3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways
Abstract
:1. Introduction
2. Results
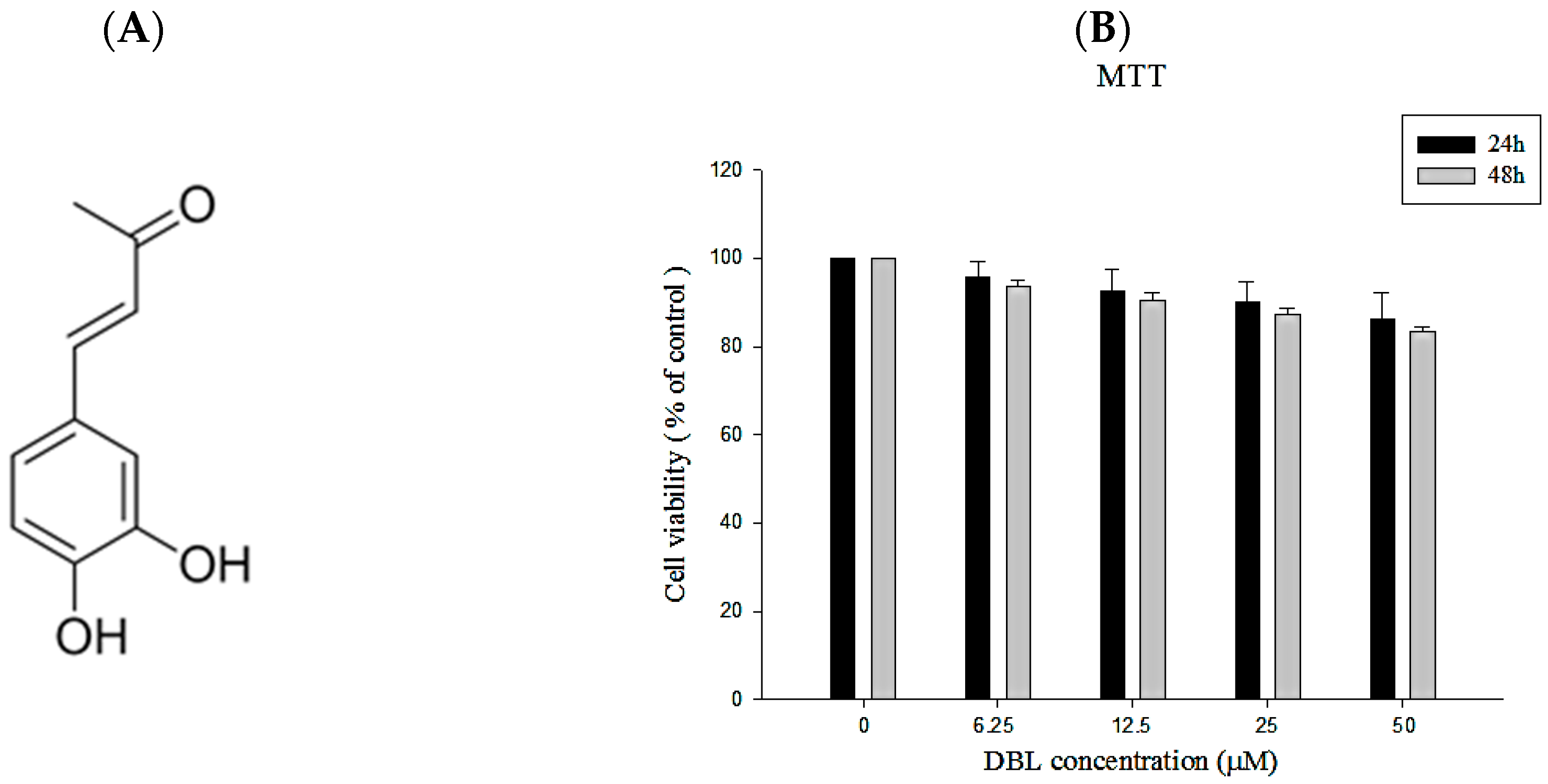
2.1. The Chemical Profile and Cytotoxicity of DBL
2.1.1. Isolation of DBL from Phellinus linteus and Its Structural Characterization
2.1.2. The Cell Viability of DBL in A549 Cells
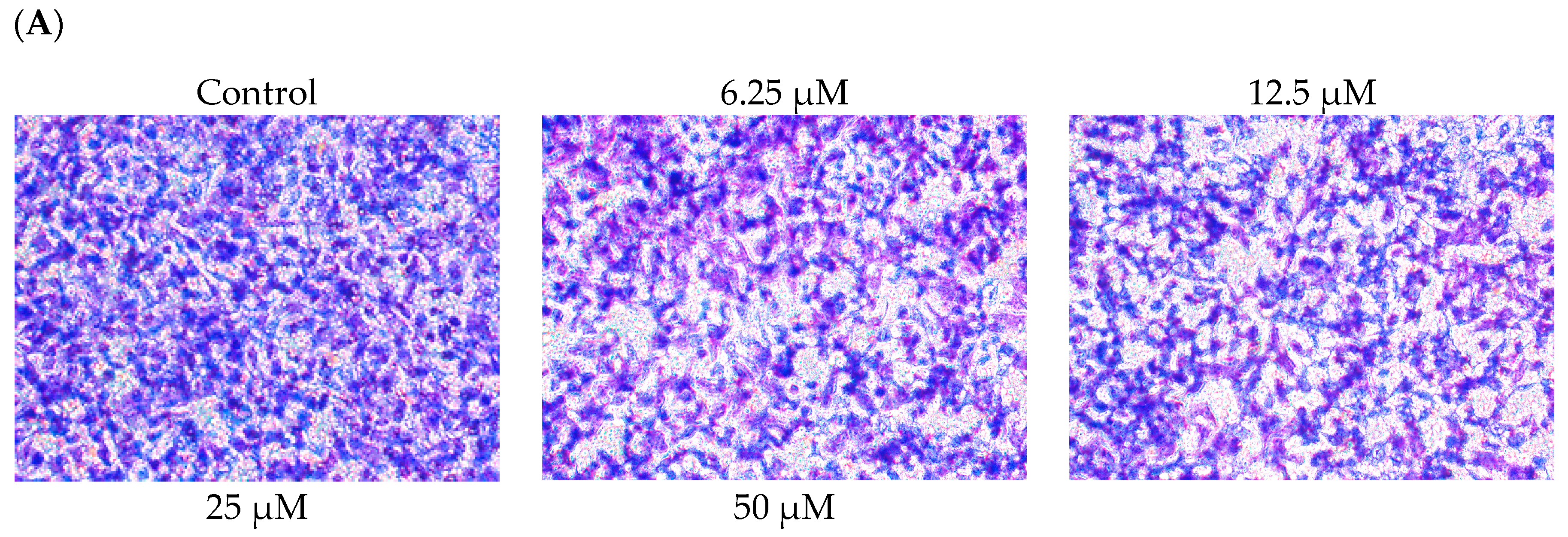
2.2. DBL Inhibits Migration, Invasion, and Adhesion Ability of A549 Cells
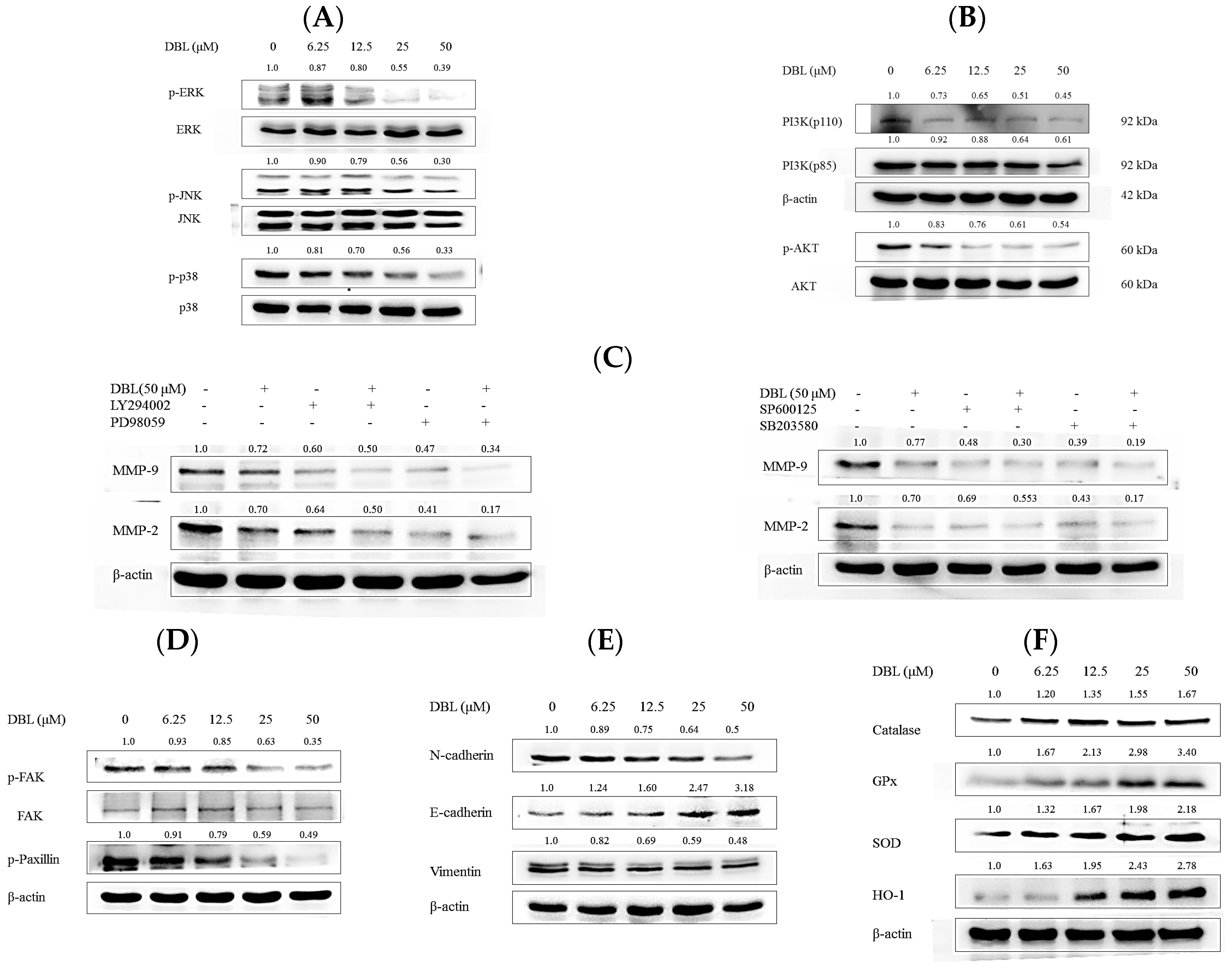
2.3. DBL Exerts Inhibitory Effects on Activity, Protein Expression, and mRNA Levels of MMPs
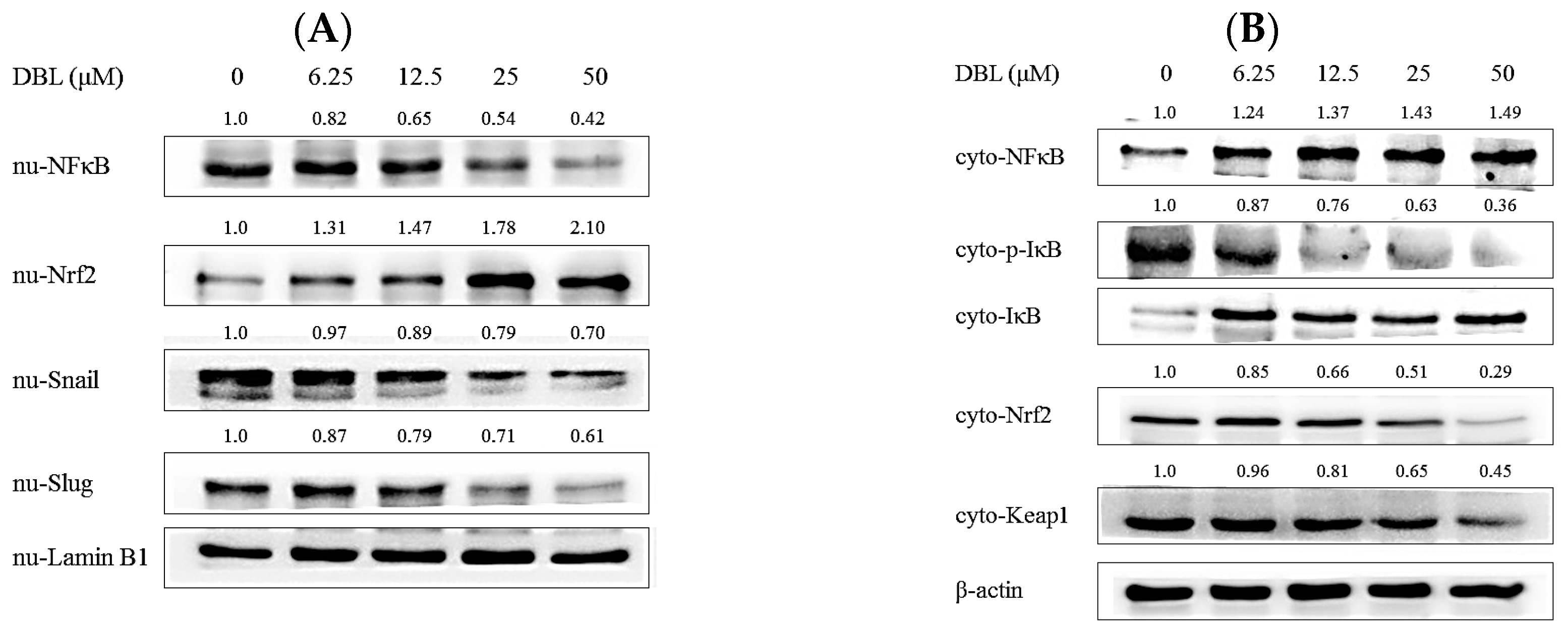
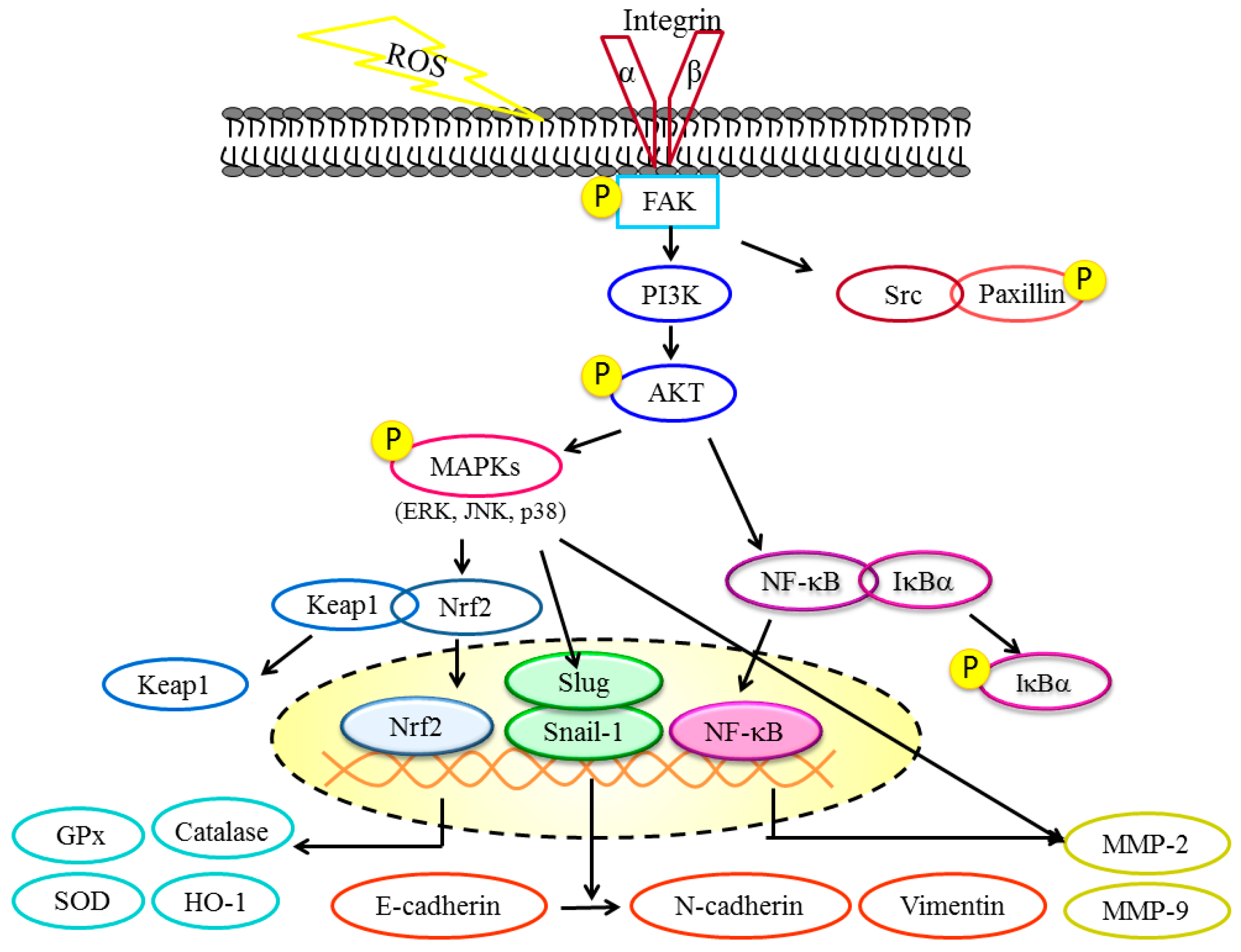
2.4. DBL Takes Part in Cancer Metastasis Suppression in Multiple Pathways, Revealed by Protein Expression
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Antibodies
4.2. Cell Lines and Cell Culture
4.3. Isolation and Determination of DBL
4.4. Cell Viability Assay
4.5. Gelatin Zymography Assay
4.6. Cell Migration and Invasion Assay
4.7. Cell Adhesion Assay
4.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.9. Nuclear and Cytosolic Protein Extraction
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Fedor, D.; Johnson, W.R.; Singhal, S. Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg. Oncol. 2013, 22, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, H.; Li, W.; Guo, Q.; He, S.; Hirasaki, Y.; Hou, W.; Hua, B.; Li, C.; Bao, Y.; et al. Anti-tumor enhancement of Fei-Liu-Ping ointment in combination with celecoxib via cyclooxygenase-2-mediated lung metastatic inflammatory microenvironment in Lewis lung carcinoma xenograft mouse model. J. Transl. Med. 2015, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 1990, 54, 159–211. [Google Scholar] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.R.; Camacho, D.F.; Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. Mechanisms of cancer cell metastasis to the bone: A multistep process. Future Oncol. 2011, 7, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Ray, J.M.; Stetler-Stevenson, W.G. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur. Respir. J. 1994, 7, 2062–2072. [Google Scholar] [PubMed]
- Wu, Z.-Y.; Lien, J.-C.; Huang, Y.-P.; Liao, C.-L.; Lin, J.-J.; Fan, M.-J.; Ko, Y.-C.; Hsiao, Y.-P.; Lu, H.-F.; Chung, J.-G. Casticin Inhibits A375.S2 Human Melanoma Cell Migration/Invasion through Downregulating NF-κB and Matrix Metalloproteinase-2 and -1. Molecules 2016, 21, 384. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J.; Kahari, V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999, 13, 781–792. [Google Scholar] [PubMed]
- Bjorklund, M.; Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta 2005, 1755, 37–69. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Chuang, C.-H.; Lee, W.-J.; Huang, C.-S. Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells. Molecules 2016, 21, 1691. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Chau, C.F.; Hsieh, Y.S.; Yang, S.F.; Yen, G.C. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis 2008, 29, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Nishida, H.; Nakamura, Y.; Konishi, T. Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone isolated from Chaga (Inonotus obliquus (persoon) Pilat). Free Radic. Biol. Med. 2009, 47, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Hu, M.L. Hispolon induces apoptosis and cell cycle arrest of human hepatocellular carcinoma Hep3B cells by modulating ERK phosphorylation. J. Agric. Food Chem. 2011, 59, 7104–7113. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Yun, B.S. Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus. Bioorg. Med. Chem. 2007, 15, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Takada, Y.; Murakami, A.; Aggarwal, B.B. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappa B-regulated gene products. J. Immunol. 2005, 174, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Gunjima, K.; Tomiyama, R.; Takakura, K.; Yamada, T.; Hashida, K.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-dihydroxybenzalacetone protects against Parkinson’s disease-related neurotoxin 6-OHDA through Akt/Nrf2/glutathione pathway. J. Cell. Biochem. 2014, 115, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Tang, Y.A.; Huang, S.M.; Juan, H.F.; Wu, L.W.; Sun, Y.C.; Wang, S.C.; Wu, K.W.; Balraj, G.; Chang, T.T.; et al. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011, 71, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kölbl, A.; Jeschke, U.; Andergassen, U. The Significance of Epithelial-to-Mesenchymal Transition for Circulating Tumor Cells. Int. J. Mol. Sci. 2016, 17, 1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, C.; Yan, S.; Liu, G.; Liu, G.; Chen, W.; Cui, Y.; Liu, Y. NADPH oxidase 1-dependent ROS is crucial for TLR4 signaling to promote tumor metastasis of non-small cell lung cancer. Tumor Biol. 2015, 36, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yao, Y.; Zhao, K.; Huang, Y.; Zhou, Y.; Zhao, L.; Guo, Q.; Lu, N. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells. Mol. Carcinog. 2016, 55, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Yang, C.M.; Su, C.M.; Liao, J.W.; Hu, M.L. Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BL/6 mice. Nutr. Cancer 2011, 63, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Kee, J.-Y.; Kim, D.-S.; Mun, J.-G.; Jeong, M.-Y.; Park, S.-H.; Choi, B.-M.; Park, S.-J.; Kim, H.-J.; Um, J.-Y.; et al. Arctigenin Inhibits Lung Metastasis of Colorectal Cancer by Regulating Cell Viability and Metastatic Phenotypes. Molecules 2016, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Activation mechanisms of matrix metalloproteinases. Biol. Chem. 1997, 378, 151–160. [Google Scholar] [PubMed]
- Olson, M.W.; Gervasi, D.C.; Mobashery, S.; Fridman, R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J. Biol. Chem. 1997, 272, 29975–29983. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Kobayashi, S.; Iwase, H.; Okada, Y. The expression of MMPs and TIMPs in human breast cancer tissues and importance of their balance in cancer invasion and metastasis. Nihon Rinsho 1995, 53, 1805–1810. [Google Scholar] [PubMed]
- Wu, W.S.; Wu, J.R.; Hu, C.T. Signal cross talks for sustained MAPK activation and cell migration: The potential role of reactive oxygen species. Cancer Metastasis Rev. 2008, 27, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.; Song, J.; Ha, S.; Hong, E. Inonotus obliquus-derived polysaccharide inhibits the migration and invasion of human non-small cell lung carcinoma cells via suppression of MMP-2 and MMP-9. Int. J. Oncol. 2014, 45, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, F.C.; Chou, P.Y.; Chien, Y.C.; Chang, W.S.; Huang, G.J.; Wu, C.H.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 378415. [Google Scholar]
- Zhang, F.-Y.; Hu, Y.; Que, Z.-Y.; Wang, P.; Liu, Y.-H.; Wang, Z.-H.; Xue, Y.-X. Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine. Int. J. Mol. Sci. 2015, 16, 23823–23848. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Chu, S.C.; Hsu, L.S.; Chen, K.S.; Lai, M.T.; Yeh, C.H.; Chen, P.N. Rubus idaeus L. reverses epithelial-to-mesenchymal transition and suppresses cell invasion and protease activities by targeting ERK1/2 and FAK pathways in human lung cancer cells. Food Chem. Toxicol. 2013, 62, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Sun, K.H.; Chen, S.Y.; Wang, H.H.; Lee, M.Y.; Tsou, Y.C.; Jwo, S.C.; Sun, G.H.; Tang, S.J. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine 2012, 59, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial—Mesenchymal and mesenchymal—Epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Uchikado, Y.; Okumura, H.; Ishigami, S.; Setoyama, T.; Matsumoto, M.; Owaki, T.; Kita, Y.; Natsugoe, S. Increased Slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 2011, 14, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Rahman, N.; Jeon, M. Cytoprotective Effect of Makgeolli Lees on Paraquat Induced Oxidative Stress in A549 Cells via Activation of Nrf2 and Antioxidant Genes. J. Microbiol. Biotechnol. 2016, 26, 277–286. [Google Scholar]
- Kim, H.; Lee, S.W.; Baek, K.M.; Park, J.S.; Min, J.H. Continuous hypoxia attenuates paraquat-induced cytotoxicity in the human A549 lung carcinoma cell line. Exp. Mol. Med. 2011, 43, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.-H.; Zhang, B.; Fan, Y.; Lin, N.-M. Keap1-Nrf2 pathway: A promising target towards lung cancer prevention and therapeutics. Chron. Dis. Transl. Med. 2015, 1, 175–186. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Repesh, L.A. A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis 1989, 9, 192–208. [Google Scholar] [PubMed]
Sample Availability: Not Available. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, W.; Deng, J.-S.; Li, P.-Y.; Liang, Y.-C.; Huang, G.-J. 3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways. Molecules 2017, 22, 537. https://doi.org/10.3390/molecules22040537
Chao W, Deng J-S, Li P-Y, Liang Y-C, Huang G-J. 3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways. Molecules. 2017; 22(4):537. https://doi.org/10.3390/molecules22040537
Chicago/Turabian StyleChao, Wei, Jeng-Shyan Deng, Pei-Ying Li, Yu-Chia Liang, and Guan-Jhong Huang. 2017. "3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways" Molecules 22, no. 4: 537. https://doi.org/10.3390/molecules22040537




