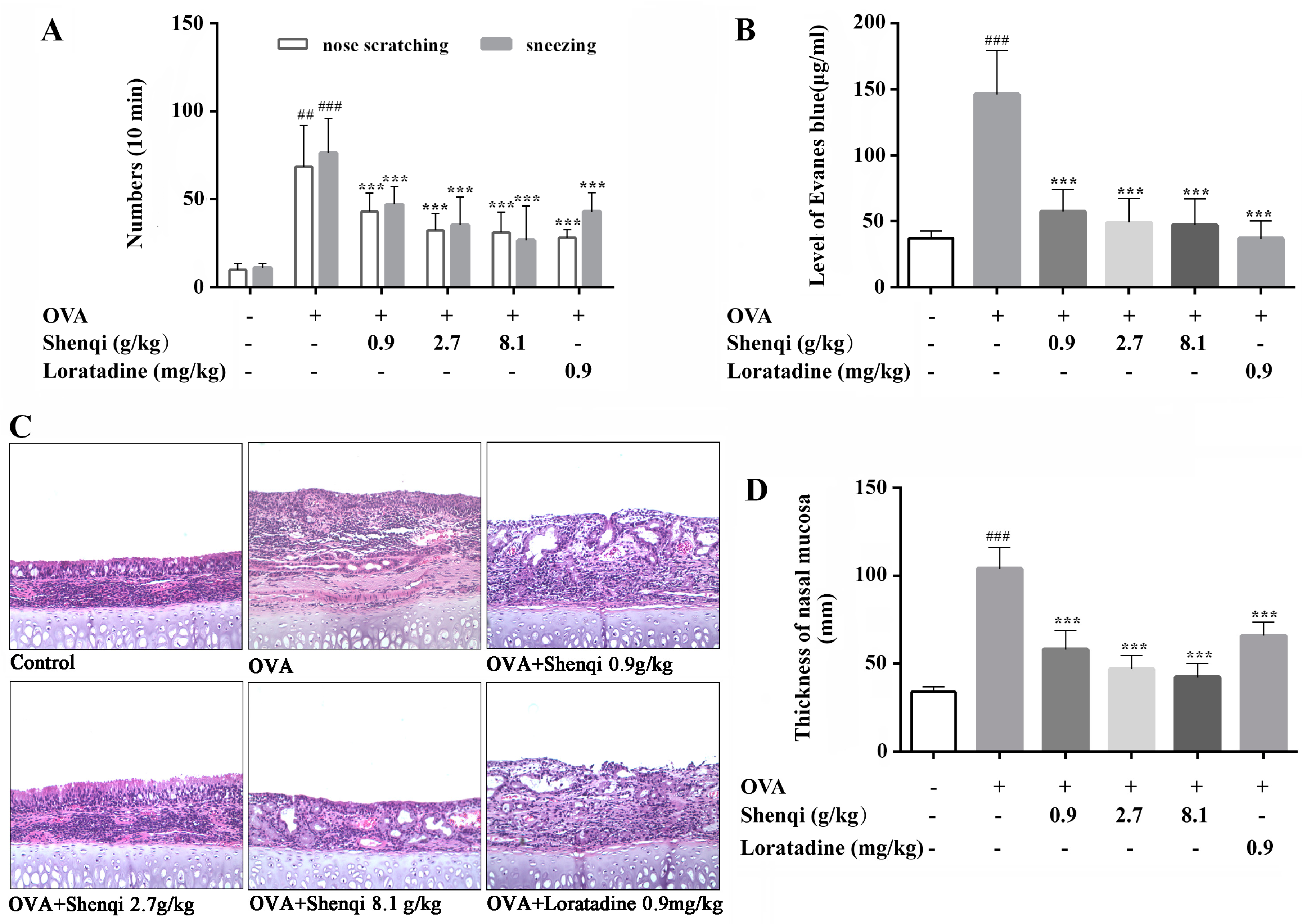
Ovalbumin is widely used to induce AR rat model, this model rat shows the characteristic rhinitis symptoms and pathological changes of AR, such as sneezing, nasal scratching, congestion, and mucosa edema. These are elicited by OVA sensitization and challenge in the in vivo model, resulting in increased levels of histamine and total IgE in the serum and the infiltration of inflammatory cells in the nasal mucosa [
12,
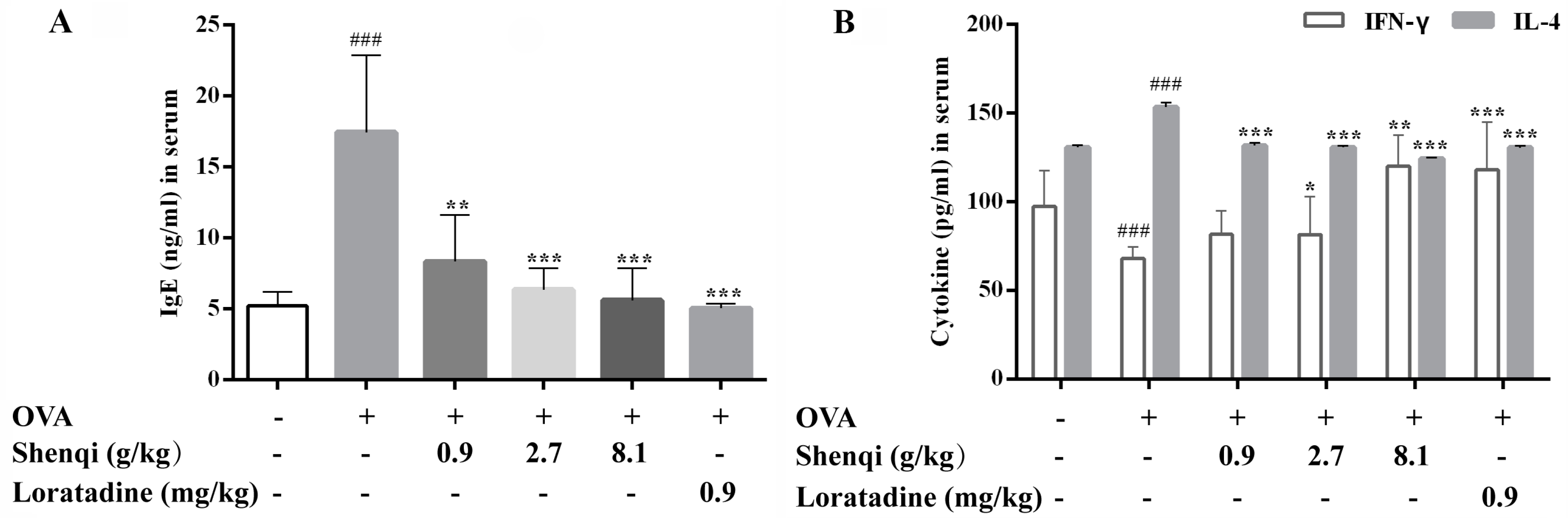
13]. In our study, OVA-induced AR model rats were used to assess the anti-allergic efficacy of Shenqi. Shenqi treatment remarkably alleviated the AR symptoms, the morphological changes of nasal mucosa, and the IgE production in OVA-sensitized rats. The results demonstrated that Shenqi possessed a treatment effect on AR, and this may be involved in suppressing IgE production in AR model rats.
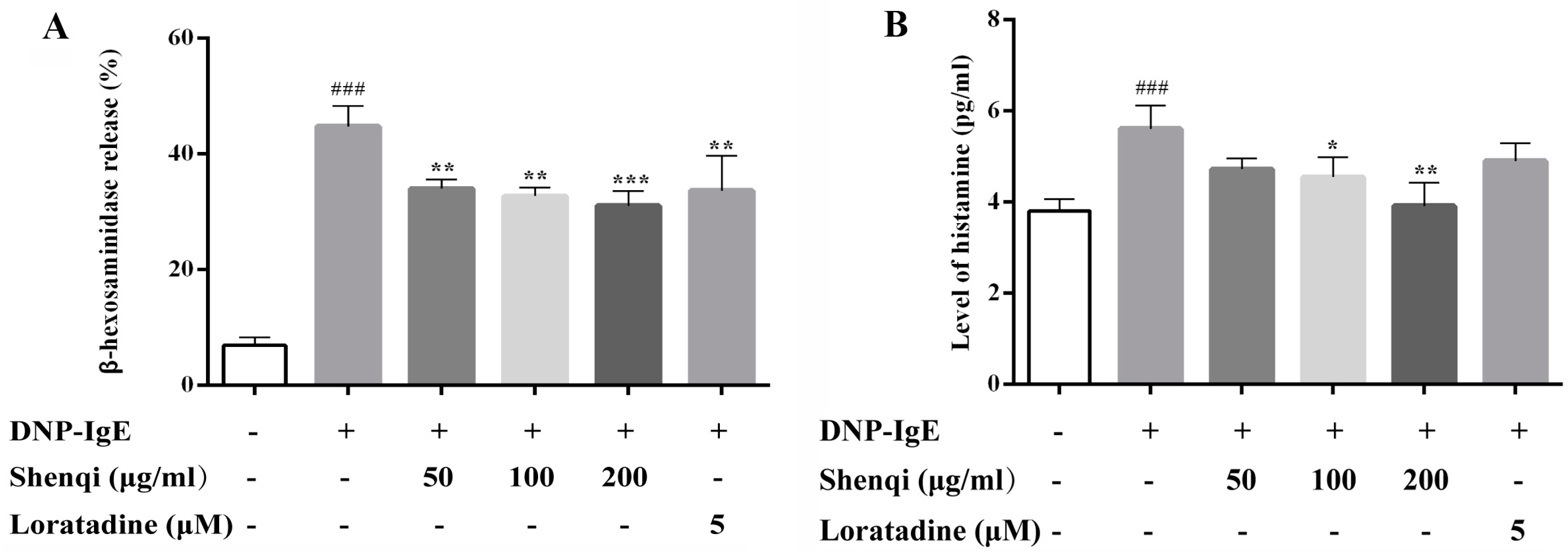
It is well known that AR is a Type I hypersensitivity allergic reaction mediated through the activity of mast cells by all kinds of allergens and resulting in a degranulation and release of inflammatory mediators, such as histamine, TNF-α, and LTC4 [
14]. Therefore, mast cells play a central role in modulating allergic reactions via granulation, and the degranulation of mast cells is considered a key target for anti-allergic drugs. Here, we tried to explore the possible targets of Shenqi in anti-allergic rhinitis. Mast cells were challenged by DNP-IgE to evaluate the efficacy of Shenqi on degranulation. Our study showed that Shenqi significantly inhibited the degranulation and release of histamine in DNP-IgE challenged RBL-2H3 mast cells. It is implied that Shenqi may prevent the activation of mast cells by IgE against the degranulation response.
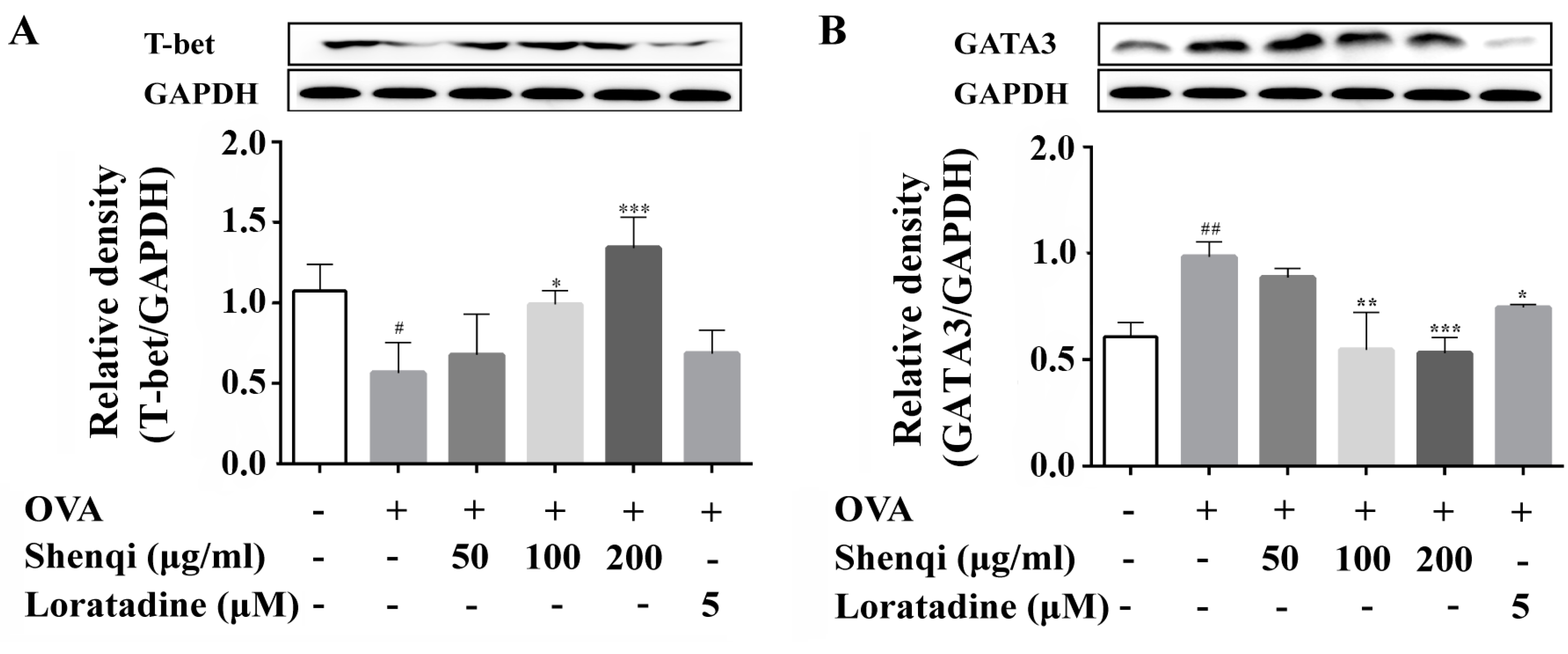
The infiltration and release of inflammatory mediators by immune cells play a crucial role in the development of allergic disease. In particular, T lymphocytes are key immune cells in allergic responses, especially Th1 and Th2 [
15]. T-bet is a Th1 special transcription factor that controls the expression of the hallmark Th1 cytokines, such as IFN-γ [
16]. Th1 lymphocytes produce IFN-γ that carries out cell-mediated immunity in auto-immune disease [
17]. GATA3 has been shown to induce the Th2 differentiation that depends on STAT6 activation, while suppressing the differentiation towards Th1 cells [
18,
19]. Th2 cells are characterized to secrete IL-4, which induces an IgE antibody production by B cells to contribute to the development of allergic responses [
20]. The Th1 or Th2 subsets also cross-regulate each other, so the balance between IFN-γ/IL-4 cytokines can determine whether the immune response is appropriate or will terminate in detrimental immunopathologies [
21,
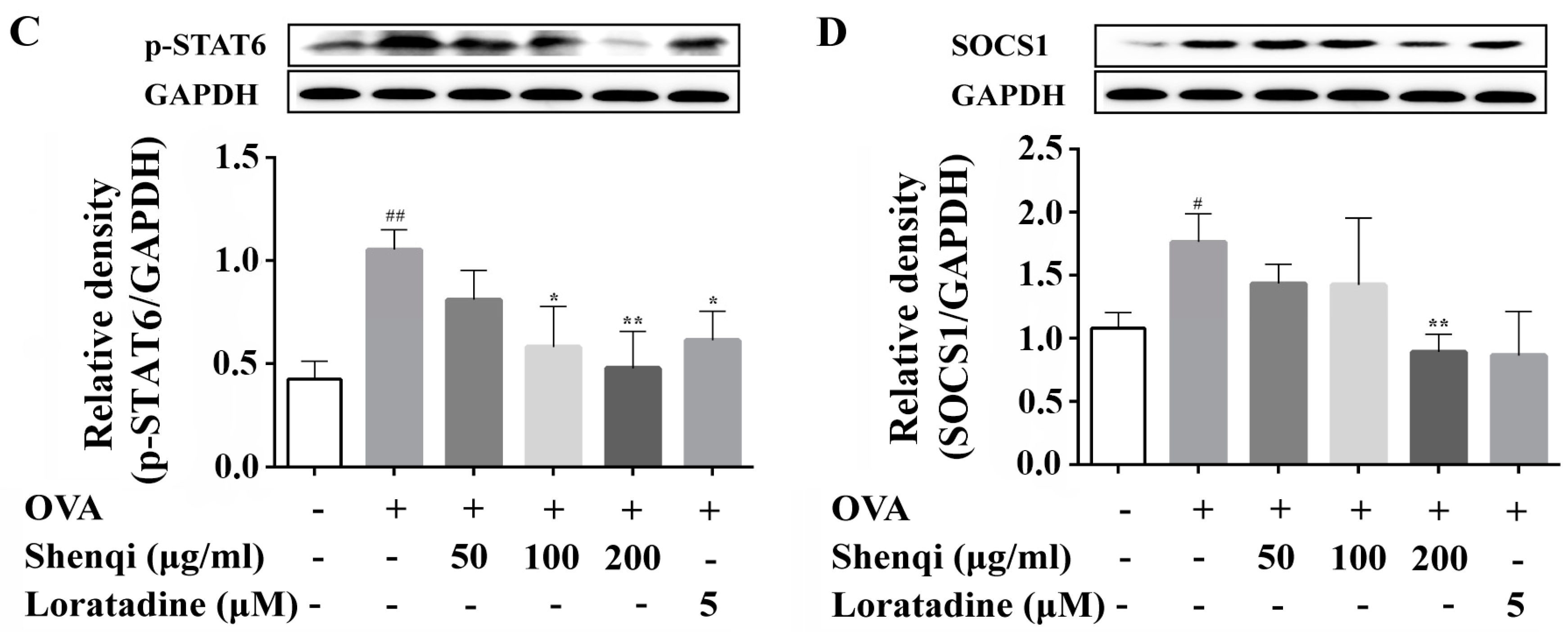
22]. Here, we measured the expression of transcription factors of GATA3 and T-bet, and the levels of IFN-γ and IL-4 in the presence or absence of Shenqi in OVA-induced primary spleen lymphocytes, to investigate the regulatory effect of Shenqi on T cell differentiation. Our findings of the present study showed that the ratio of IFN-γ/IL-4 was obviously reduced in OVA-stimulated primary lymphocytes, and the imbalance was considered towards Th2 (dominant by IL-4) with a reduction of Th1/Th2. However, we demonstrated that Shenqi directly improved the imbalanced ratio of IFN-γ/IL-4 by inhibiting the production of IL-4. The reduction of IL-4 was due to the decreased expression of Th2-specific transcription factor GATA3 and p-STAT6, or the increased expression of Th1-specific transcription factor T-bet. In addition, an upregulated SOCS1 expression correlated with downregulated T-bet and higher expression levels of GATA3 and IL-4 [
23]. Our study also showed that Shenqi treatment prevented the higher expression of SOCS1 in OVA-stimulated lymphocytes compared with OVA-stimulated lymphocytes not treated with Shenqi.
4. Materials and Methods
4.1. Cells and Reagents
RBL-2H3 cell was gifted by Professor Shigang Li (China Three Gorges University). The cells were cultured in Dulbecco’s modification of Eagle’s medium Dulbecco (DMEM, Invitrogen, NY, USA) containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, NY, USA), at 37 °C with 5% CO2 in a humidified atmosphere. Primary spleen lymphocytes were isolated from Wistar rat and cultured in RPMI Medium 1640 (Invitrogen, Carlsbad, NY, USA) with 10% FBS, 100 U/mL penicillin (Gibco, Grand Island, NY, USA), and 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA). Ovalbumin (OVA grade V), 4-niroPheny 1-N-acety-β-d-glucosaminide, and anti-dinitrophenyl IgE antibody (anti-DNP IgE) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA), and DNP-bovine serum albumin (BSA) was purchased from Invitrogen (Carlsbad, NY, USA). The following primary antibodies of GATA3 (ab99428), T-bet (ab91103), p-STAT6 (Tyr641, ab54461), and SOCS1 (ab62584) were purchased from Abcam (Cambridge, MA, USA). Evans blue was purchased from Tokyo chemical industry Co., Ltd. (Tokyo, Japan), and histamine and IgE ELISA kits were purchased from Shanghai Jianglai industrial Limited By Share Ltd. (Shanghai, China). IL-4 and IFN-γ ELISA kits were purchased from Hangzhou Cluster Technology Limited Biotechnology Co., Ltd. (Hangzhou, China). Loratadine was purchased from Shanghai Schering-Plough Pharmaceutical Co., Ltd. (Shanghai, China). Shenqi was provided by Xinjiang Jin Shi Kang Pharmaceutical Co., LTD. (Urumchi, China).
4.2. Preparation of Shenqi
The herb formula Shenqi consisted of 10 different traditional Chinese herbs, including the following: 20 g of
Angelicae dahuricae radix; 10 g each of
Scutellariae radix,
Lonicerae japonicae flos,
Menthae haplocalycis herrrba,
Astragali radix, and
Codonopsis radix; 30 g each of
Astragali radix,
Codonopsis radix, and
Agrimoniae herba; and 5 g of
Asari radix et rhizome (
Table 1). The voucher specimens of the 10 herbs were deposited at Herbarium, Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing, China, with voucher numbers as follows:
Angelica dahurica (Fisch. exHoffm.) Benth. et Hook. f., ID-S-600;
Scutellaria baicalensis Georgi, ID-S-2523;
Lonicera japonica Thunb., ID-S-1107;
Mentha haplocalyx Briq., ID-S-1747;
Astragalus membranaceus (Fisch.) Bunge, ID-S-2173;
Codonopsis tangshen Oliv., ID-S-480;
Isatis indigotica Fort., ID-S-2084;
Taraxacum mongolicum Hand.–Mazz., ID-S-2528;
Agrimonia pilosa Ledeb., ID-S-1086;
Asarum sieboldii Miq., ID-S-1193.
Shenqi is manufactured by Xinjiang Jinshikang Pharmaceutical Co., Ltd. (Urumchi, China). Briefly, each herb was sliced to an appropriate size. The mixture of Asari radix, rhizome, and Menthae haplocalycis herrrba was extracted in distilled water (1:8, w/v) for 5 h, and volatile oil was collected and formed a clathrate compound to be used. The mixture of Scutellariae radix, Lonicerae japonicae flos, Codonopsis tangshen Oliv, Taraxaci herba, and Agrimoniae herba was decocted in boiling distilled water (1:8, w/v) for 1 h three times. Angelicae dahuricae radix, Isatidis folium, and Astragali radix were mixed to extract in 75% ethanol (1:8, w/v) for 1 h twice. Above liquid extraction was filtered using a 100 mesh filter and condensed under the reduced pressure to obtain concentrations of 1.20–1.25 relative density, respectively. Concentrations were combined and vacuum dried at 60 °C to yield a powder, and the powder was then mixed with the clathrate compound and dried at 50 °C to yield a new powder. One gram of yielded powder was equivalent to 1.9 g of the total raw herbs.
4.3. Animals
A total of 108 male Wistar rats (180–200 g) was purchased from Beijing Wei Tong Li Hua experimental animal technical Co., Ltd. (Beijing, China). Rats were maintained in the Animal Resource Facility. They were fed standard laboratory chow and water ad libitum, and kept under a 12 h dark/light cycle. The animal experiment was conducted with an approval form and under the supervision of the Laboratory Animal Welfare and Ethics Committee of the Institute of Materia Medica, Chinese Academy of Medical Sciences (No. 571/2016). Animal surgeries were performed under sodium pentobarbital anesthesia with minimal suffering.
4.4. OVA-Induced AR Rat Models and Shenqi Treatment
Rats were sensitized by OVA as follows. OVA (0.3 mg/mL) diluted in aluminum hydroxide gel and was used to sensitize rats seven times via intraperitoneal injection on Days 1, 3, 5, 7, 9, 11, and 13. Then, sensitized rats were intranasally challenged by daily dropping with OVA diluted with sterile physiological saline (20 μL/nostril, 50 mg/mL) from Days 15 to 21 [
24].
As the OVA-induced allergic rhinitis model was successfully established, rats were randomly divided into six groups (n = 18 per group) that received different agents by daily intragastric administration for 7 days. There was a control group and a model group with distilled water, three Shenqi treatment groups at doses of 0.9, 2.7, and 8.1 g/kg, respectively, and a positive agent group with Loratadine at a dose of 0.9 mg/kg.
4.5. The Effects of Shenqi on OVA-Induced Rats
4.5.1. Evaluation of Nasal Symptoms
On the 30th day of the experiment, rats were stimulated by OVA (20 μL/nostril, 50 mg/mL), dissolved in physiological saline solution into the bilateral nasal cavities, and then placed into the observation cage. Instances of sneezing and nose scratching were counted for 10 min after the OVA intranasal stimulation to evaluate the degree of allergic responses. Rats in the normal control were processed with saline.
4.5.2. Permeability of Nasal Mucosa
The permeability of nasal mucosa was detected to evaluate the protection effect of Shenqi on OVA-induced impairment of rat nasal mucosa. Briefly, rats were anesthetized with 10% chloral hydrate (0.3 mL/100 g), and 1% Evans blue (1 mL) was then injected in the tail vein. Tracheotomy was performed and perfused with preheated (37 °C) 1% OVA into a nasal cavity by peristaltic pump at a speed of 0.2 mL/min. Perfusion fluid was collected every 15 min to 120 min. Collected perfusion fluid samples were centrifuged at 3000 g for 15 min, and for 100 μL of each sample, supernatants were obtained to detect the absorbance at 610 nm using a microplate reader. The permeability of nasal mucosa was evaluated by calculating the total level of Evans blue in perfusion fluid.
4.5.3. Histological Analysis of Nasal Mucosa
After the experiment, rats were sacrificed by intraperitoneal overdose of chloral hydrate, and the nasal mucosa was separated and fixed in 4% neutral buffered formalin at room temperature. After fixation, tissues were then paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E) to examine eosinophils lesions and nasal mucosa swelling.
4.6. Degranulation and Histamine Release Assay for RBL-2H3 Cells
Degranulation was estimated by detecting β-hexosaminidase release according to the reference of Wang et al. [
25]. Briefly, RBL-2H3 cells were seeded in 96-well plates at a density of 2.5 × 104 cells/well and sensitized overnight with mouse monoclonal anti-dinitrophenyl immunoglobulin E (DNP-IgE) antibody (1 μg/mL). The cells were washed twice with Tyrode’s solution (137 mM NaCl, 2.7 mM KCl, 0.4 mM NaH
2PO
4, 1 mM MgCl
2, 12 mM NaHCO
3, and 1.8 mM CaCl
2, hepes 20 mM, BSA 1 mg/mL, pH 7.4). Then, the cells were treated with Shenqi (50, 100, and 200 μg/mL) or Loratadine (5 μM, positive control) at 37 °C for 1 h, and antigen dinitrophenyl-bovine serum albumin (DNP-BSA) (200 ng/mL) was then added to stimulate for another 1 h at 37 °C to induce degranulation. The reaction was stopped by putting the plate in an ice bath. Fifty microliters of supernatant was collected to measure the level of histamine via an ELISA kit according to the manufacturer’s instructions.
To determine the β-hexosaminidase release, 50 μL of supernatant was incubated with 50 μL of substrate (1 mM
p-nitrophenly-
N-acetyl-β-
d-glucosaminide in 0.1 M citrate buffer, pH 5.0) in 96-well plates at 37 °C for 1 h. The reaction liquid was collected to measure the concentration of β-hexosaminidase in supernatant. The total β-hexosaminidase activity in the cells was also detected. Cells were lysed with an equal volume of 0.1% Triton-X 100 at 37 °C for 1 h. The reaction was terminated by adding 150 μL of 0.1 M carbonate buffer (Na
2CO
3/NaHCO
3, pH 10.0). Lysates were collected to measure the concentration of β-hexosaminidase as total level. The absorbance was measured at 405 nm by a microplate reader (Synergy H1, BioTeck, VT, USA). The β-hexosaminidase release rate was calculated by the following formula:
4.7. Cytokines Measurement
After the animal experiment, AR rats were sacrificed, and blood samples were collected. All blood samples were centrifuged at 3000 g for 15 min at 4 °C. Serum was collected and conserved at −20 °C. The levels of cytokines IL-4, IFN-γ, and IgE were measured via ELISA.
Primary rat spleen lymphocytes were obtained from normal Wistar rats by isolation kit (Tianjin Hao Yang biological manufacture Co., Ltd., Tianjin, China) according to the manufacturer’s instructions. The isolated primary cells (6 × 106 cells/mL) were seeded in 6-well plates overnight and pretreated with Shenqi (50, 100, and 200 μg/mL) or Loratadine (5 μM) at 37 °C for 1 h, and the cells were then stimulated with OVA (200 μg/mL) for another 72 h. The concentration of IL-4 and IFN-γ in the cell culture supernatants were measured using ELISA kits according to the manufacturer’s instruments. The cells were prepared to detect the protein expression via Western blot.
4.8. Western Blot
Primary spleen lymphocytes treated with Shenqi and OVA as above described were harvested and resuspended in lysis buffer with RIPA (Beyotime, Shanghai, China) on ice for 30 min to obtain total protein. Concentration of protein was detected with a BCA protein assay kit (Thermo, Rockford, IL, USA) and loading buffer was added to the sample. The sample was then heated at 100 °C for 5 min. Equal amounts of protein from the different samples were separated by 10% sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE), and then transferred to PVDF membranes. After being blocked with 5% nonfat milk for 1 h, the PVDF membranes were incubated overnight at 4 °C with the primary antibodies that recognized GATA3, T-bet, p-STAT6, and SOCS1. Anti-rabbit horseradish-linked IgG was used as the secondary antibody. Signals were detected using electrochemiluminescence (ECL, Millipore, MA, USA).
4.9. Statistical Analysis
Data are presented as means ± SEM. unless otherwise indicated. The statistical significance of difference among multiple groups was analyzed by a one-way ANOVA—Ordinary (GraphPad software, Prism 5.0, GraphPad software Inc., San Diego, CA, USA). The significance of differences between the normal and control groups was evaluated using Student’s t-tests. p < 0.05 was considered significant.