Determination of Ultra-trace Rhodium in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Cloud Point Extraction Using 2-(5-Iodo-2-Pyridylazo)-5-Dimethylaminoaniline as a Chelating Agent
Abstract
:1. Introduction
2. Result and Discussion
2.1. Effect of pH
2.2. Effect of the Amount of Chelating Agent
2.3. Effect of Triton X-114 Concentration
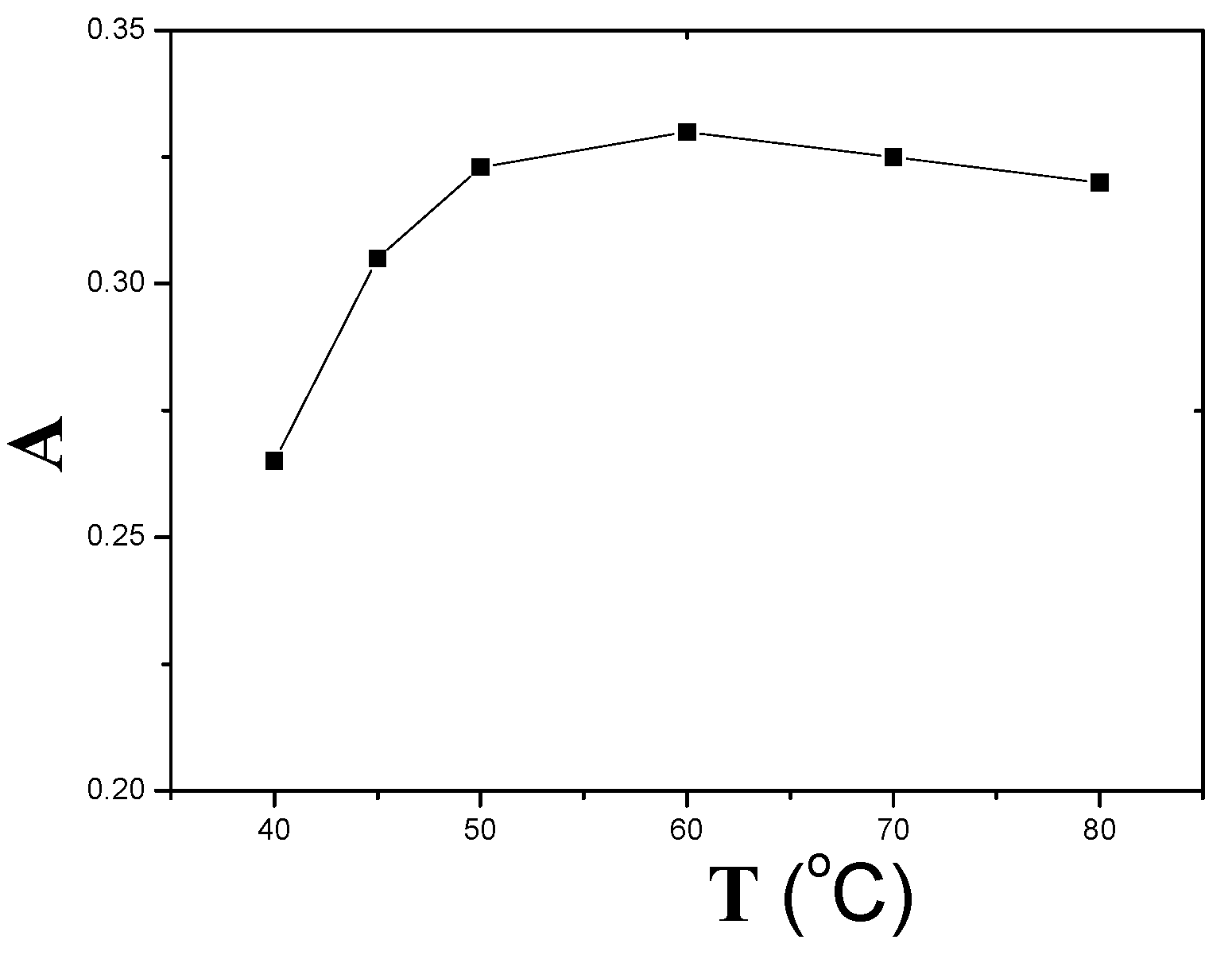
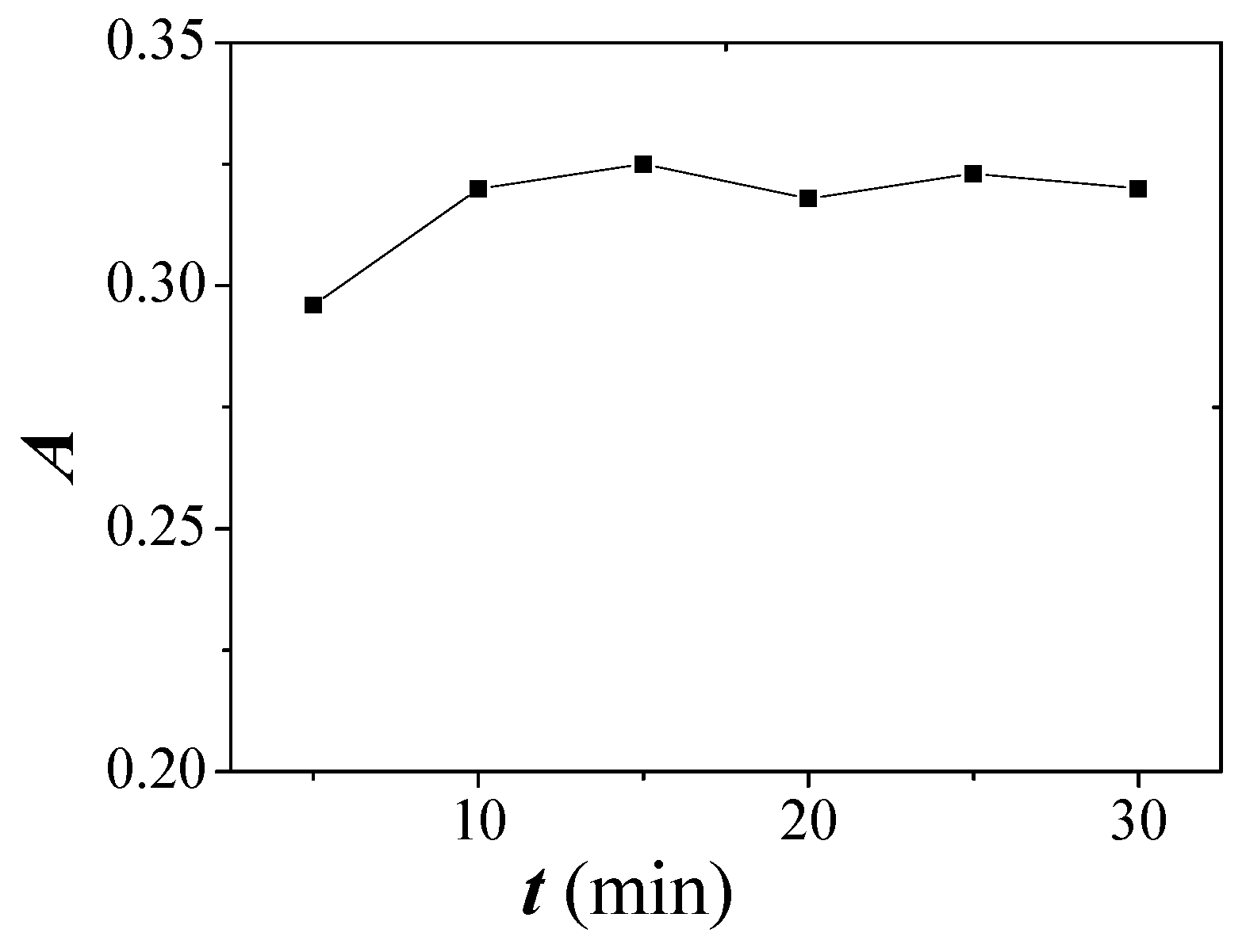
2.4. Effect of Equilibration Temperature and Time
2.5. Interference Studies
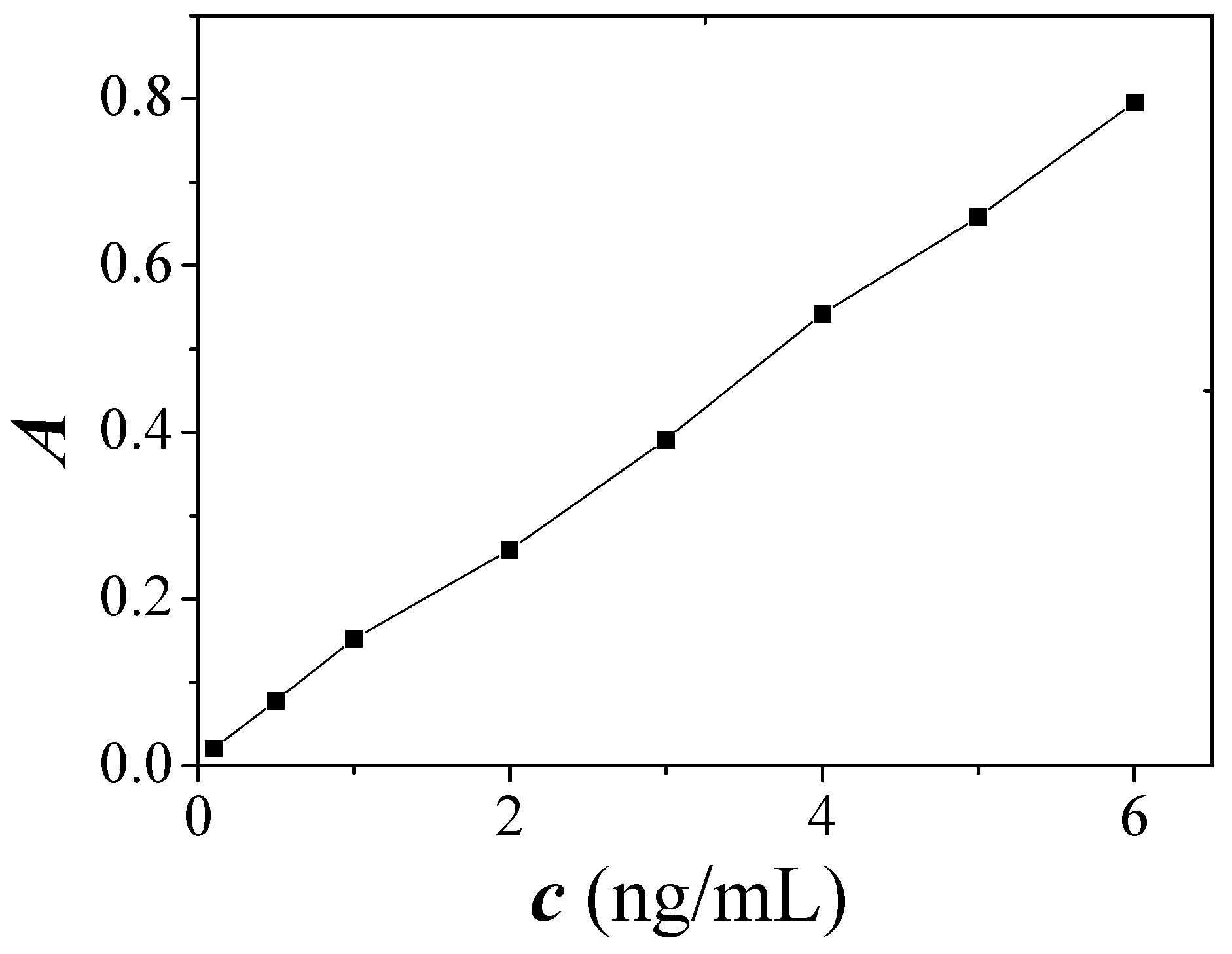
2.6. Calibration Curve, Detection Limit and Precision
3. Application of Real Water Samples
4. Experimental Section
4.1. Apparatus
4.2. Reagents and Solutions
4.3. Procedure
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dubiella-Jackowska, A.; Polkowska, Z.; Namiesnik, J. Platinum group elements: A challenge for environmental analytics. Polish J. Environ. Stud. 2007, 16, 329–345. [Google Scholar]
- Moldovan, M.; Palacios, M.; Gomez, M.; Morrison, G.; Rauch, S.; McLeod, C. Environmental risk of particulate and soluble platinum group elements released from gasoline and diesel engine catalytic converters. Sci. Total Environ. 2002, 296, 199–208. [Google Scholar] [CrossRef]
- Rojas, F.S.; Ojeda, C.B.; Pavon, J.M.C. Graphite furnace atomic absorption spectrometric determination of rhodium after online ion-exchange preconcentration. Anal. Lett. 2004, 37, 2685–2700. [Google Scholar] [CrossRef]
- Rojas, F.S.; Ojeda, C.B.; Pavon, J.M.C. Application of flow injection on-line electrothermal atomic absorption spectrometry to the determination of rhodium. Annali Di Chimica 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Sylwester, H.; Joanna, K.; Monika, S.; Jerzy, G. Simultaneous determination of platinum and rhodium with hydroxylamine and acetone oxime by catalytic adsorptive stripping voltammetry (CAdSV). Electroanalysis 2005, 17, 1841–1846. [Google Scholar]
- Paraskevas, M.; Tsopelas, F.; Ochsenkühn-petropoulou, M. Determination of Pt and Pd in particles emitted from automobile exhaust catalysts using ion-exchange matrix separation and voltammetric detection. Microchim. Acta 2012, 176, 235–242. [Google Scholar] [CrossRef]
- Van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Stripping voltammetric determination of palladium, platinum and rhodium in freshwater and sediment samples from South African water resources. J. Environ. Sci. Health. Part A Tox. Hazard Subst. Environ. Eng. 2012, 47, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Kormali, S.M.; Schweikert, E.A. Trace determination of rhodium using proton activation followed by X-ray counting. J. Radioanal. Nucl. Chem. 1986, 100, 197–201. [Google Scholar] [CrossRef]
- Li, X.L.; Ebihara, M. determination of all platinum-group elements in mantle-derived xenoliths by neutron activation analysis with NiS fire-assay preconcentration. J. Radioanal. Nucl. Chem. 2003, 255, 131–135. [Google Scholar] [CrossRef]
- Fan, Z.; Jing, Z.; Yang, F.; Hu, B. Determination of platinum, palladium and rhodium in biological and environmental samples by low temperature electrothermal vaporization inductively coupled plasma atomic emission spectrometry with diethyldithiocarbamate as chemical modifier. Anal. Chim. Acta 2004, 510, 45–51. [Google Scholar] [CrossRef]
- Kovacevic, R.; Todorovic, M.; Manojlovic, D.; Muticet, J. Development of inductively coupled plasma atomic emission spectrometry for palladium and rhodium determination in platinum-based alloy. J. Iran Chem. Soc. 2008, 5, 336–341. [Google Scholar] [CrossRef]
- Vanhaecke, F.; Resano, M.; Pruneda-Lopez, M.; Moens, L. Determination of platinum and rhodium in environmental matrixes by solid sampling-electrothermal vaporization-inductively coupled plasma mass spectrometry. Anal. Chem. 2002, 74, 6040–6048. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, J.; Meisel, T. Determination of anthropogenic input of Ru, Rh, Pd, Re, Os, Ir and Pt in soils along Austrian motorways by isotope dilution ICP-MS. Sci. Total Environ. 2004, 325, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Niemela, M.; Kola, H.; Peramaki, P.; Piispanen, J.; Poikolainen, J. Comparison of microwave-assisted digestion methods and selection of internal standards for the determination of Rh, Pd and Pt in dust samples by ICP-MS. Microchim. Acta 2005, 150, 211–217. [Google Scholar] [CrossRef]
- Afzali, D.; Ghazizadeh, A.; Salari, H. Ion pair-dispersive liquid-liquid microextraction of trace amount of rhodium ion in water and road dust samples prior to flame atomic absorption spectrometry determination. Quim. Nova 2011, 34, 1124–1128. [Google Scholar] [CrossRef]
- Hossain, K.Z.; Honjo, T. Separation of trace amounts of rhodium (III) with tri-n-butyl phosphate from nitric acid and sodium trichloroacetate media. Fresenius J. Anal. Chem. 2000, 368, 480–483. [Google Scholar] [CrossRef]
- Zhen, H.; Yang, S.; Wang, J.; Sun, W. Highly selective determination of rhodium(III) using silica gel surface-imprinted solid-phase extraction. Int. J. Environ. Anal. Chemi. 2011, 91, 1013–1023. [Google Scholar] [CrossRef]
- Shelar, Y.S.; Kuchekar, S.R.; Han, S.H. Extraction spectrophotometric determination of rhodium(III) with o-methylphenyl thiourea. J. Saudi Chem. Soc. 2015, 19, 616–627. [Google Scholar] [CrossRef]
- Rojas, F.S.; Ojeda, C.B.; Pavón, J.M.C. Determination of rhodium and platinum by electrothermal atomic absorption spectrometry after preconcentration with a chelating resin. J. Braz. Chem. Soc. 2007, 18, 1270–1275. [Google Scholar] [CrossRef]
- Ojeda, C.B.; Rojas, F.S. Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim. Acta 2012, 177, 1–21. [Google Scholar] [CrossRef]
- Ojeda, C.B.; Rojas, F.S. Separation and Preconcentration by a cloud point extraction procedure for determination of metals: An overview. Anal. Bioanal. Chem. 2009, 394, 759–782. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shao, X. Cloud point extraction and its applications to the separation and analysis of organic components. Prog. Chem. 2006, 18, 482–484. [Google Scholar]
- Han, Q.; Huo, Y.; Liu, Y.; Yang, X.; Gao, M. Synthesis of a new reagent 2-(5-iodo-2-pyridylazo)-5-dimethylaminoaniline and its color reaction with rhodium. J. Instrum. Anal. 2010, 29, 797–801. [Google Scholar]
- Silva, M.F.; Cerutti, E.S.; Martinez, L.D. Coupling cloud point extraction to instrumental detection systems for metal analysis. Microchim. Acta 2006, 155, 349–364. [Google Scholar] [CrossRef]
- Mohammed, A.K.; Alaa, S.A. Determination of rhodium in metallic alloy and water samples using cloud point extraction coupled with spectrophotometric technique. Spectrochim. Acta Part A 2015, 136, 1955–1961. [Google Scholar]
- Roushani, M.; Baghelani, Y.M.; Abbasi, S.; Mohammadi, S.Z. Ligandless cloud point extraction of trace amounts of palladium and rhodium in road dust samples using span 80 prior to their determination by flame atomic absorption spectrometry. Quim. Nova 2014, 37, 308–1311. [Google Scholar] [CrossRef]
- Suvardhan, K.; Suresh, K.K.; Rekha, D.; Subrahmanyam, P.; Kiran, K.; Jayaraj, B.; Ramanaiah, S.; Janardhanam, K.; Chiranjeevi, P. Determination of rhodium in water samples using cloud point extraction (CPE) coupled with flame atomic absorption spectrometry (FAAS). Microchim. Acta 2007, 157, 237–244. [Google Scholar] [CrossRef]
- Da Silva, M.A.M.; Frescura, V.L.A.; Curtius, A.J. Determination of noble metals in biological samples by electrothermal vaporization inductively coupled plasma mass spectrometry, following cloud point extraction. Spectrochim. Acta Part B 2001, 56, 1941–1949. [Google Scholar] [CrossRef]
- Simitchiev, K.; Stefanova, V.; Kmetov, V.; Andreev, G.; Kovachev, N.; Canals, A. Microwave-assisted cloud point extraction of Rh, Pd and Pt with 2-mercaptobenzothiazole as preconcentration procedure prior to ICP-MS analysis of pharmaceutical products. J. Anal. At. Spectrom. 2008, 23, 717–726. [Google Scholar] [CrossRef]
- Shokoufi, N.; Shemirani, F. Laser induced-thermal lens spectrometry after cloud point extraction for the determination of trace amounts of rhodium. Talanta 2007, 73, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of 5-I-PADMA, are not available from the authors, but can be synthesized according to literature [23].
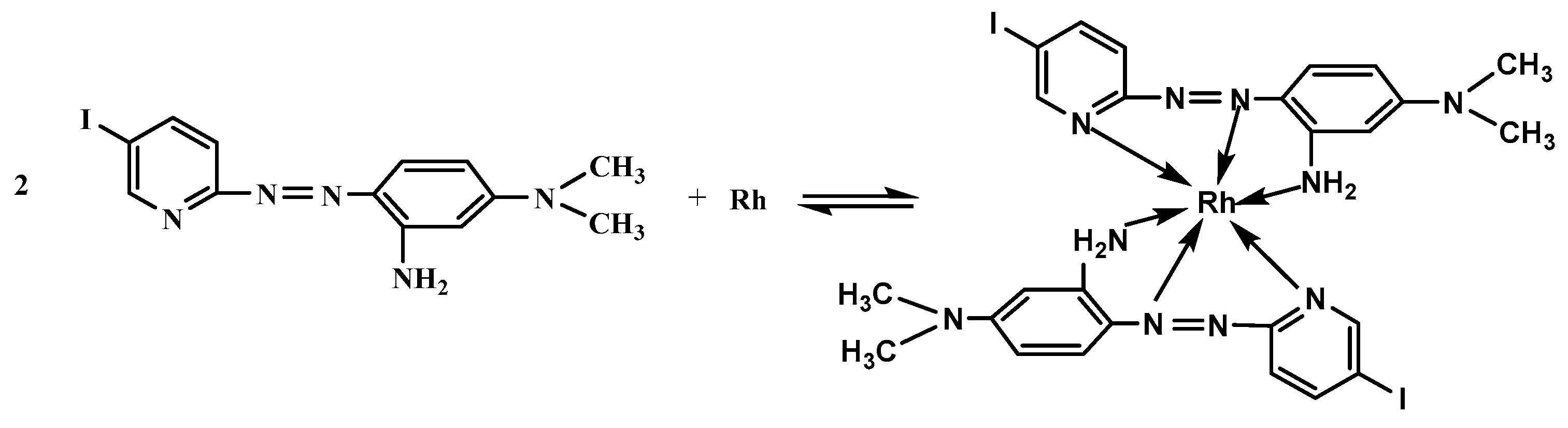
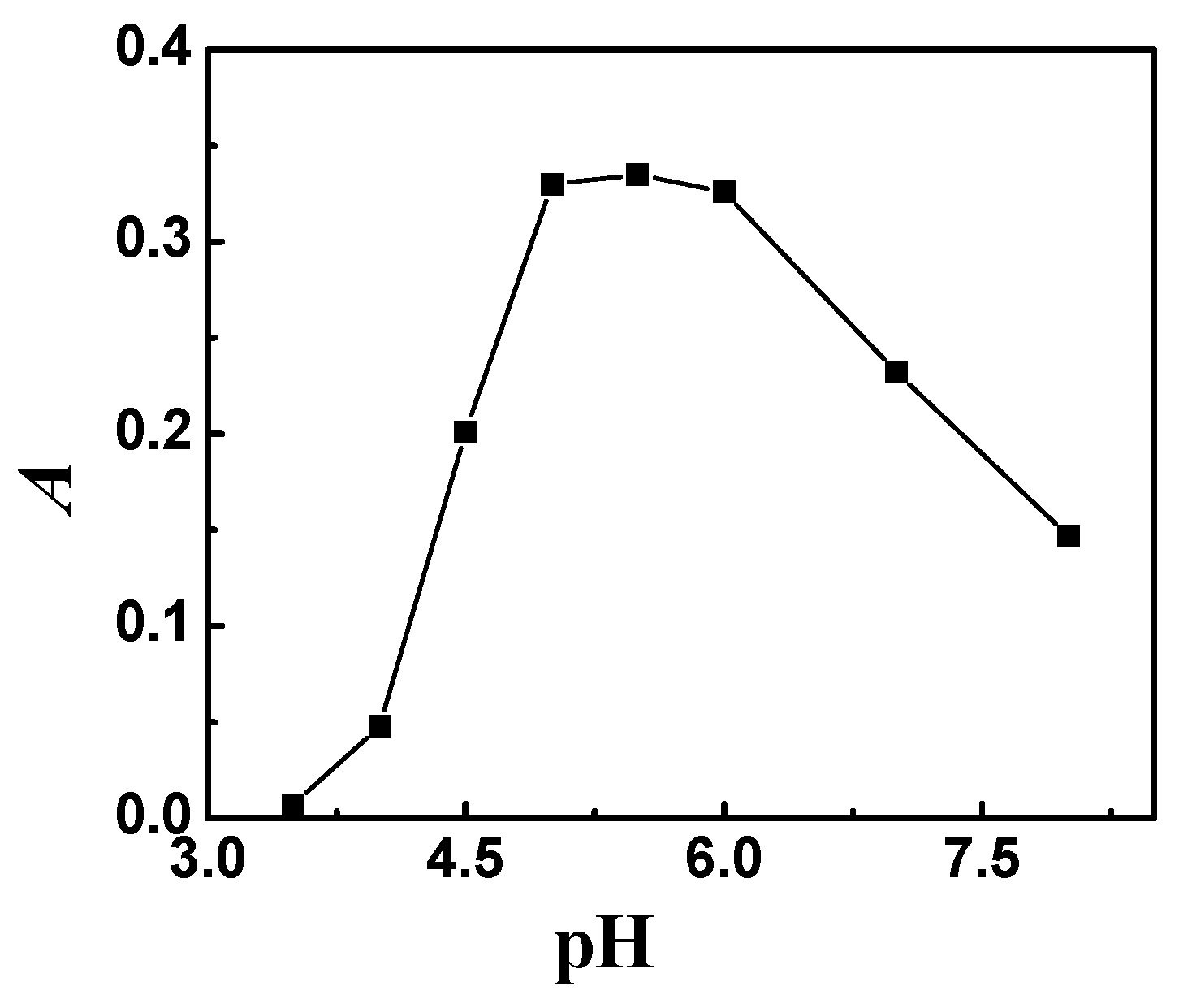
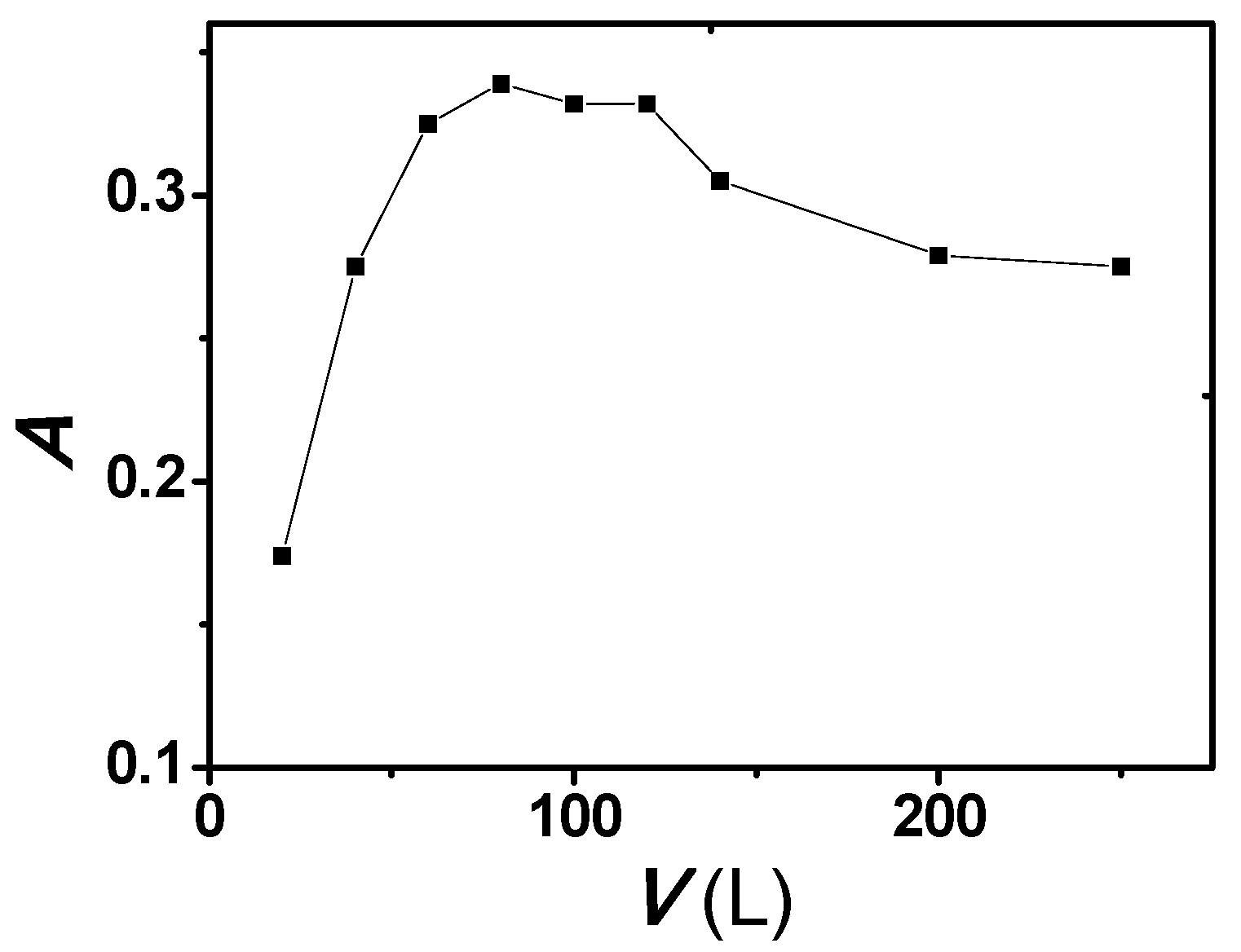
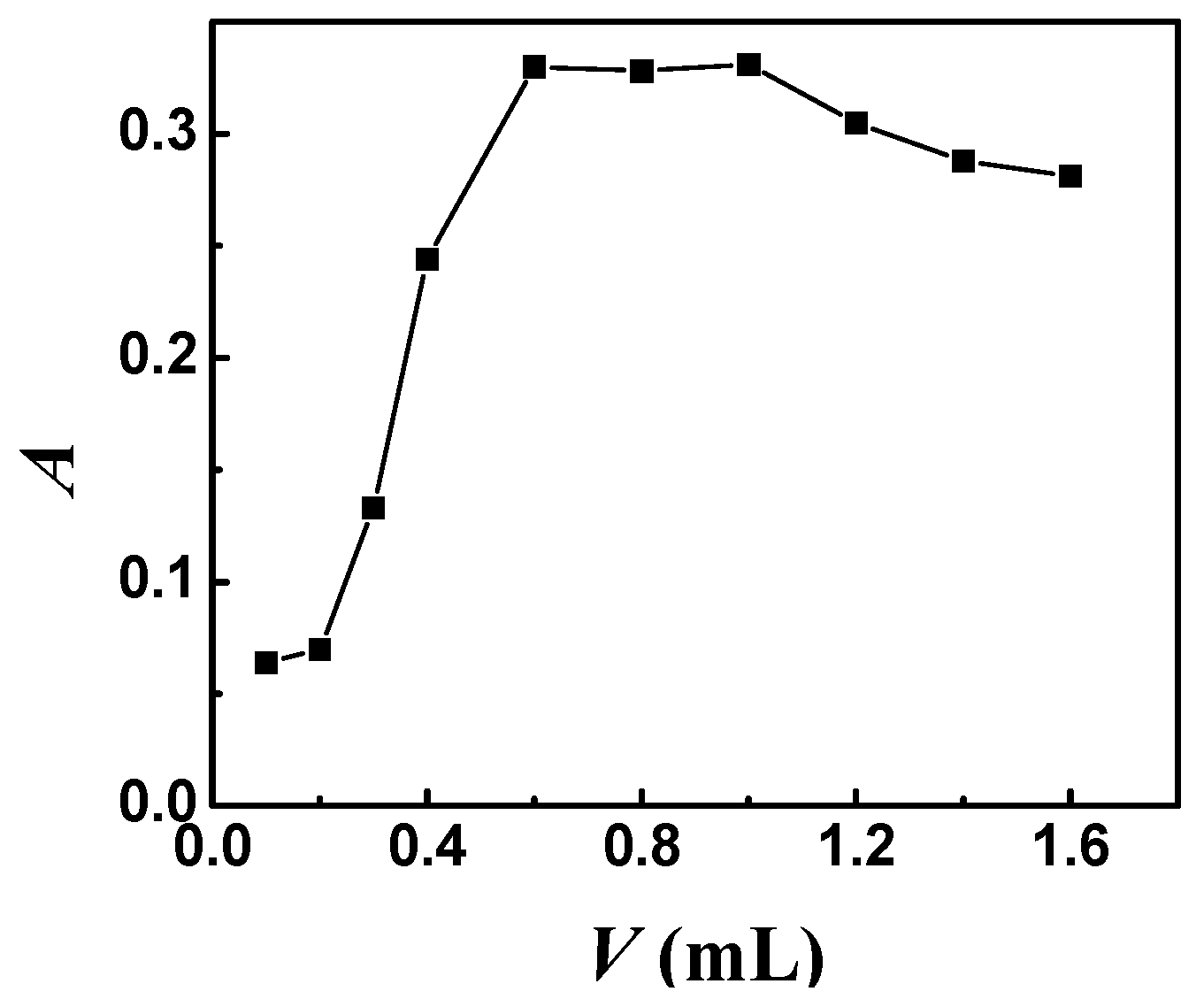
| Species | Foreign Species to Rh Ratio (w/w) | Species | Foreign Species to Rh Ratio (w/w) |
|---|---|---|---|
| Li+, Na+, K+, Mg2+, Ca2+, Sr2+, Zn2+, F−, Cl−, Br−, NO3− | 2500 | Cu2+, Ni2+, W(VI) | 500 |
| Ba2+, I−, SO42− | 2000 | Ag+, Pt(IV), Ir(IV), Ru(III), Fe3+ | 200 |
| Cd2+, Cr3−, As(V) | 1500 | Co2+ | 100 |
| Pb2+ | 1000 | Pd2+ | 80 |
| Mn2+, Al3+, Mo(IV) | 800 |
| Reagent | Detection System | Surfactant | Limit of Detection (ng/mL) | Reference |
|---|---|---|---|---|
| 5-(4′-Nitro-2′,6′-dichlorophenylazo)-6-hydroxypyrimidine-2,4-dione | SP | Triton X-114 | 0.15 | [25] |
| - | FAAS | Span 80 | 1.2 | [26] |
| 2-propylpiperidine-1-carbodithioate | FAAS | Triton X-114 | 0.052 | [27] |
| O,O-Diethydithiophosphate | ICP/MS | Triton X-114 | 0.003 | [28] |
| 2-Mercaptobenzothiazole | ICP/MS | Triton X-100 | 0.001 | [29] |
| 1-(2-Pyridylazo)-2-naphthol | TLS | Triton X-114 | 0.06 | [30] |
| 2-(5-Iodo-2-pyridylazo)-5-dimethylaminoaniline | GFAAS | Triton X-114 | 0.023 | This work |
| Sample | Added (ng/mL) | Found (ng/mL) | Average (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Tap water | - | ND | - | - | - |
| 1.0 | 0.950, 0.960, 0.930 1.02, 0.970, 1.01 | 0.973 | 97.3 | 3.6 | |
| 3.0 | 3.22, 3.10, 2.94 2.96, 3.16, 3.22 | 3.10 | 103.3 | 3.9 | |
| Reservoir water | - | ND | - | - | - |
| 2.0 | 1.94, 2.05, 2.08 1.98, 1.94, 2.08 | 2.01 | 101 | 3.3 | |
| 4.0 | 3.92, 4.06, 3.90 3.92, 3.86, 4.07 | 3.96 | 98.9 | 2.2 | |
| Well water | - | ND | - | - | - |
| 1.0 | 0.970, 0.980, 1.00 0.930, 0.960, 1.04 | 0.980 | 98.0 | 2.9 | |
| 3.0 | 3.02, 2.86, 2.90 2.82, 2.88, 2.92 | 2.90 | 96.7 | 2.4 | |
| River water | - | ND | - | - | - |
| 2.0 | 1.96, 1.95, 2.08 1.92, 2.06, 1.92 | 1.98 | 99.0 | 3.6 | |
| 4.0 | 3.88, 3.80, 3.88 4.06, 3.92, 3.98 | 3.92 | 98.0 | 2.3 |
| Lamp current | 6.0 mA |
| Wavelength | 343.5 nm |
| Slit | 0.2 nm |
| Filter coefficient | 0.10 |
| Pressure (Ar) | 0.60 Mpa |
| Injected volume | 10.0 μL |
| Drying temp. | 120 °C (Ramp 5 s, hold 10 s) |
| Ashing temp. | 1100 °C (Ramp 10 s, hold 15 s) |
| Atomization temp. | 2300 °C (Ramp 0 s, hold 3 s) |
| Cleaning temp. | 2500 °C (Ramp 1 s, hold 3 s) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Huo, Y.; Wu, J.; He, Y.; Yang, X.; Yang, L. Determination of Ultra-trace Rhodium in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Cloud Point Extraction Using 2-(5-Iodo-2-Pyridylazo)-5-Dimethylaminoaniline as a Chelating Agent. Molecules 2017, 22, 487. https://doi.org/10.3390/molecules22040487
Han Q, Huo Y, Wu J, He Y, Yang X, Yang L. Determination of Ultra-trace Rhodium in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Cloud Point Extraction Using 2-(5-Iodo-2-Pyridylazo)-5-Dimethylaminoaniline as a Chelating Agent. Molecules. 2017; 22(4):487. https://doi.org/10.3390/molecules22040487
Chicago/Turabian StyleHan, Quan, Yanyan Huo, Jiangyan Wu, Yaping He, Xiaohui Yang, and Longhu Yang. 2017. "Determination of Ultra-trace Rhodium in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Cloud Point Extraction Using 2-(5-Iodo-2-Pyridylazo)-5-Dimethylaminoaniline as a Chelating Agent" Molecules 22, no. 4: 487. https://doi.org/10.3390/molecules22040487






