The Effect and Mechanism of Transdermal Penetration Enhancement of Fu’s Cupping Therapy: New Physical Penetration Technology for Transdermal Administration with Traditional Chinese Medicine (TCM) Characteristics
Abstract
:1. Background
2. Methods
2.1. Materials
2.2. Preparation of Indomethacin Patches
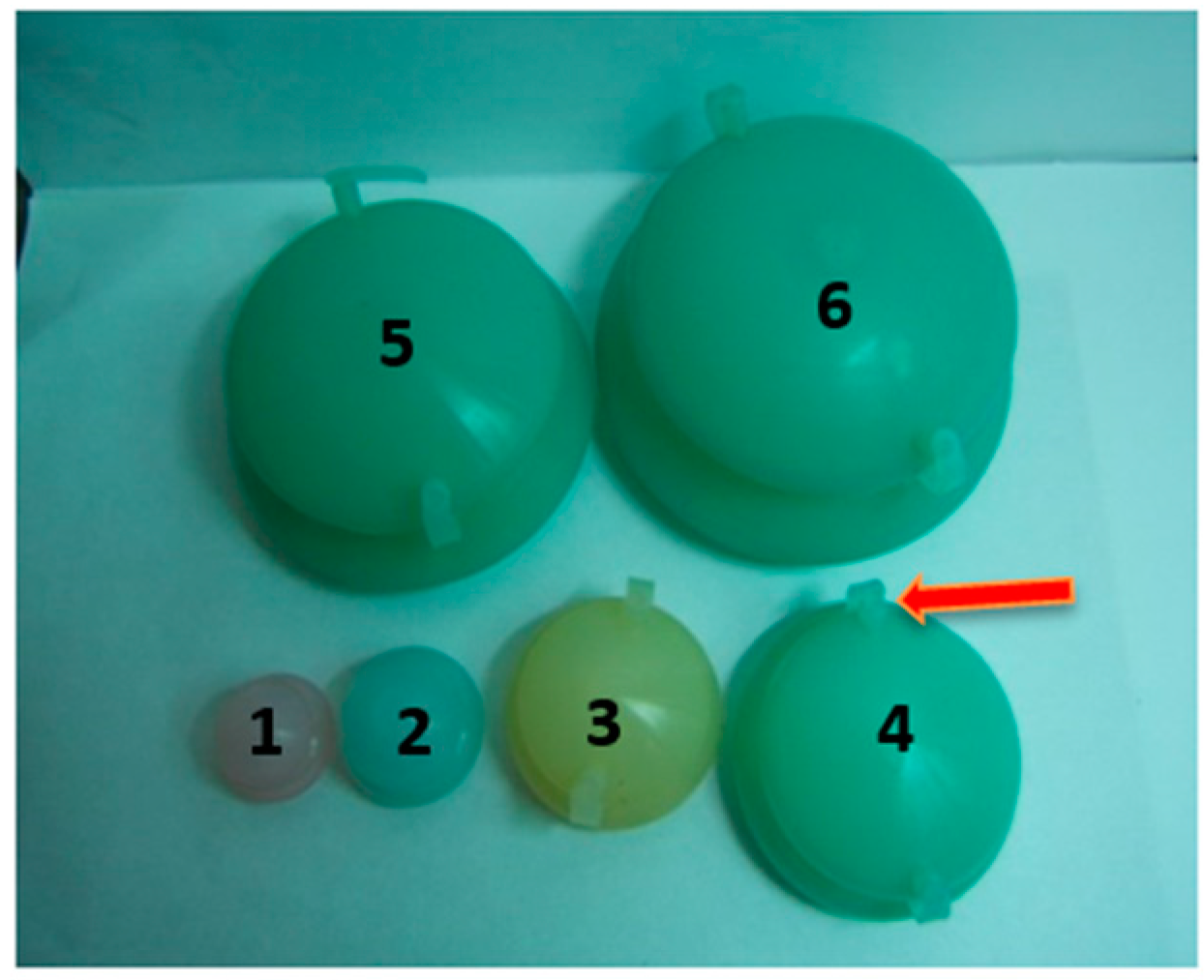
2.3. Standard Operation of FCT Penetration Enhancement
3. In Vitro Transdermal Penetration Enhancement Study
3.1. Quantitative Methods
3.2. In Vitro Transdermal Tests
4. In Vivo Transdermal Penetration Enhancement
4.1. Quantitative Method
4.2. In Vivo Percutaneous Penetration Enhancement Test
5. Penetration Enhancement Synergism Study
6. Skin Structure Study by SEM and TEM
6.1. Grouping and Treatment of Animals
6.2. Sample Preparation and Observation
7. Results
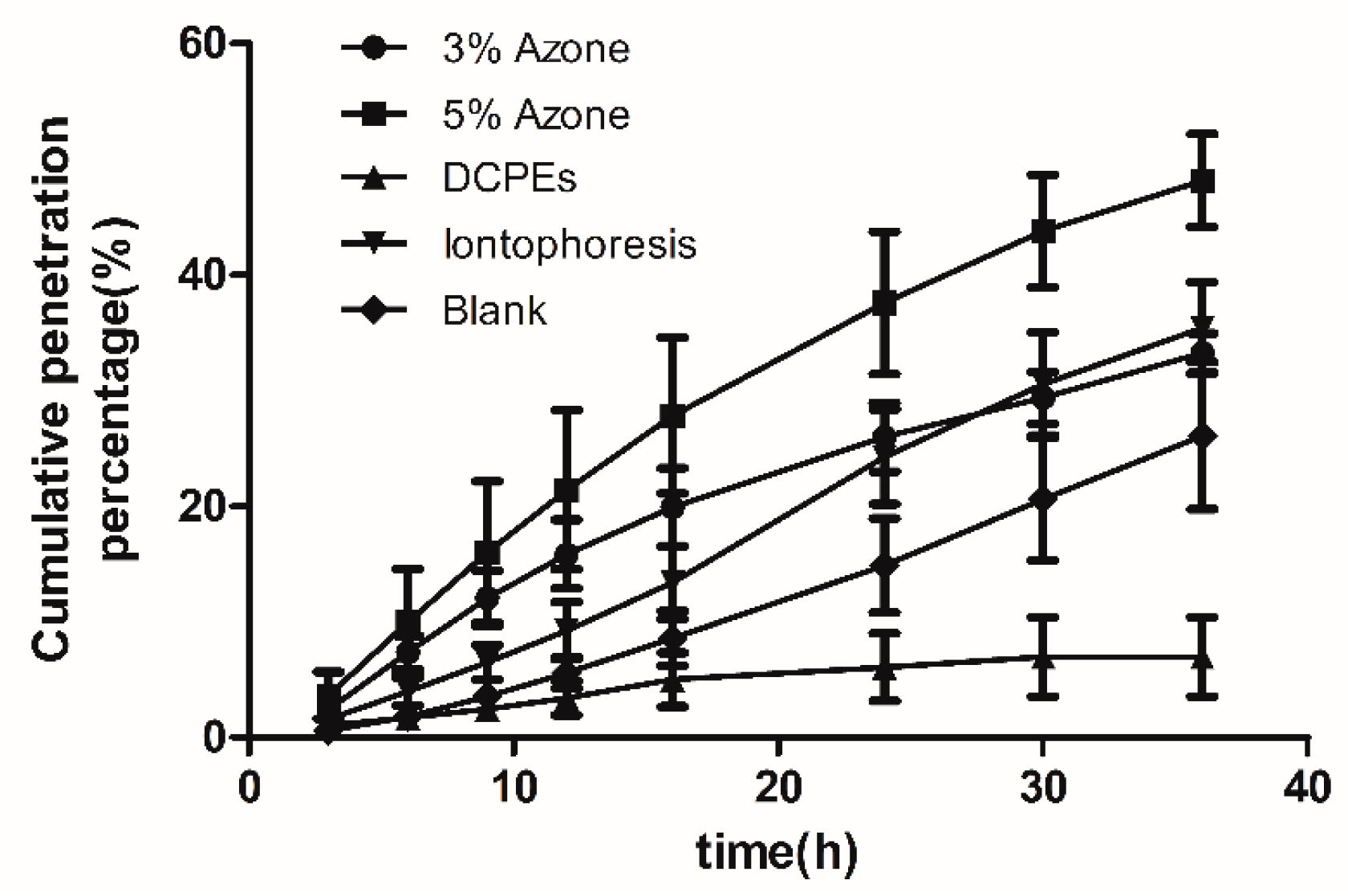
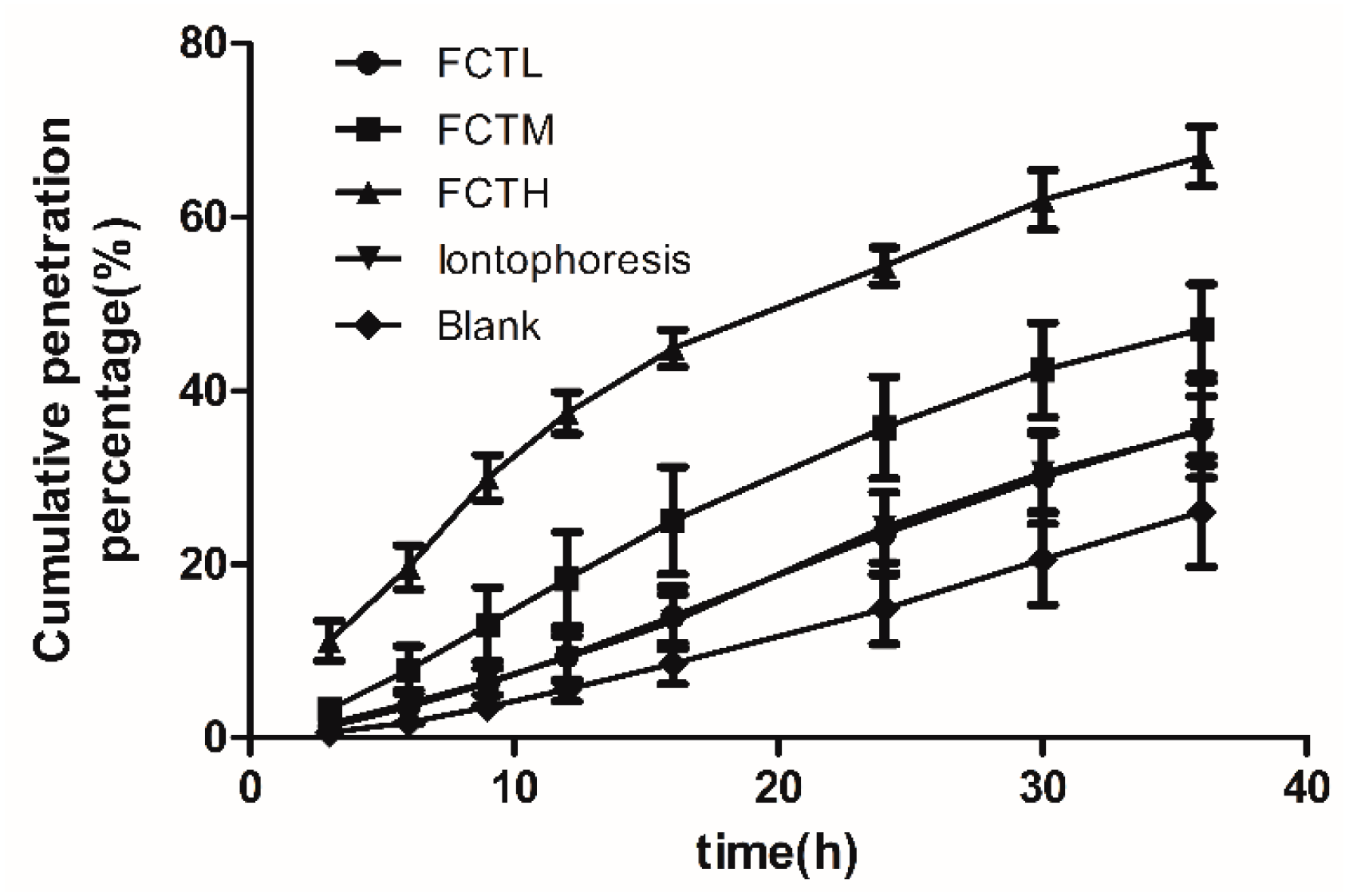
7.1. Analysis of In Vitro Results
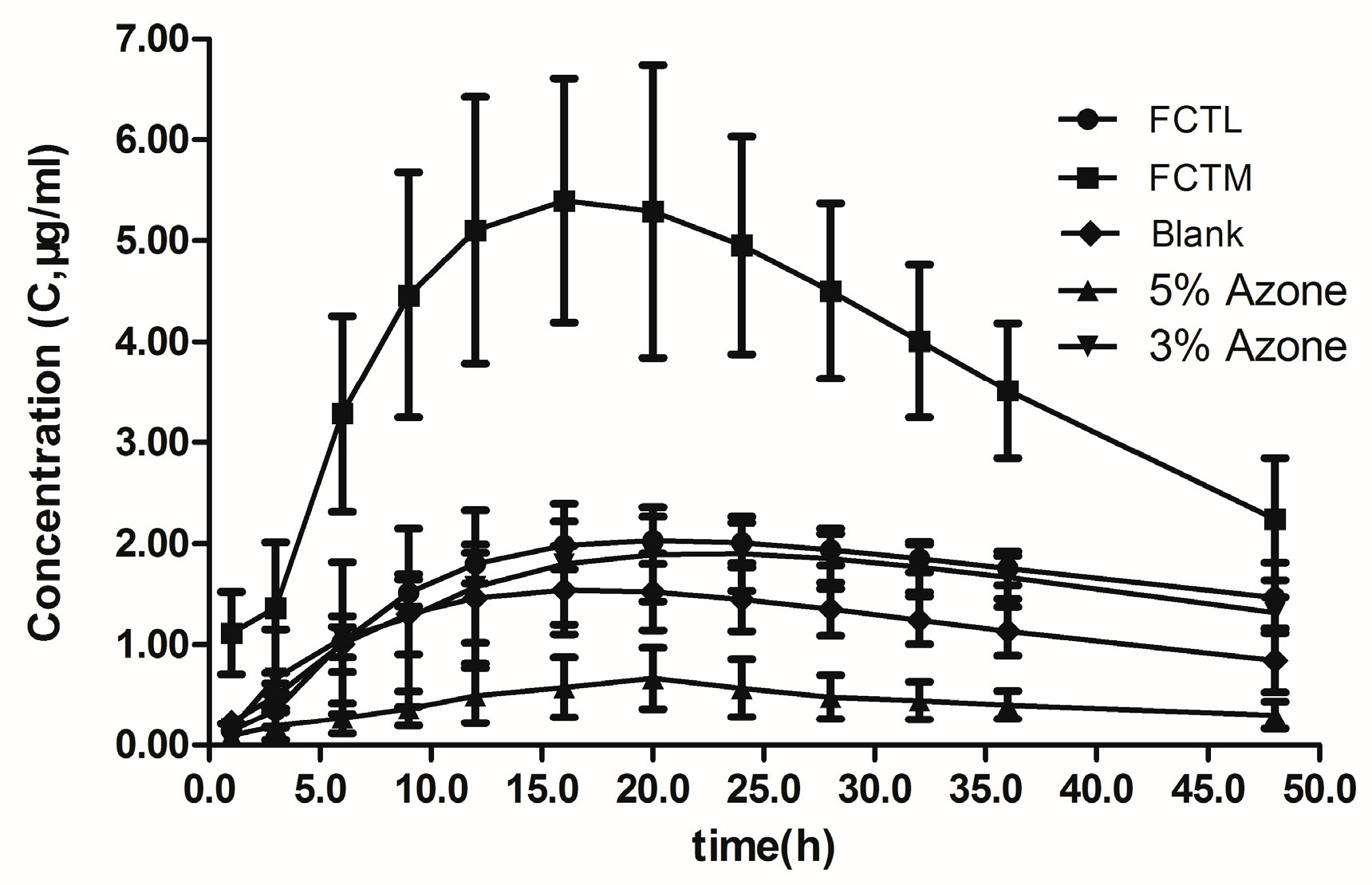
7.2. Analysis of In Vivo Pharmacokinetic Results
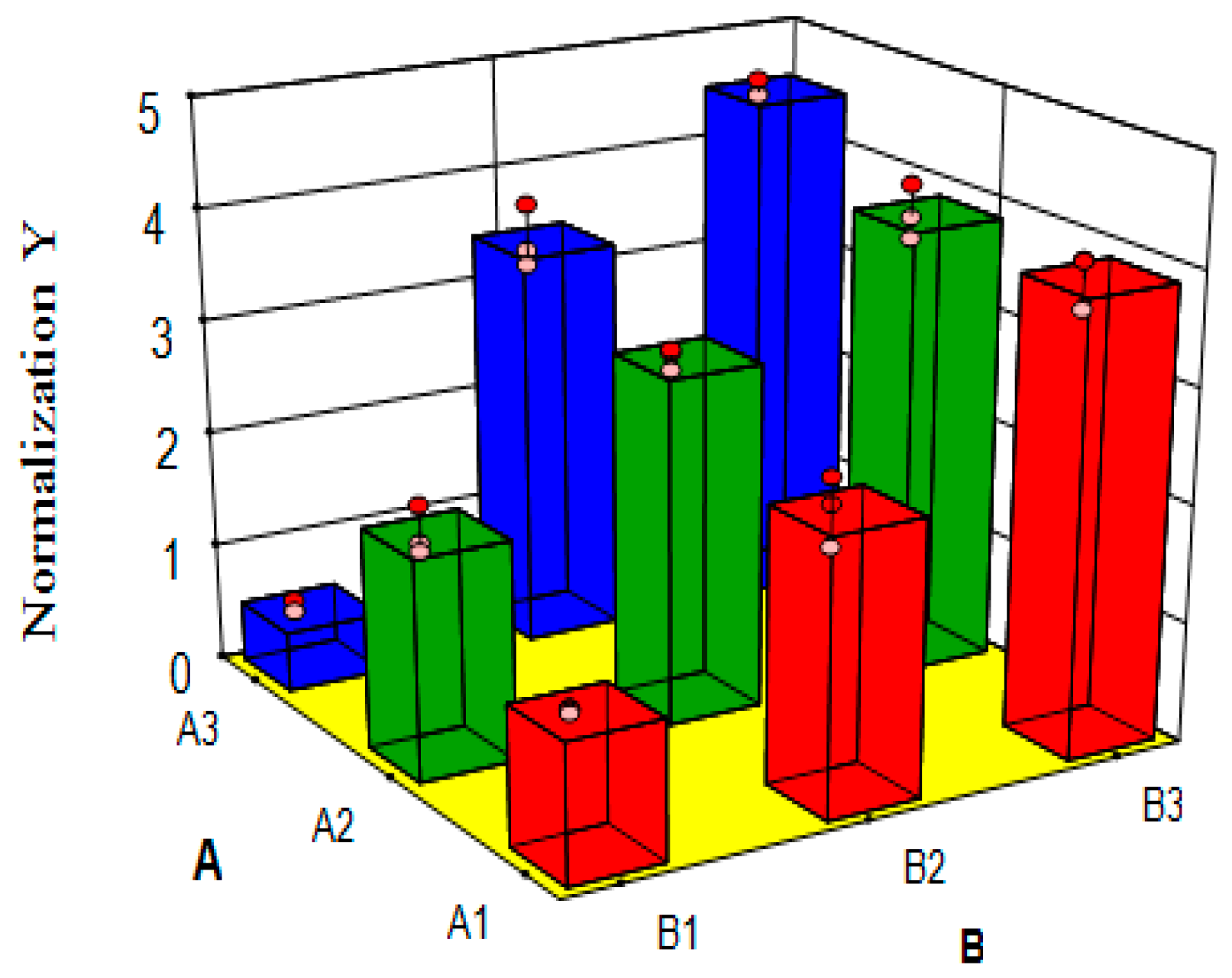
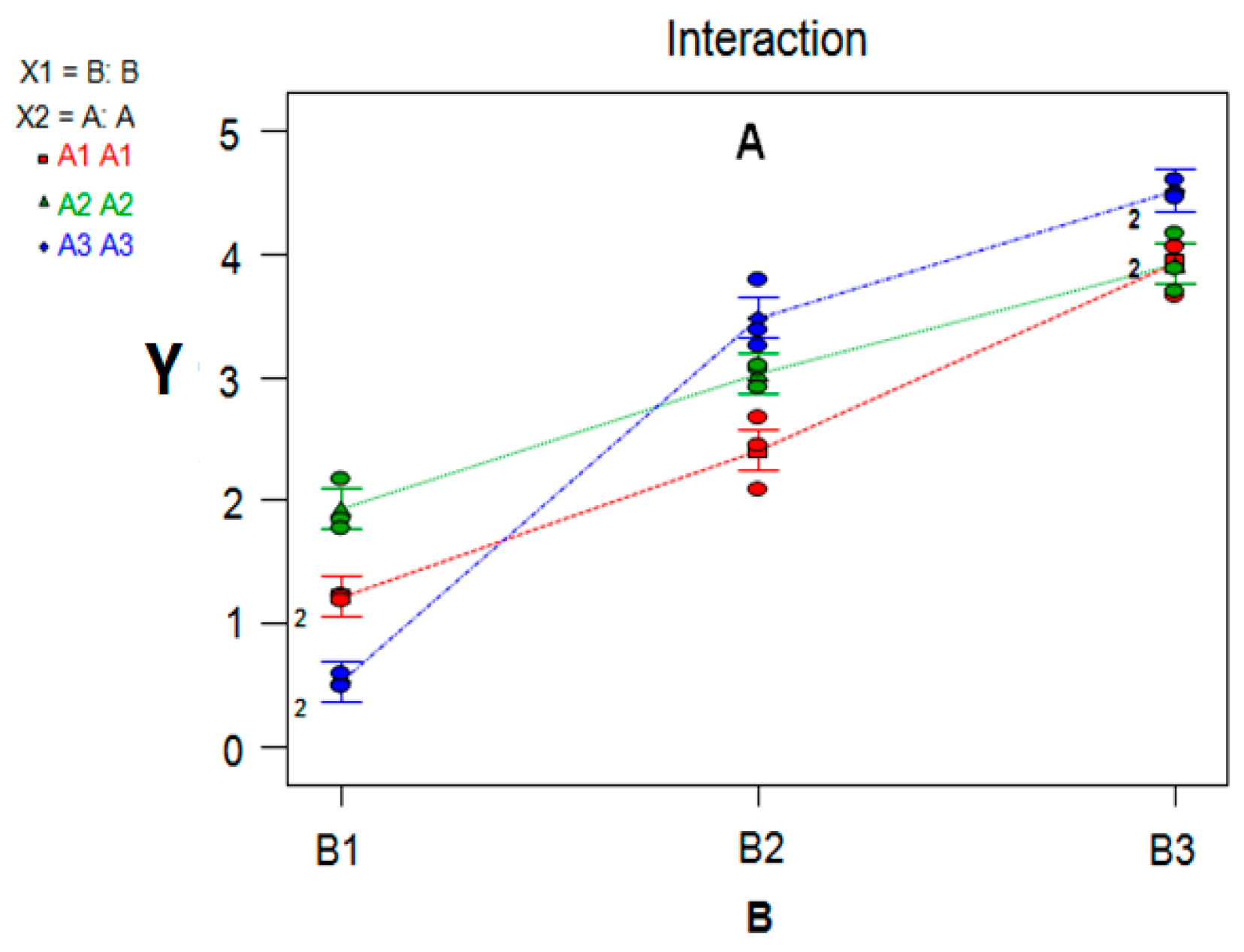
7.3. Variance Analysis of Synergism Study
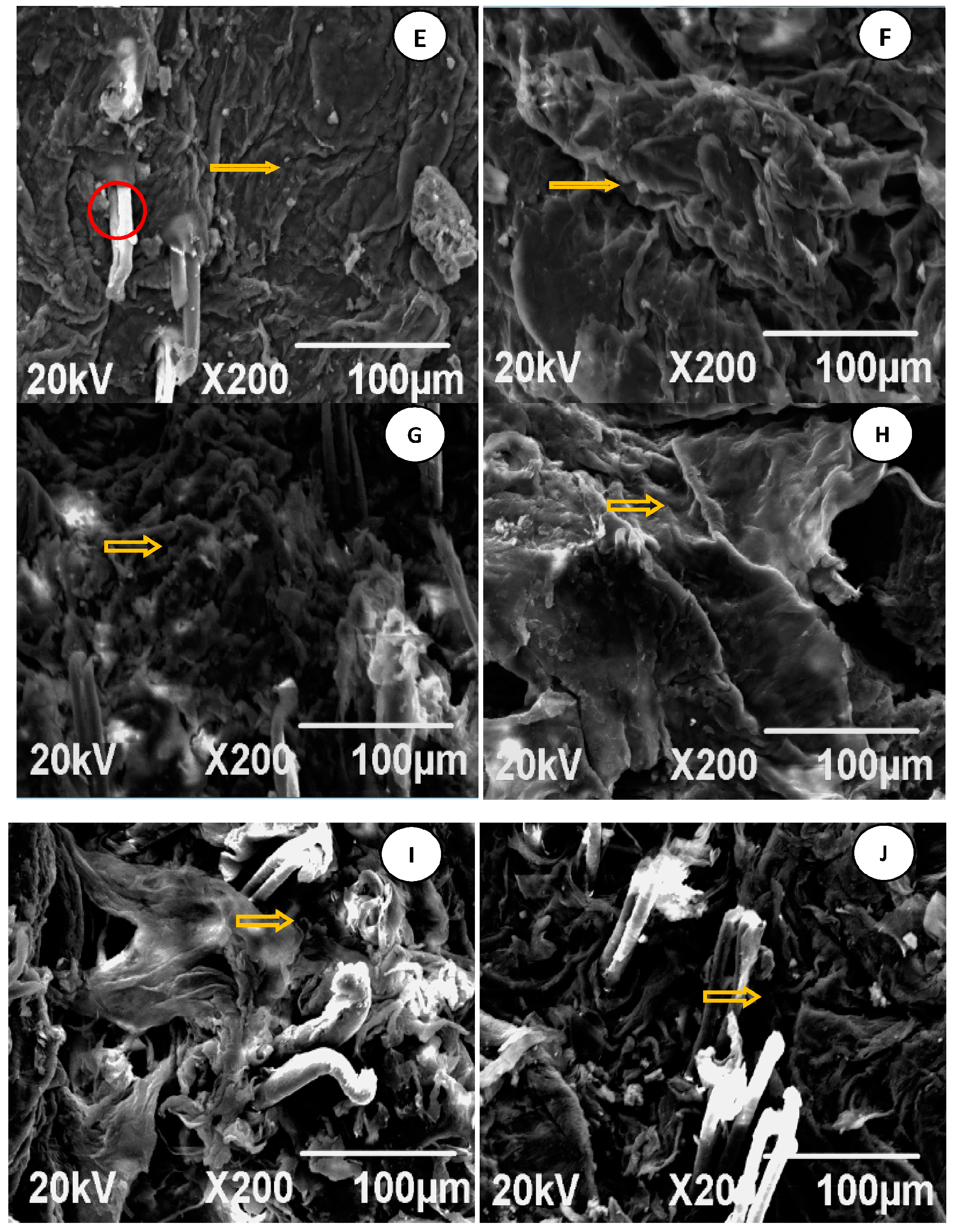
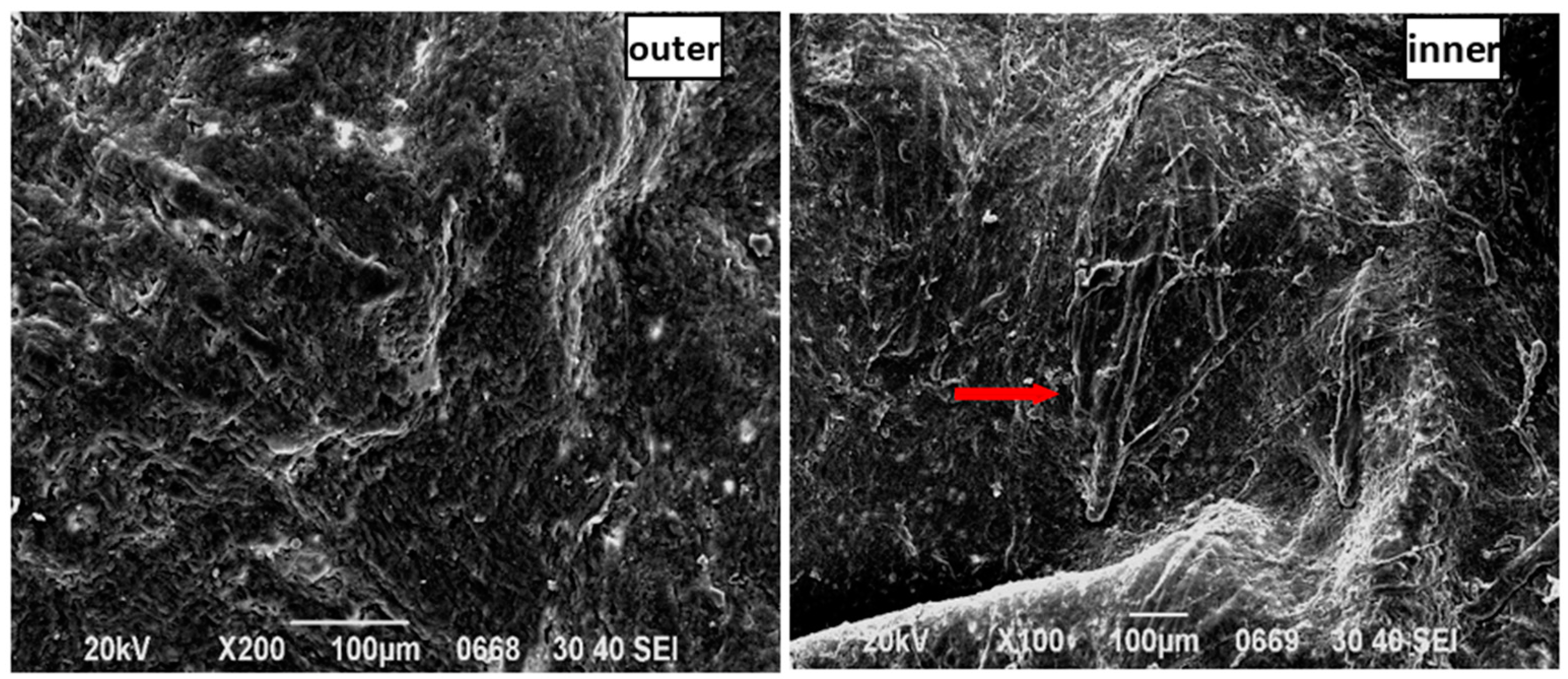
7.4. Analysis of SEM Images
7.5. Analysis of TEM Images
8. Discussion
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Artusi, M.; Nicoli, S.; Colombo, P.; Bettini, R.; Sacchi, A.; Santi, P. Effect of chemical enhancers and iontophoresis on thiocolchicoside permeation across rabbit and human skin in vitro. J. Pharm. Sci. 2004, 93, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Bloksgaard, M.; Brewer, J.R.; Pashkovski, E.; Ananthapadmanabhan, K.P.; Sorensen, J.A.; Bagatolli, L.A. Effect of detergents on the physicochemical properties of skin stratum corneum: A two-photon excitation fluorescence microscopy study. Int. J. Cosmet. Sci. 2014, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Bonilla-Martinez, D.; Villegas-Gonzalez, M.A.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vazquez, A.L. Microneedles: A valuable physical enhancer to increase transdermal drug delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.A.; Chong, Y.F.; Cock, A. The mechanical properties of the skin epidermis in relation to targeted gene and drug delivery. Biomaterials 2007, 28, 4968–4977. [Google Scholar] [CrossRef] [PubMed]
- Mack Correa, M.C.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Pamornpathomkul, B.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Investigation of the mechanism of enhanced skin penetration by ultradeformable liposomes. Int. J. Nanomed. 2014, 9, 3539–3550. [Google Scholar]
- Mathur, V.; Satrawala, Y.; Rajput, M. Physical and chemical penetration enhancers in transdermaldrug delivery system. Asian J. Pharm. 2010, 4, 173–182. [Google Scholar] [CrossRef]
- Polat, B.E.; Deen, W.M.; Langer, R.; Blankschtein, D. A physical mechanism to explain the delivery of chemical penetration enhancers into skin during transdermal sonophoresis—Insight into the observed synergism. J. Control. Release 2012, 158, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, H.; Zheng, J.; Mou, D.; Wan, J.; Zhang, J.; Shi, T.; Zhao, Y.; Xu, H.; Yang, X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J. Control. Release 2009, 139, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kalluri, H.; Banga, A.K. Effects of chemical and physical enhancement techniques on transdermal delivery of cyanocobalamin (Vitamin B12) in vitro. Pharmaceutics 2011, 3, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.; Kruithof, A.C.; Liebl, H.; Tomerius, M.; Bouwstra, J.; Lademann, J.; Meinke, M. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy. Laser Phys. Lett. 2010, 7, 242–246. [Google Scholar] [CrossRef]
- Qvist, M.H.; Hoeck, U.; Kreilgaard, B.; Madsen, F.; Hovgaard, L.; Frokjaer, S. Application of confocal laser scanning microscopy in characterization of chemical enhancers in drug-in-adhesive transdermal patches. AAPS J. 2002, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Patel, G.; Baria, A. Formulation and evaluation of transdermal patch of aceclofenac. Int. J. Drug Deliv. 2009, 1, 41–51. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; El-Massik, M.A.; Abdallah, O.Y. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Baurecht, D.; Holper, E.; Mahrhauser, D.; Valenta, C. Validation of the combined ATR-FTIR/tape stripping technique for monitoring the distribution of surfactants in the stratum corneum. Int. J. Pharm. 2014, 472, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, T.; Kankate, L.; Kramer, K.D. Structure and dynamics of drug-carrier systems as studied by parelectric spectroscopy. Adv. Drug Deliv. Rev. 2007, 59, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Leichtnam, M.L.; Rolland, H.; Wuthrich, P.; Guy, R.H. Identification of penetration enhancers for testosterone transdermal delivery from spray formulations. J. Control. Release 2006, 113, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Sun, Y.; Liu, L.; Ma, X.; Hu, X.; Fan, J.; Zhao, Y. Folate-functionalized nanoparticles for controlled ergosta-4,6,8(14),22-tetraen-3-one delivery. Int. J. Pharm. 2013, 441, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Design and evaluation of transdermal drug delivery system for curcumin as an anti-inflammatory drug. Drug Dev. Ind. Pharm. 2009, 35, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Csizmazia, E.; Eros, G.; Berkesi, O.; Berko, S.; Szabo-Revesz, P.; Csanyi, E. Ibuprofen penetration enhance by sucrose ester examined by ATR-FTIR in vivo. Pharm. Dev. Technol. 2012, 17, 125–128. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds IM (indomethacin) and naproxen are available from the authors. |


 ) indomethacin residual in the matrix patch; (
) indomethacin residual in the matrix patch; (  ) indomethacin residual in skin; (
) indomethacin residual in skin; (  ) transmission indomethacin.
) transmission indomethacin.
 ) indomethacin residual in the matrix patch; (
) indomethacin residual in the matrix patch; (  ) indomethacin residual in skin; (
) indomethacin residual in skin; (  ) transmission indomethacin.
) transmission indomethacin.


| FCT Groups | Cupping Operation | Cupping Number | Time |
|---|---|---|---|
| FCTL | Retaining cupping | 2 | 6.0 min |
| FCTM | Shaking cupping | 3 | 11.0 min |
| FCTH | Moving cupping | 4 | 15.0 min |
| Time | Flow Rate (mL/min) | Aqueous Phase (%) | Organic Phase (%) | Curve |
|---|---|---|---|---|
| 0.0 | 0.3 | 64.0 | 36.0 | 6 |
| 1.0 | 0.3 | 54.0 | 46.0 | 6 |
| 2.1 | 0.3 | 48.0 | 52.0 | 6 |
| 4.5 | 0.3 | 25.0 | 75.0 | 6 |
| 5.0 | 0.3 | 10.0 | 90.0 | 6 |
| 6.0 | 0.3 | 10.0 | 90.0 | 6 |
| 6.5 | 0.3 | 64.0 | 36.0 | 6 |
| 7.5 | 64.0 | 36.0 | 38.0 | 6 |
| Compound | Parent (m/z) | Daughter (m/z) | Dwell (s) | Cone (V) | Collision (V) |
|---|---|---|---|---|---|
| IM number 1 | 358.1198 | 138.9997 | 0.49 | 18 | 20 |
| IM number 2 | 358.1198 | 111.9691 | 0.49 | 16 | 48 |
| Naproxen | 231.1855 | 185.0841 | 0.49 | 16 | 14 |
| Levels | CPE (A) | FCT (B) |
|---|---|---|
| 1 | Blank (A1) | Blank (B1) |
| 2 | 3% Azone (A2) | FCTL (B2) |
| 3 | 5% Azone (A3) | FCTM (B3) |
| No. | Arrangement | Indexes | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | AUC(0–t) | AUC(0–∞) | AUMC(0–t) | AUMC(0–∞) | Cmax | Y | |
| 1 | A2 | B2 | 111.719 | 210.015 | 2838.514 | 13,136.654 | 3.805 | 3.073 |
| 2 | A3 | B2 | 180.273 | 232.491 | 4164.901 | 11,384.695 | 5.072 | 3.800 |
| 3 | A3 | B1 | 23.021 | 37.343 | 526.322 | 2601.184 | 0.783 | 0.594 |
| 4 | A2 | B3 | 183.711 | 246.637 | 4072.945 | 11,759.021 | 7.699 | 4.176 |
| 5 | A1 | B2 | 78.320 | 168.944 | 1992.035 | 17,113.618 | 2.548 | 2.678 |
| 6 | A3 | B3 | 213.673 | 287.876 | 4611.003 | 12,129.244 | 7.849 | 4.613 |
| 7 | A2 | B2 | 120.784 | 206.598 | 2982.561 | 9664.643 | 3.750 | 2.924 |
| 8 | A3 | B2 | 138.879 | 222.301 | 3203.711 | 10,611.719 | 4.571 | 3.262 |
| 9 | A3 | B3 | 216.276 | 265.211 | 4522.868 | 10,476.852 | 8.332 | 4.487 |
| 10 | A1 | B2 | 75.333 | 155.939 | 1955.883 | 14,172.779 | 2.655 | 2.452 |
| 11 | A2 | B1 | 78.905 | 130.606 | 1865.553 | 10,963.154 | 2.681 | 2.176 |
| 12 | A3 | B1 | 21.087 | 30.890 | 534.802 | 1450.696 | 0.782 | 0.497 |
| 13 | A3 | B1 | 22.260 | 31.663 | 539.604 | 1243.887 | 0.779 | 0.493 |
| 14 | A2 | B3 | 167.116 | 236.699 | 3990.846 | 11,035.755 | 6.723 | 3.891 |
| 15 | A2 | B3 | 176.351 | 219.040 | 3875.847 | 8132.089 | 6.972 | 3.706 |
| 16 | A3 | B2 | 139.788 | 233.713 | 3275.264 | 11,562.142 | 4.694 | 3.391 |
| 17 | A1 | B2 | 76.484 | 148.867 | 2014.481 | 8270.574 | 2.585 | 2.092 |
| 18 | A2 | B1 | 66.105 | 138.919 | 1837.867 | 6682.340 | 2.331 | 1.849 |
| 19 | A1 | B1 | 56.227 | 70.071 | 1479.349 | 2427.651 | 2.296 | 1.234 |
| 20 | A1 | B3 | 184.696 | 257.779 | 4263.475 | 9408.590 | 7.277 | 4.074 |
| 21 | A1 | B3 | 205.119 | 262.471 | 4028.318 | 10,487.270 | 6.175 | 4.066 |
| 22 | A3 | B3 | 218.340 | 260.470 | 4459.897 | 10,219.833 | 8.483 | 4.469 |
| 23 | A2 | B2 | 138.911 | 201.867 | 3177.125 | 9951.217 | 4.199 | 3.103 |
| 24 | A2 | B1 | 68.813 | 122.355 | 1846.695 | 6065.224 | 2.398 | 1.778 |
| 25 | A1 | B3 | 159.919 | 225.284 | 3750.857 | 10,929.334 | 5.941 | 3.667 |
| 26 | A1 | B1 | 55.597 | 70.376 | 1374.053 | 1969.218 | 2.621 | 1.221 |
| 27 | A1 | B1 | 56.397 | 68.021 | 1309.445 | 2121.255 | 2.436 | 1.190 |
| Group | QA9 (μg/cm2) | QA24 (μg/cm2) | ER9 | ER24 |
|---|---|---|---|---|
| Blank | 23.72 ± 5.60 | 98.98 ± 12.82 | 1.06 ± 0.26 | 1.11 ± 0.30 |
| 3% Azone | 70.72 ± 17.22 | 150.4 ± 22.86 | 2.98 ± 1.20 | 1.52 ± 0.27 |
| 5% Azone | 62.97 ± 27.53 | 146.02 ± 34.86 | 2.65 ± 1.16 | 1.48 ± 0.35 |
| DCPEs | 9.82 ± 3.66 | 27.34 ± 5.35 | 0.36 ± 0.21 | 0.23 ± 0.12 |
| Iontophoresis | 42.17 ± 12.64 | 153.89 ± 33.99 | 1.78 ± 0.53 | 1.55 ± 0.34 |
| FCTL | 41.79 ± 17.72 | 156.21 ± 35.56 | 1.76 ± 0.82 | 1.58 ± 0.39 |
| FCTM | 84.88 ± 26.37 | 232.13 ± 35.04 | 3.58 ± 1.11 | 2.35 ± 0.35 |
| FCTH | 199.08 ± 14.48 | 361.54 ± 14.73 | 8.39 ± 0.61 | 3.65 ± 0.15 |
| Parameters | Units | Blank Group | FCTL Group | FCTM Group | 3% Azone Group |
|---|---|---|---|---|---|
| t1/2 | h | 11.54 ± 2.90 | 26.98 ± 12.76 | 12.21 ± 1.43 | 21.24 ± 2.33 |
| Ke | 1/h | 0.06 ± 0.01 | 0.03 ± .0.01 | 0.06 ± 0.01 | 0.03 ± 0.00 |
| V1/F | L/kg | 4.47 ± 357.78 | 7900.62 ± 2567.20 | 0.00 ± 0.00 | 0.01 ± 0.00 |
| AUC(0–t) | μg/mL∙h | 60.66 ± 4.77 | 76.95 ± 3.46 | 202.54 ± 34.44 | 69.72 ± 7.37 |
| AUC(0–∞) | μg/mL∙h | 74.05 ± 8.66 | 140.01 ± 2.48 | 261.05 ± 41.86 | 128.45 ± 40.35 |
| Ka | 1/h | 0.07 ± 0.01 | 0.11 ± 0.04 | 0.07 ± 0.00 | 0.07 ± 0.03 |
| t1/2Ka | h | 9.84 ± 1.94 | 7.38 ± 1.27 | 9.47 ± 0.83 | 14.08 ± 2.35 |
| Tlag | h | 1.58 ± 0.06 | 1.98 ± 0.65 | 1.55 ± 0.37 | 1.03 ± 0.42 |
| Statistical Moment | Units | Blank Group | FCTL Group | FCTM Group | 3% Azone Group |
|---|---|---|---|---|---|
| AUC(0–t) | μg/mL∙h | 60.21 ± 2.56 | 76.86 ± 6.07 | 198.72 ± 72.28 | 69.89 ± 10.13 |
| AUC(0–∞) | μg/mL∙h | 71.42 ± 9.80 | 133.22 ± 61.40 | 266.93 ± 106.45 | 135.23 ± 23.07 |
| AUMC(0–t) | μg/mL∙h2 | 1389.21 ± 135.64 | 1974.49 ± 166.81 | 4615.58 ± 1490.33 | 1812.18 ± 148.18 |
| AUMC(0–∞) | μg/mL∙h2 | 2299.00 ± 434.96 | 9511.82 ± 4546.77 | 11,145.18 ± 3525.72 | 7884.63 ± 6115.12 |
| MRT(0–t) | h | 23.0 ± 2.0 | 25.7 ± 0.6 | 23.5 ± 1.1 | 26.3 ± 2.1 |
| MRT(0–∞) | h | 31.5 ± 3.5 | 59.6 ± 17.1 | 40.7 ± 16.0 | 54.1 ± 16.5 |
| VRT(0–t) | h2 | 125.6 ± 6.5 | 142.9 ± 18.2 | 141.3 ± 5.5 | 143.5 ± 5.5 |
| VRT(0–∞) | h2 | 582.0 ± 619.7 | 3056.1 ± 4545.2 | 1111.2 ± 538.5 | 2671.0 ± 1042.3 |
| t1/2z | h | 13.8 ± 9.8 | 30.3 ± 13.2 | 17.8 ± 7.5 | 30.6 ± 5.9 |
| Tmax | h | 18.0 ± 5.2 | 28.0 ± 2.8 | 19.2 ± 3.4 | 24.7 ± 8.9 |
| Cmax | μg/mL | 2.39 ± 0.38 | 2.61 ± 0.31 | 8.00 ± 2.15 | 2.21 ± 0.55 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 43.85 | 8 | 5.48 | 145.05 | <0.0001 | *** |
| A-A | 0.95 | 2 | 0.48 | 12.62 | 0.0004 | *** |
| B-B | 38.43 | 2 | 19.21 | 508.41 | <0.0001 | *** |
| A × B | 4.47 | 4 | 1.12 | 29.59 | <0.0001 | *** |
| Pure error | 0.68 | 18 | 0.04 | |||
| Cor total | 44.53 | 26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.-J.; Zhang, Y.-P.; Xu, J.; Sun, X.-B.; Yang, F.-F. The Effect and Mechanism of Transdermal Penetration Enhancement of Fu’s Cupping Therapy: New Physical Penetration Technology for Transdermal Administration with Traditional Chinese Medicine (TCM) Characteristics. Molecules 2017, 22, 525. https://doi.org/10.3390/molecules22040525
Xie W-J, Zhang Y-P, Xu J, Sun X-B, Yang F-F. The Effect and Mechanism of Transdermal Penetration Enhancement of Fu’s Cupping Therapy: New Physical Penetration Technology for Transdermal Administration with Traditional Chinese Medicine (TCM) Characteristics. Molecules. 2017; 22(4):525. https://doi.org/10.3390/molecules22040525
Chicago/Turabian StyleXie, Wei-Jie, Yong-Ping Zhang, Jian Xu, Xiao-Bo Sun, and Fang-Fang Yang. 2017. "The Effect and Mechanism of Transdermal Penetration Enhancement of Fu’s Cupping Therapy: New Physical Penetration Technology for Transdermal Administration with Traditional Chinese Medicine (TCM) Characteristics" Molecules 22, no. 4: 525. https://doi.org/10.3390/molecules22040525






