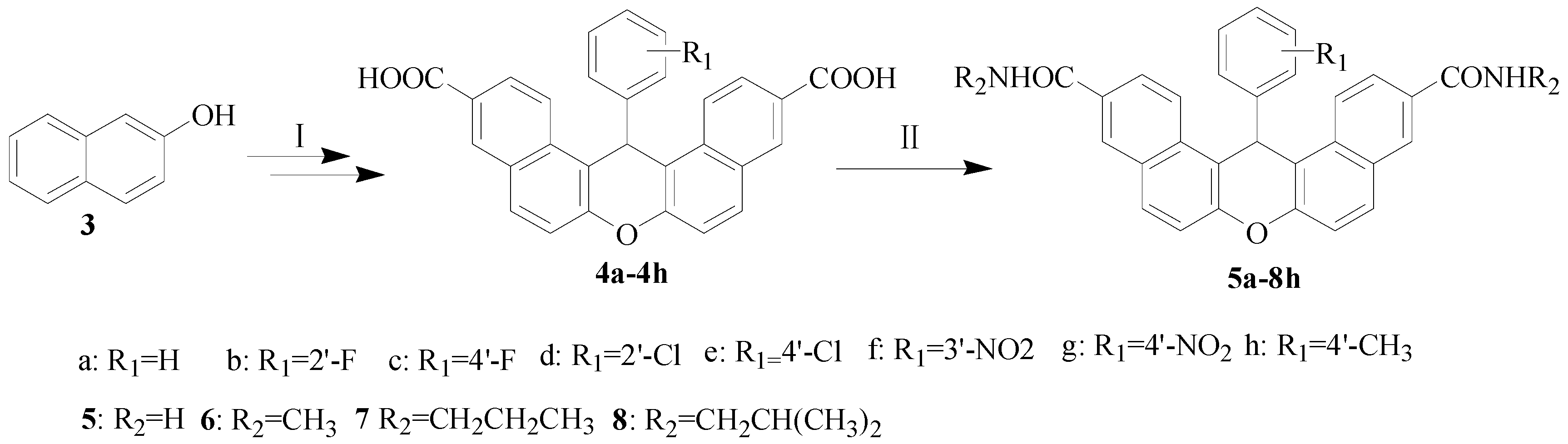
2.1. Chemistry
The synthetic route for the carboxamide derivatives
5a–
h,
6a–
h,
7a–
h and
8a–
h is outlined in
Scheme 1.
14-Phenyl-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5a): White solid. Yield 84.2%. m.p. 229–231 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 8.9 Hz, 2H, H-1, 13), 8.49 (s, 2H, H-4, 10), 8.10 (s, 2H, CONH × 2), 8.04 (overlapping t, J = 8.9 Hz, 4H), 7.72–7.57 (m, 4H), 7.44 (s, 2H, CONH × 2), 7.15 (t, J = 7.6 Hz, 2H, H-3′, 5′), 6.98 (t, J = 7.2 Hz, 1H, H-4′), 6.79 (s, 1H, H-14). IR (KBr) ν: 3422, 1655, 1623, 1595, 1466, 1396, 1244, 1081 cm−1. HR-MS (ESI) calcd for C29H21N2O3 [M + H]+ 445.1552, found 445.1559.
14-(2-Fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5b): White solid. Yield 83.7%. m.p. 229–230 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.51 (d, J = 1.5 Hz, 2H, H-4, 10), 8.47 (d, J = 9.0 Hz, 2H, H-1, 13), 8.10 (s, 2H, CONH × 2), 8.07(dd, J = 9.0, 1.7 Hz, 2H, H-2, 12), 8.06 (d, J = 9.0 Hz, 2H, H-5, 9), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.62–7.57 (m, 1H, H-6′), 7.45 (s, 2H, CONH × 2), 7.14–6.97 (m, 3H, H-3′, 4′, 5′), 6.90 (s, 1H, H-14). IR (KBr) ν: 3409, 1658, 1624, 1595, 1467, 1397, 1256, 1245 cm−1. HR-MS (ESI) calcd for C29H20FN2O3 [M + H]+ 463.1458, found 463.1455.
14-(4-Fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5c): White solid. Yield 87.9%. m.p. 241–243 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 9.0 Hz, 2H, H-1, 13), 8.51 (d, J = 1.6 Hz, 2H, H-4, 10), 8.12 (s, 2H, CONH × 2), 8.06 (dd, J = 9.0, 1.7 Hz, 2H, H-2, 12), 8.04 (d, J = 8.9 Hz, 2H, H-5, 9), 7.72–7.62 (m, 2H, H-2′, 6′), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.45 (s, 2H, CONH × 2), 6.99 (t, J = 8.9 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14). IR (KBr) ν: 3394, 3157, 3049, 1655, 1623, 1506, 1466, 1401, 1245 cm−1. HR-MS (ESI) calcd for C29H20FN2O3 [M + H]+ 463.1458, found 463.1469.
14-(2-Chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5d): White solid. Yield 82.5%. m.p. 235–236 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.65 (d, J = 9.0 Hz, 2H, H-1, 13), 8.51 (d, J = 1.5 Hz, 2H, H-4, 10), 8.10 (s, 2H, CONH × 2), 8.08 (dd, J = 9.0, 1.7 Hz, 2H, H-2, 12), 8.07 (d, J = 9.0 Hz, 2H, H-5, 9), 7.64 (d, J = 8.9 Hz, 2H, H-6, 8), 7.55 (d, J = 7.4 Hz, 1H, H-6′), 7.45 (s, 2H, CONH × 2), 7.34 (dd, J = 8.0, 1.1 Hz, 1H, H-3′), 7.15 (t, J = 7.0 Hz, 1H, H-5′), 7.07 (td, J = 7.8, 1.6 Hz, 1H, H-4′), 6.88 (s, 1H, H-14). IR (KBr) ν: 3377, 3192, 1657, 1623, 1594, 1467, 1396, 1254 cm−1. HR-MS (ESI) calcd for C29H20ClN2O3 [M + H]+ 479.1162, found 479.1170.
14-(4-Chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5e): White solid. Yield 82.3%. m.p. 231–233 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.72 (d, J = 8.9 Hz, 2H, H-1, 13), 8.50 (d, J = 1.6 Hz, 2H, H-4, 10), 8.11 (s, 2H, CONH × 2), 8.06 (dd, J = 8.9, 1.6 Hz, 2H, H-2, 12), 8.05 (d, J = 8.9 Hz, 2H, H-5, 9), 7.65 (d, J = 8.6 Hz, 2H, H-2′, 6′), 7.64 (d, J = 8.9 Hz, 2H, H-6, 8), 7.45 (s, 2H, CONH × 2), 7.23 (d, J = 8.6 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14). IR (KBr) ν: 3396, 3185, 1656, 1623, 1593, 1466, 1398, 1253 cm−1. HR-MS (ESI) calcd for C29H20ClN2O3 [M + H]+ 479.1162, found 479.1168.
14-(3-Nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5f): White solid. Yield 81.8%. m.p. 225–227 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.81 (d, J = 9.0 Hz, 2H, H-1, 13), 8.56 (t, J = 1.9 Hz, 1H, H-2′), 8.51 (d, J = 1.6 Hz, 2H, H-4, 10), 8.12 (s, 2H, CONH × 2), 8.11–8.06 (m, 5H), 7.87 (br.d, J = 7.6 Hz, 1H, H-4′), 7.68 (d, J = 8.9 Hz, 2H, H-6, 8), 7.48 (t, J = 8.1 Hz, 1H, H-5′), 7.46 (s, 2H, CONH × 2), 7.04 (s, 1H, H-14). IR (KBr) ν: 3434, 3186, 1664, 1622, 1594, 1521, 1465, 1397, 1348, 1253 cm−1. HR-MS (ESI) calcd for C29H20N3O5 [M + H]+ 490.1403, found 490.1411.
14-(4-Nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5g): White solid. Yield 83.7%. m.p. 238–240 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.75 (d, J = 9.0 Hz, 2H, H-1, 13), 8.51 (d, J = 1.5 Hz, 2H, H-4, 10), 8.12 (s, 2H, CONH × 2), 8.10-8.02 (m, 6H), 7.93 (d, J = 8.9 Hz, 2H, H-2′, 6′), 7.67 (d, J = 8.9 Hz, 2H, H-6, 8), 7.46 (s, 2H, CONH × 2), 7.00 (s, 1H, H-14). IR (KBr) ν: 3382, 3191, 1661, 1623, 1607, 1594, 1516, 1466, 1397, 1345, 1254 cm−1. HR-MS (ESI) calcd for C29H20N3O5 [M + H]+ 490.1403, found 490.1398.
14-(4-Methylphenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (5h): White solid. Yield 84.2%. m.p. 229–231 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.72 (d, J = 9.0 Hz, 2H, H-1, 13), 8.50 (d, J = 1.4 Hz, 2H, H-4, 10), 8.12 (s, 2H, CONH × 2), 8.05 (dd, J = 9.0, 1.6 Hz, 2H, H-2, 12), 8.02 (d, J = 9.0 Hz, 2H, H-5, 9), 7.62 (d, J = 8.9 Hz, 2H, H-6, 8), 7.50 (d, J = 8.1 Hz, 2H, H-2′, 6′), 7.45 (s, 2H, CONH × 2), 6.95 (d, J = 8.0 Hz, 2H, H-3′, 5′), 6.74 (s, 1H, H-14), 2.05 (s, 3H, CH3). IR (KBr) ν: 3398, 3193, 1656, 1623, 1594, 1466, 1398, 1255, 1244 cm−1. HR-MS (ESI) calcd for C30H23N2O3 [M + H]+ 459.1709, found 459.1701.
N3,N11-Dimethyl-14-phenyl-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6a): White solid. Yield 87.1%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 9.0 Hz, 2H, H-1, 13), 8.63 (q, J = 4.5 Hz, 2H, CONH × 2), 8.45 (br.s, 2H, H-4, 10), 8.03 (overlapping d, J = 8.9 Hz, 4H), 7.62 (overlapping d, J = 8.7 Hz, 4H), 7.13 (t, J = 7.6 Hz, 2H, H-3′, 5′), 6.96 (t, J = 7.3 Hz, 1H, H-4′), 6.77 (s, 1H, H-14), 2.82 (d, J = 4.4 Hz, 6H, CH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.9, 149.4, 145.8, 132.6, 131.0, 130.6, 130.4, 129.0, 128.6, 128.4, 126.9, 125.4, 124.1, 118.9, 118.0, 36.9, 26.8. IR (KBr) ν: 3434, 1638, 1620, 1546, 1464, 1400, 1251 cm−1. HR-MS (ESI) calcd for C31H25N2O3 [M + H]+ 473.1865, found 473.1869.
N3,N11-Dimethyl-14-(2-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6b): White solid. Yield 85.4%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.61 (br.q, J = 4.3 Hz, 2H, CONH × 2), 8.44 (overlapping d, J = 5.5 Hz, 4H), 8.03 (overlapping t, J = 7.5 Hz, 4H), 7.58 (overlapping t, J = 8.6 Hz, 3H), 7.13–6.92 (m, 3H), 6.86 (s, 1H, H-14), 2.82 (d, J = 4.1 Hz, 6H, CH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.8, 159.0 (d, J = 244.3 Hz), 149.6, 132.6, 131.9 (d, J = 13.3 Hz), 131.4 (d, J = 3.2 Hz), 131.0, 131.0, 130.3, 129.5 (d, J = 8.3 Hz), 128.8, 125.7, 125.7, 123.0, 118.9, 116.3 (d, J = 22.5 Hz), 115.7, 31.6, 26.8. IR (KBr) ν: 3358, 1641, 1622, 1542, 1488, 1465, 1400, 1310, 1253 cm−1. HR-MS (ESI) calcd for C31H24FN2O3 [M + H]+ 491.1771, found 491.1778.
N3,N11-Dimethyl-14-(4-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6c): White solid. Yield 86.6%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 8.9 Hz, 2H, H-1, 13), 8.68–8.57 (m, 2H, CONH × 2), 8.45 (br.s, 2H, H-4, 10), 8.03 (overlapping d, J = 8.9 Hz, 4H), 7.70–7.58 (m, 4H), 6.97 (t, J = 8.7 Hz, 2H, H-3′, 5′), 6.81 (s, 1H, H-14), 2.82 (d, J = 4.3 Hz, 6H, CH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.9, 161.0 (d, J = 243.3 Hz), 149.3, 142.0, 132.6, 131.1, 130.8, 130.5, 130.2 (d, J = 8.0 Hz), 128.7, 125.5, 124.0, 118.9, 117.8, 115.7 (d, J = 21.2), 36.0, 26.8. IR (KBr) ν: 3306, 1642, 1549, 1506, 1464, 1401, 1252 cm−1. HR-MS (ESI) calcd for C31H24FN2O3 [M + H]+ 491.1771, found 491.1762.
N3,N11-Dimethyl-14-(2-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6d): White solid. Yield 89.3%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.60 (overlapping d, J = 8.7 Hz, 4H), 8.45 (br.s, 2H, H-4, 10), 8.04 (overlapping d, J = 8.8 Hz, 4H), 7.59 (d, J = 8.9 Hz, 2H, H-6, 8), 7.49 (d, J = 7.3 Hz, 1H, H-6′), 7.32 (d, J = 7.7 Hz, 1H, H-3′), 7.11 (t, J = 7.4 Hz, 1H, H-5′), 7.03 (t, J = 7.2 Hz, 1H, H-4′), 6.79 (s, 1H, H-14), 2.82 (d, J = 4.2 Hz, 6H, CH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.8, 149.7, 142.8, 132.7, 132.3, 131.1, 131.0, 130.6, 130.5, 130.4, 129.2, 128.8, 128.7, 125.5, 123.6, 119.0, 116.7, 35.2, 26.8. IR (KBr) ν: 3428, 1640, 1550, 1465, 1400, 1252 cm−1. HR-MS (ESI) calcd for C31H24ClN2O3 [M + H]+ 507.1475, found 507.1481.
N3,N11-Dimethyl-14-(4-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6e): White solid. Yield 82.7%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.71 (d, J = 9.0 Hz, 2H, H-1, 13), 8.56 (br.q, J = 4.6 Hz, 2H, CONH × 2), 8.44 (br.s, 2H, H-4, 10), 8.03 (overlapping t, J = 8.4 Hz, 4H), 7.67–7.58 (m, 4H), 7.21 (d, J = 8.5 Hz, 2H, H-3′, 5′), 6.81 (s, 1H, H-14), 2.83 (d, J = 4.5 Hz, 6H, CONCH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.9, 149.4, 144.7, 132.5, 131.6, 131.2, 130.8, 130.5, 130.1, 129.0, 128.6, 125.5, 124.0, 118.9, 117.5, 36.2, 26.8. IR (KBr) ν: 3350, 1642, 1548, 1488, 1465, 1400, 1253 cm−1. HR-MS (ESI) calcd for C31H24ClN2O3 [M + H]+ 507.1475, found 507.1483.
N3,N11-Dimethyl-14-(3-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6f): White solid. Yield 84.9%. m.p. >300 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.80 (d, J = 9.0 Hz, 2H, H-1, 13), 8.64–8.55 (m, 3H), 8.46 (s, 2H, H-4, 10), 8.10–8.00 (m, 5H), 7.86 (br.d, J = 8.0 Hz, 1H, H-4′), 7.67 (d, J = 8.9 Hz, 2H, H-6, 8), 7.46 (t, J = 8.0 Hz, 1H, H-5′), 7.02 (s, 1H, H-14), 2.82 (d, J = 4.4 Hz, 6H, CONCH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.8, 149.5, 148.3, 147.7, 134.8, 132.5, 131.2, 131.2, 130.7, 130.5, 128.8, 125.7, 123.8, 122.5, 122.2, 119.0, 117.0, 36.3, 26.8. IR (KBr) ν: 3449, 3352, 1641, 1546, 1524, 1464, 1401, 1350, 1348, 1255 cm−1. HR-MS (ESI) calcd for C31H24N3O5 [M + H]+ 518.1716, found 518.1722.
N3,N11-Dimethyl-14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6g): White solid. Yield 84.2%. m.p. 285–287 °C. 1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.75 (d, J = 8.9 Hz, 2H, H-1, 13), 8.60 (br.d, J = 4.3 Hz, 2H, CONH × 2), 8.45 (s, 2H, H-4, 10), 8.12–7.98 (m, 6H), 7.92 (d, J = 8.6 Hz, 2H, H-2′, 6′), 7.65 (d, J = 8.9 Hz, 2H, H-6, 8), 6.99 (s, 1H, H-14), 2.82 (d, J = 4.0 Hz, 6H, CH3 × 2). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.8, 152.8, 149.4, 146.4, 132.5, 131.2, 131.2, 130.5, 129.5, 128.7, 125.7, 124.3, 123.9, 119.0, 116.7, 36.7, 26.8. IR (KBr) ν: 3353, 1645, 1550, 1523, 1465, 1399, 1344, 1255 cm−1. HR-MS (ESI) calcd for C31H24N3O5 [M + H]+ 518.1716, found 518.1710.
N3,N11-Dimethyl-14-(4-methylphenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (6h): White solid. Yield 85.7%. m.p. >300 °C.1H-NMR (300 MHz, DMSO-d6) δ (in ppm): 8.71 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (q, J = 4.5 Hz, 2H, CONH × 2), 8.43 (d, J = 1.4 Hz, 2H, H-4, 10), 8.04–7.96 (m, 4H), 7.60 (d, J = 8.9 Hz, 2H, H-6, 8), 7.48 (d, J = 8.1 Hz, 2H, H-2′, 6′), 6.92 (d, J = 8.0 Hz, 2H, H-3′, 5′), 6.72 (s, 1H, H-14), 2.82 (d, J = 4.5 Hz, 6H, CONCH3 × 2), 2.03 (s, 3H, Ar-CH3). 13C-NMR (75 MHz, DMSO-d6) δ (in ppm): 166.9, 149.3, 142.9, 136.0, 132.6, 131.0, 130.5, 130.4, 129.5, 128.6, 128.3, 125.3, 124.1, 118.9, 118.0, 36.5, 26.8, 20.9. IR (KBr) ν: 3356, 1641, 1548, 1466, 1400, 1309, 1253 cm−1. HR-MS (ESI) calcd for C32H27N2O3 [M + H]+ 487.2022, found 487.2031.
N3,N11-Dipropyl-14-phenyl-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7a): White solid. Yield 87.8%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 8.9 Hz, 2H, H-1, 13), 8.57 (t, J = 5.5 Hz, 2H, CONH × 2), 8.44 (s, 2H, H-4, 10), 8.06–8.00 (m, 4H), 7.69–7.55 (m, 4H), 7.15 (t, J = 7.6 Hz, 2H, H-3′, 5′), 6.98 (t, J = 7.5 Hz, 1H, H-4′), 6.78 (s, 1H, H-14), 3.30–3.13 (m, 4H, NHCH2CH2CH3 × 2), 1.56 (m, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3272, 2959, 2927, 1638, 1551, 1463, 1250 cm−1. HR-MS (ESI) calcd for C35H33N2O3 [M + H]+ 529.2491, found 529.2498.
N3,N11-Dipropyl-14-(2-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7b): White solid. Yield 91.2%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.57 (t, J = 5.6 Hz, 2H, CONH × 2), 8.47 (d, J = 8.9 Hz, 2H, H-1, 13), 8.46 (s, 2H, H-4, 10), 8.07 (d, J = 9.0 Hz, 2H, H-5, 9), 8.04 (dd, J = 8.9, 1.5 Hz, 2H, H-2, 12), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.58 (t, J = 7.8 Hz, 1H, H-6′), 7.13–7.05 (m, 2H, H-3′, 5′), 7.04–6.90 (m, 1H, H-4′), 6.89 (s, 1H, H-14), 3.34–3.19 (m, 4H, NHCH2CH2CH3 × 2), 1.56 (h, J = 7.2 Hz, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3270, 2960, 2934, 1632, 1551, 1464, 1252 cm−1. HR-MS (ESI) calcd for C35H32FN2O3 [M + H]+ 547.2397, found 547.2404.
N3,N11-Dipropyl-14-(4-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7c): White solid. Yield 88.7%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.73 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (t, J = 5.7 Hz, 2H, CONH × 2), 8.45 (d, J = 1.6 Hz, 2H, H-4, 10), 8.06–8.00 (m, 4H), 7.76–7.54 (m, 4H), 6.98 (t, J = 8.9 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14), 3.35–3.14 (m, 4H, NHCH2CH2CH3 × 2), 1.57 (h, J = 7.3 Hz, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3292, 2962, 2931, 2873, 1634, 1549, 1507, 1462, 1399, 1248 cm−1. HR-MS (ESI) calcd for C35H32FN2O3 [M + H]+ 547.2397, found 547.2391.
N3,N11-Dipropyl-14-(2-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7d): White solid. Yield 84.6%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.67 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (t, J = 5.7 Hz, 2H, CONH × 2), 8.47 (d, J = 1.5 Hz, 2H, H-4, 10), 8.09 (d, J = 9.0 Hz, 2H, H-5, 9), 8.05 (dd, J = 8.9, 1.7 Hz, 2H, H-2, 12), 7.65 (d, J = 8.9 Hz, 2H, H-6, 8), 7.54 (d, J = 7.8 Hz, 1H, H-6′), 7.34 (d, J = 6.8 Hz, 1H, H-3′), 7.15 (t, J = 7.0 Hz, 1H, H-5′), 7.07 (t, J = 7.6 Hz, 1H, H-4′), 6.89 (s, 1H, H-14), 3.35–3.16 (m, 4H, NHCH2CH2CH3 × 2), 1.56 (m, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3260, 2960, 2926, 2872, 1639, 1550, 1463, 1398, 1250 cm−1. HR-MS (ESI) calcd for C35H32ClN2O3 [M + H]+ 563.2101, found 563.2112.
N3,N11-Dipropyl-14-(4-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7e): White solid. Yield 82.5%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.72 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (t, J = 5.7 Hz, 2H, CONH × 2), 8.46 (d, J = 1.7 Hz, 2H, H-4, 10), 8.06 (d, J = 8.9 Hz, 2H, H-5, 9), 8.03 (dd, J = 9.0, 1.8 Hz, 2H, H-2, 12), 7.64 (d, J = 8.6 Hz, 2H, H-2′, 6′), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.22 (d, J = 8.6 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14), 3.36–3.19 (m, 4H, NHCH2CH2CH3 × 2), 1.57(h, J = 7.3 Hz, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3302, 2962, 2931, 1636, 1547, 1462, 1399, 1251 cm−1. HR-MS (ESI) calcd for C35H32ClN2O3 [M + H]+ 563.2101, found 563.2096.
N3,N11-Dipropyl-14-(3-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7f): White solid. Yield 86.3%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.80 (d, J = 9.0 Hz, 2H, H-1, 13), 8.62–8.56 (m, 3H), 8.47 (d, J = 1.6 Hz, 2H, H-4, 10), 8.17–7.99 (m, 5H), 7.87 (dd, J = 8.2, 1.4 Hz, 1H, H-4′), 7.68 (d, J = 8.9 Hz, 2H, H-6, 8), 7.47 (t, J = 8.0 Hz, 1H, H-5′), 7.03 (s, 1H, H-14), 3.38–3.19 (m, 4H, NHCH2CH2CH3 × 2), 1.56 (m, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3243, 2959, 2921, 2868, 1630, 1526, 1462, 1400, 1348, 1319, 1243 cm−1. HR-MS (ESI) calcd for C35H32N3O5 [M + H]+ 574.2342, found 574.2332.
N3,N11-Dipropyl-14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7g): White solid. Yield 85.3%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 9.0 Hz, 2H, H-1, 13), 8.59 (t, J = 5.7 Hz, 2H, CONH × 2), 8.46 (d, J = 1.7 Hz, 2H, H-4, 10), 8.09 (d, J = 9.0 Hz, 2H, H-3′, 5′), 8.06–8.01(m, 4H), 7.93 (d, J = 8.9 Hz, 2H, H-2′, 6′), 7.67 (d, J = 8.9 Hz, 2H, H-6, 8), 7.00 (s, 1H, H-14), 3.36–3.14(m, 4H, NHCH2CH2CH3 × 2), 1.56 (h, J = 7.3 Hz, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3273, 2966, 2925, 2872, 1639, 1622, 1529, 1463, 1399, 1345, 1250 cm−1. HR-MS (ESI) calcd for C35H32N3O5 [M + H]+ 574.2342, found 574.2350.
N3,N11-Dipropyl-14-(4-methylphenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (7h): White solid. Yield 89.9%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.71 (d, J = 9.0 Hz, 2H, H-1, 13), 8.57 (t, J = 5.7Hz, 2H, CONH × 2), 8.44 (d, J = 1.6 Hz, 2H, H-4, 10), 8.03 (d, J = 8.9 Hz, 2H, H-5, 9), 8.01 (dd, J = 9.0, 1.8 Hz, 2H, H-2, 12), 7.62 (d, J = 8.9 Hz, 2H, H-6, 8), 7.49 (d, J = 8.1 Hz, 2H, H-2′, 6′), 6.94 (d, J = 8.0 Hz, 2H, H-3′, 5′), 6.73 (s, 1H, H-14), 3.33–3.16 (m, 4H, NHCH2CH2CH3 × 2), 2.05 (s, 3H, Ar-CH3), 1.57 (h, J = 7.3 Hz, 4H, NHCH2CH2CH3 × 2), 0.91 (t, J = 7.4 Hz, 6H, NHCH2CH2CH3 × 2). IR (KBr) ν: 3289, 2959, 2925, 2856, 1639, 1552, 1511, 1462, 1398, 1317, 1241 cm−1. HR-MS (ESI) calcd for C36H35N2O3 [M + H]+ 543.2648, found 543.2640.
N3,N11-Diisobutyl-14-phenyl-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8a): White solid. Yield 84.3%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (t, J = 5.8 Hz, 2H, CONH × 2), 8.45 (d, J = 1.1 Hz, 2H, H-4, 10), 8.07–8.00 (m, 4H), 7.64 (d, J = 8.9 Hz, 2H, H-6, 8), 7.63 (d, J = 7.5 Hz, 2H, H-2′, 6′), 7.15 (t, J = 7.7 Hz, 2H, H-3′, 5′), 6.98 (t, J = 7.3 Hz, 1H, H-4′), 6.78 (s, 1H, H-14), 3.22–3.03 (m, 4H, NHCH2 × 2), 1.94–1.82 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3298, 2958, 2924, 1635, 1550, 1462, 1242 cm−1. HR-MS (ESI) calcd for C37H37N2O3 [M + H]+ 557.2804, found 557.2809.
N3,N11-Diisobutyl-14-(2-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8b): White solid. Yield 82.7%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.57 (t, J = 5.8 Hz, 2H, CONH × 2), 8.50–8.44 (m, 4H), 8.08 (d, J = 9.0 Hz, 2H, H-5, 9), 8.05 (dd, J = 9.0, 1.6 Hz, 2H, H-2, 12), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.58 (t, J = 7.8 Hz, 1H, H-6′), 7.17–7.05 (m, 2H, H-3′, 5′), 7.04–6.95 (m, 1H, H-4′), 6.89 (s, 1H, H-14), 3.17–3.09 (m, 4H, NHCH2 × 2), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3295, 2958, 2925, 1637, 1550, 1463, 1251 cm−1. HR-MS (ESI) calcd for C37H36FN2O3 [M + H]+ 575.2710, found 575.2715.
N3,N11-Diisobutyl-14-(4-fluorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8c): White solid. Yield 83.8%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.73 (d, J = 9.0 Hz, 2H, H-1, 13), 8.59 (t, J = 5.8 Hz, 2H, CONH × 2), 8.46 (d, J = 1.6 Hz, 2H, H-4, 10), 8.07–8.00 (m, 4H), 7.76–7.54 (m, 4H), 6.98 (t, J = 8.9 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14), 3.21–3.07 (m, 4H, NHCH2 × 2), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3299, 2958, 2925, 1635, 1549, 1507, 1462, 1399, 1248 cm−1. HR-MS (ESI) calcd for C37H36FN2O3 [M + H]+ 575.2710, found 575.2719.
N3,N11-Diisobutyl-14-(2-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8d): White solid. Yield 87.5%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.66 (d, J = 9.0 Hz, 2H, H-1, 13), 8.59 (t, J = 5.8 Hz, 2H, CONH × 2), 8.47 (d, J = 1.3 Hz, 2H, H-4, 10), 8.12–8.03 (m, 4H), 7.64 (d, J = 8.9 Hz, 2H, H-6, 8), 7.53 (d, J = 7.7 Hz, 1H, H-6′), 7.34 (d, J = 8.0 Hz, 1H, H-3′), 7.14 (t, J = 7.1 Hz, 1H, H-5′), 7.07 (t, J = 6.9 Hz, 1H, H-4′), 6.87 (s, 1H, H-14), 3.13 (t, J = 6.4 Hz, 4H, NHCH2 × 2), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3281, 2960, 2926, 1638, 1547, 1463, 1397, 1249 cm−1. HR-MS (ESI) calcd for C37H36ClN2O3 [M + H]+ 591.2414, found 591.2420.
N3,N11-Diisobutyl-14-(4-chlorophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8e): White solid. Yield 81.6%. m.p. 266–268 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.72 (d, J = 9.0 Hz, 2H, H-1, 13), 8.58 (t, J = 5.8 Hz, 2H, CONH × 2), 8.46 (d, J = 1.7 Hz, 2H, H-4, 10), 8.06 (d, J = 8.9 Hz, 2H, H-5, 9), 8.03 (dd, J = 8.9, 1.8 Hz, 2H, H-2, 12), 7.64 (d, J = 8.6 Hz, 2H, H-2′, 6′), 7.63 (d, J = 8.9 Hz, 2H, H-6, 8), 7.22 (d, J = 8.6 Hz, 2H, H-3′, 5′), 6.82 (s, 1H, H-14), 3.20–3.06 (m, 4H, NHCH2 × 2), 1.93–1.80 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3319, 2958, 2926, 1637, 1546, 1463, 1398, 1250 cm−1. HR-MS (ESI) calcd for C37H36ClN2O3 [M + H]+ 591.2414, found 591.2418.
N3,N11-Diisobutyl-14-(3-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8f): White solid. Yield 85.1%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.80 (d, J = 9.0 Hz, 2H, H-1, 13), 8.62–8.56 (m, 3H), 8.47 (d, J = 1.6 Hz, 2H, H-4, 10), 8.17–7.99 (m, 5H), 7.87 (dd, J = 8.2, 1.4 Hz, 1H, H-4′), 7.68 (d, J = 8.9 Hz, 2H, H-6, 8), 7.48 (t, J = 8.0 Hz, 1H, H-5′), 7.03 (s, 1H, H-14), 3.23–3.02 (m, 4H, NHCH2 × 2), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3293, 2960, 2926, 1640, 1532, 1462, 1396, 1350, 1246 cm−1. HR-MS (ESI) calcd for C37H36N3O5 [M + H]+ 602.2655, found 602.2650.
N3,N11-Diisobutyl-14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8g): White solid. Yield 82.6%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.74 (d, J = 8.9 Hz, 2H, H-1, 13), 8.59 (t, J = 5.7 Hz, 2H, CONH × 2), 8.47 (d, J = 1.6 Hz, 2H, H-4, 10), 8.09 (d, J = 9.0 Hz, 2H, H-3′, 5′), 8.06–8.01(m, 4H), 7.93 (d, J = 8.9 Hz, 2H, H-2′, 6′), 7.67 (d, J = 8.9 Hz, 2H, H-6, 8), 7.00 (s, 1H, H-14), 3.23–3.02 (m, 4H, NHCH2 × 2), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3320, 2958, 2926, 1640, 1546, 1514, 1463, 1346, 1250 cm−1. HR-MS (ESI) calcd for C37H36N3O5 [M + H]+ 602.2655, found 602.2648.
N3,N11-Diisobutyl-14-(4-methylphenyl)-14H-dibenzo[a,j]xanthene-3,11-dicarboxamide (8h): White solid. Yield 84.6%. m.p. >300 °C. 1H-NMR (400 MHz, DMSO-d6) δ (in ppm): 8.71 (d, J = 9.0 Hz, 2H, H-1, 13), 8.57 (t, J = 5.8 Hz, 2H, CONH × 2), 8.45 (d, J = 1.6 Hz, 2H, H-4, 10), 8.03 (d, J = 8.9 Hz, 2H, H-5, 9), 8.01 (dd, J = 8.9, 1.8 Hz, 2H, H-2, 12), 7.62 (d, J = 8.9 Hz, 2H, H-6, 8), 7.49 (d, J = 8.1 Hz, 2H, H-2′, 6′), 6.94 (d, J = 8.0 Hz, 2H, H-3′, 5′), 6.73 (s, 1H, H-14), 3.23–3.02 (m, 4H, NHCH2 × 2), 2.05 (s, 3H, Ar-CH3), 1.94–1.81 (m, 2H, CH(CH3)2 × 2), 0.91 (d, J = 6.7 Hz, 12H, CH(CH3)2 × 2). IR (KBr) ν: 3340, 2956, 2922, 1641, 1548, 1463, 1397, 1316, 1249 cm−1. HR-MS (ESI) calcd for C38H39N2O3 [M + H]+ 571.2961, found 571.2967.