Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to α-Farnesene Biosynthesis
Abstract
:1. Introduction
2. Results
2.1. Flower Developmental Stages and Major Volatile Compound Emissions
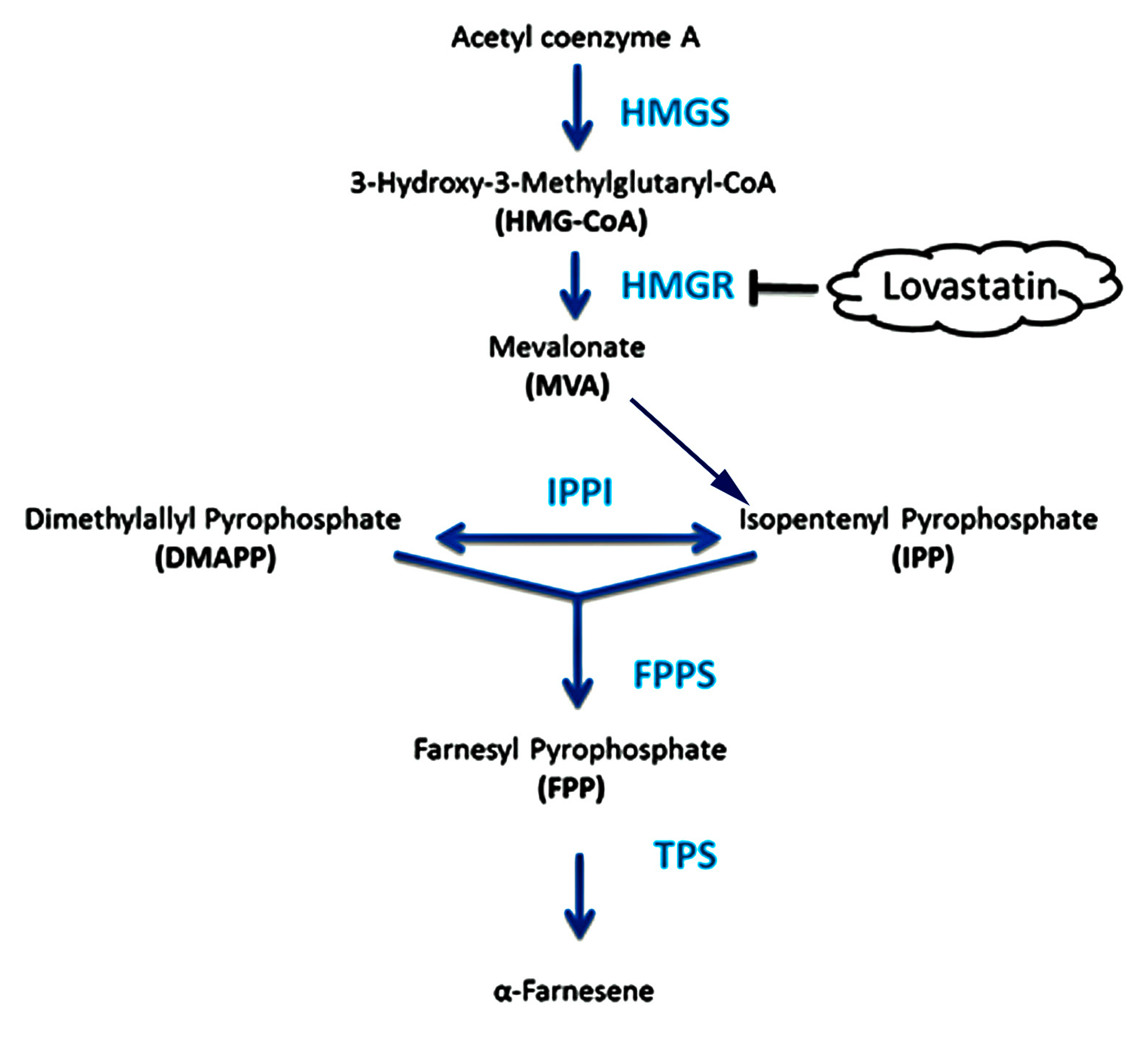
2.2. Analysis of Key Genes Related to α-Farnesene Biosynthesis
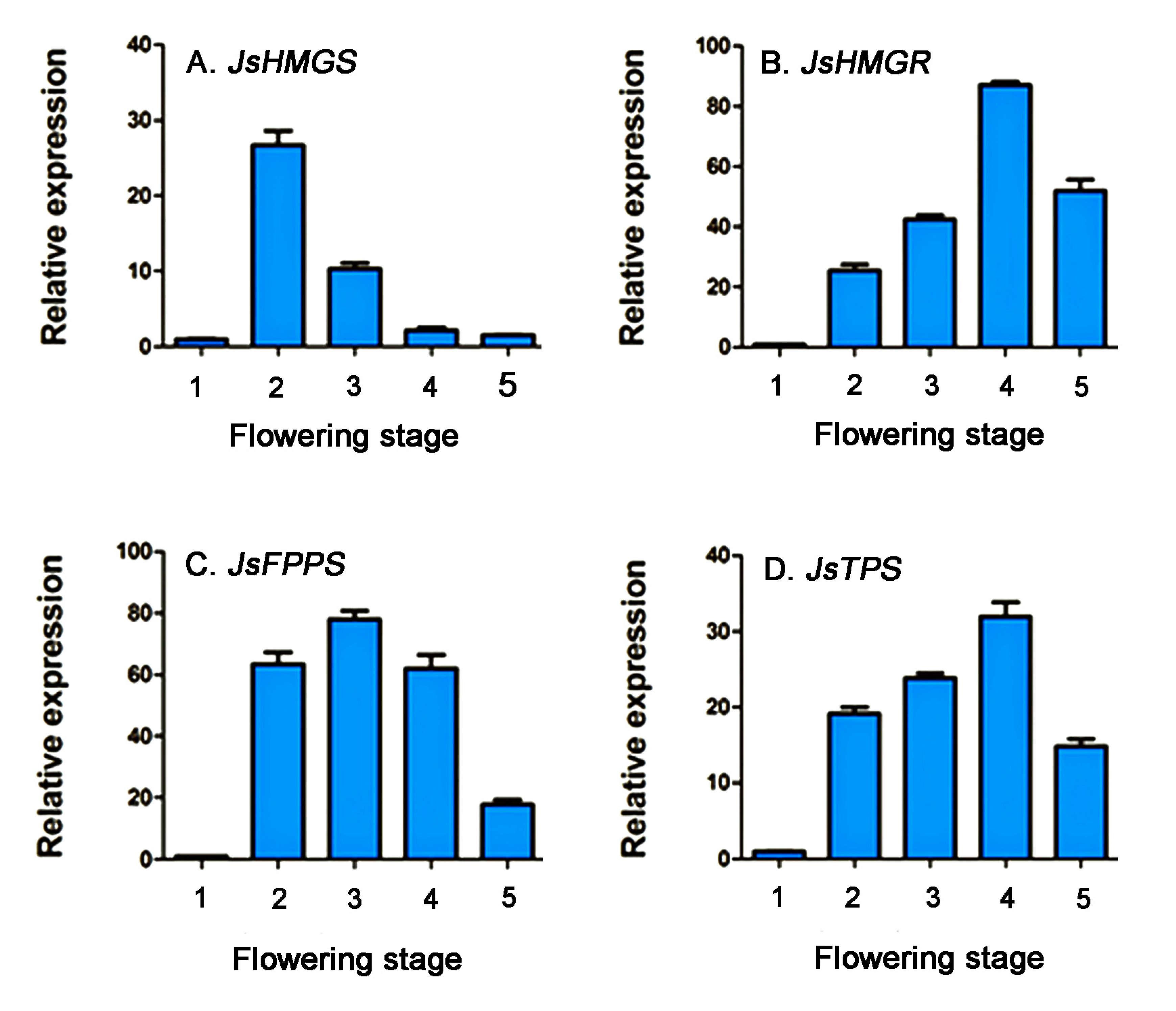
2.3. Relative Expression of Genes Related to α-Farnesene Biosynthesis
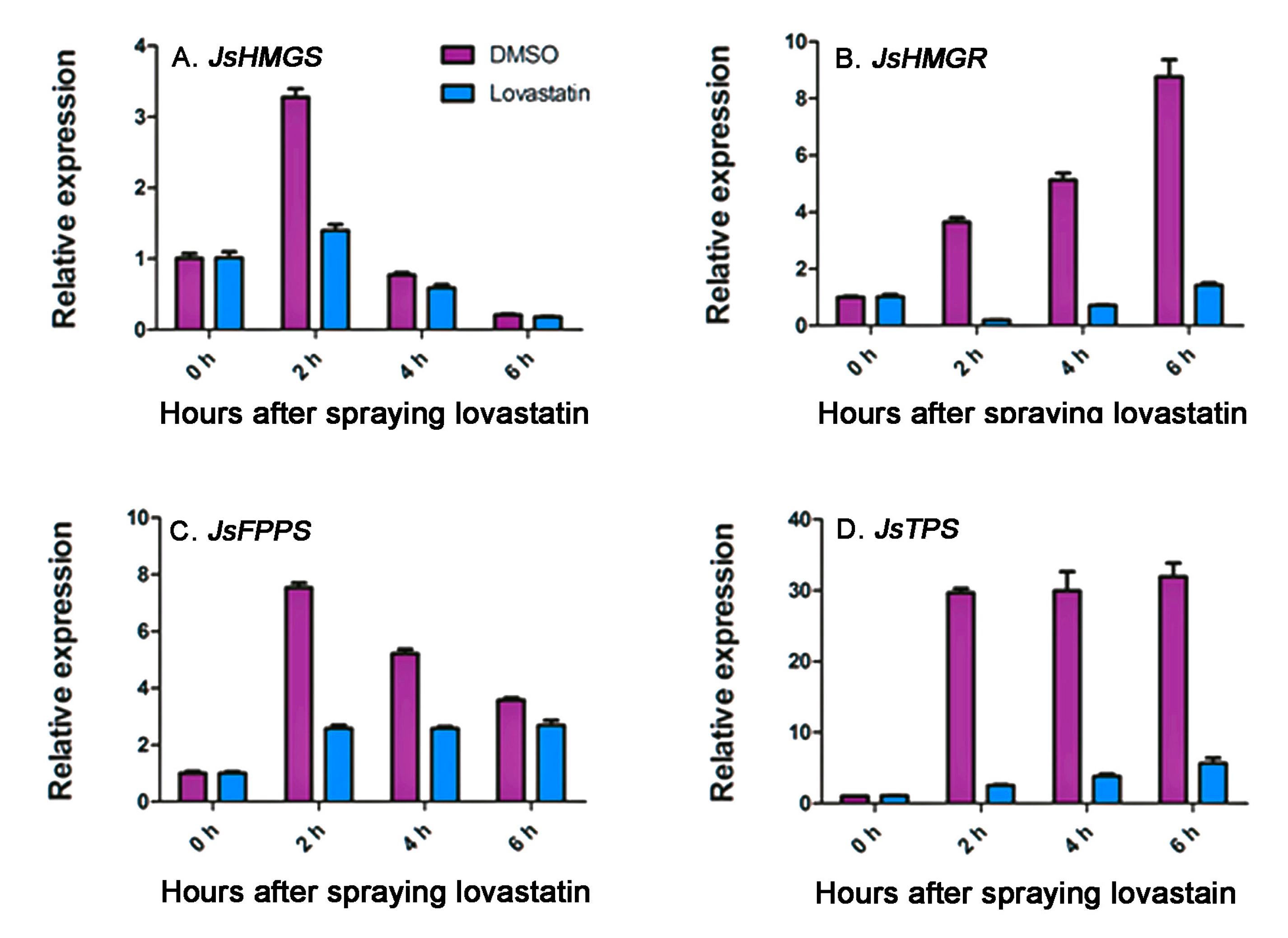
2.4. Effects of Lovastatin on Gene Expression and Volatile Compound Emission
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Aroma Analysis
4.3. Cloning Full-Length cDNA of JsHMGS, JsHMGR, JsFPPS, and JsTPS
4.4. Quantitative Real-Time PCR Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HMGS | 3-Hydroxy-3-methylglutaryl coenzyme A synthase |
| HMGR | 3-Hydroxy-3-methylglutaryl coenzyme A reductase |
| FPPS | Farnesyl pyrophosphate synthase |
| TPS | Terpene synthase |
References
- Huxley, A.; Griffiths, M. The New Royal Horticultural Society, Dictionary of Gardening; The Macmillan Press and The Stockton Press: London, UK, 1999. [Google Scholar]
- Lawless, J. The Illustrated Encyclopedia of Essential Oils: The Complete Guide to the Use of Oils in Aromatherapy and Herbalism; Element Books Limited: London, UK, 1995. [Google Scholar]
- Yuniarto, A.; Kurnia, I.; Ramadhan, M. Anti-obesity effect of ethanolic extract of jasmine flowers (Jasminum sambac Ait) in high-fat diet-induced mice: Potent inhibitor of pancreatic lipase enzyme. Int. J. Adv. Pharm. Biol. Chem. 2015, 4, 18–22. [Google Scholar]
- Ye, N.; Yang, G.; Zheng, N.; Yang, J.; Wang, Z.; Liang, X. Effects of wet-scenting process and RJF on the aroma constituent of jasmine scented tea. J. Tea Sci. 2005, 26, 65–71. [Google Scholar]
- Lim, T.K. Jasminum sambac. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; Volume 8, pp. 529–540. [Google Scholar]
- Fuzhou City Government. Jasmine and Tea Culture System of Fuzhou City. Globally Important Agricultural Heritage Systems (GIASH) Initiative 2014. FAO. Available online: http://www.fao.org/3/a-bp789e.pdf (accessed on 20 February 2014).
- Pichersky, E.; Dudareva, N. Scent engineering: Toward the goal of controlling how flowers smell. Trends. Biotechnol. 2007, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Tamboong, B.; Rideout, J. Secondary metabolites from Jasminum sambac and cananga odorata. ACGC Chem. Commun. 2003, 16, 40–47. [Google Scholar]
- Pragadheesh, V.S.; Yadav, A.; Chanotiya, C.S.; Rout, P.K.; Uniyal, G.C. Monitoring the emission of volatile organic compounds from flowers of Jasminum sambac using solid-phase micro-extraction fibers and gas chromatography with mass spectrometry detection. Nat. Prod. Commun. 2011, 6, 1333–1338. [Google Scholar] [PubMed]
- Bera, P.; Mukherjee, C.; Mitra, A. Enzymatic production and emission of floral scent volatiles in Jasminum sambac. Plant Sci. 2017, 256, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar] [PubMed]
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.X.; Xu, H.R.; Wang, X.C. A novel quality evaluation index and strategies to identify scenting quality of jasmine tea based on headspace volatiles analysis. Food Sci. Biotechnol. 2013, 22, 331–340. [Google Scholar] [CrossRef]
- Ma, L.M.; Dobhal, S.; Timmons, C. Dried teas and herbs. In The Microbiological Safety of Low Water Activity Foods and Spices, Food Microbiology and Food Safety; Gurtler, J.B., Doyle, M.P., Kornacki, J.L., Eds.; Springer: New York, NY, USA, 2014; pp. 329–344. [Google Scholar]
- Rupasinghe, H.P.V.; Paliyath, G.; Murr, D.P. Biosynthesis of α-farnesene and its relation to superficial scald development in delicious’ apples. J. Am. Soc. Hortic. Sci. 1998, 123, 882–886. [Google Scholar]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of c- and o-glycosyl flavone in blood orange (Citrus sinensis (L.) osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Curry, E.A. Lovastatin inhibits α-farnesene synthesis without affecting ethylene production during fruit ripening in golden supreme’ apples. J. Am. Soc. Hortic. Sci. 2000, 125, 105–110. [Google Scholar]
- Caelles, C.; Ferrer, A.; Balcells, L.; Hegardt, F.; Boronat, A. Isolation and structural characterization of a cdna encoding arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme a reductase. Plant Mol. Biol. 1989, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Abdin, M.Z. Over-expression of hmg-coa reductase and amorpha-4,11-diene synthase genes in Artemisia annua L. and its influence on artemisinin content. Plant Cell Rep. 2011, 30, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Ayora-Talavera, T.; Chappell, J.; Lozoya-Gloria, E.; Loyola-Vargas, V. Overexpression in Catharanthus roseus hairy roots of a truncated hamster 3-hydroxy-3-methylglutaryl-coa reductase gene. Appl. Biochem. Biotechnol. 2002, 97, 135–145. [Google Scholar] [CrossRef]
- Manzano, D.; Fernandez-Busquets, X.; Schaller, H.; Gonzalez, V.C.; Boronat, A.; Arro, M.; Ferrer, A. The metabolic imbalance underlying lesion formation in Arabidopsis thaliana overexpressing farnesyl diphosphate synthase (isoform 1S) leads to oxidative stress and is triggered by the developmental decline of endogenous HMGR activity. Planta 2004, 219, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Korth, K.; Stermer, B.; Bhattacharyya, M.; Dixon, R. HMG-CoA reductase gene families that differentially accumulate transcripts in potato tubers are developmentally expressed in floral tissues. Plant Mol. Biol. 1997, 33, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bertomeu, J.; Sales, E.; Ros, R.; Arrillaga, I.; Segura, J. Up-regulation of an n-terminal truncated 3-hydroxy-3-methylglutaryl coa reductase enhances production of essential oils and sterols in transgenic lavandula latifolia. Plant Biotechnol. J. 2007, 5, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cui, G.; Zhou, S.-F.; Zhang, X.; Huang, L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme a reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J. Plant Physiol. 2011, 168, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.X.; Scott, A.I.; Bennett, G.N. Overexpression, purification, and characterization of the thermostable mevalonate kinase from Methanococcus jannaschii. Protein Expr. Purif. 1999, 17, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Schauvinhold, I.; Larson, M.; Xu, R.; Charbonneau, A.L.; Schmidt, A.; Wilkerson, C.; Last, R.L.; Pichersky, E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 10865–10870. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Kotamreddy, J.N.; Samanta, T.; Maiti, S.; Mitra, A. Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat. Prod. Res. 2015, 29, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Lovastatin and beyond: The history of the hmg-coa reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent advances in volatiles of teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time pcr data by comparative ct method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Chemical Compound | Retention Index | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 |
|---|---|---|---|---|---|---|
| (mg/kg FW) | ||||||
| Methyl acetate | 986 | 0.20 ± 0.01 z | 1.80 ± 0.30 | 3.43 ± 1.33 | 2.40 ± 0.14 | 2.63 ± 0.76 |
| (Z)-3-Hexenyl acetate | 1008 | 0.22 ± 0.05 | 1.88 ± 0.43 | 0.79 ± 0.08 | 0.34 ± 0.12 | 0.39 ± 0.13 |
| α-Ocimene | 1016 | 0.02 ± 0.00 | 0.48 ± 0.15 | 0.86 ± 0.21 | 0.38 ± 0.02 | 0.21 ± 0.02 |
| Methyl benzoate | 1095 | 0.01 ± 0.00 | 0.36 ± 0.15 | 1.56 ± 0.78 | 1.97 ± 0.10 | 1.09 ± 0.51 |
| Linalool | 1107 | 3.89 ± 0.82 | 41.86 ± 4.99 | 22.93 ± 5.06 | 21.53 ± 1.05 | 8.38 ± 0.40 |
| Benzyl acetate | 1167 | 0.03 ± 0.00 | 4.66 ± 1.93 | 14.30 ± 2.83 | 10.56 ± 3.15 | 6.01 ± 0.12 |
| Methyl salicylate | 1193 | 0.03 ± 0.00 | 0.42 ± 0.12 | 1.58 ± 1.10 | 1.64 ± 0.18 | 0.54 ± 0.15 |
| Elemene | 1336 | 0.00 ± 0.00 | 0.61 ± 0.07 | 1.00 ± 0.19 | 0.90 ± 0.10 | 0.36 ± 0.05 |
| Ethyl decanoate y | 1395 | 0.99 ±0.01 | 1.00 ±0.00 | 1.00 ±0.01 | 1.00 ±0.00 | 0.99 ±0.00 |
| Caryophyllene | 1420 | 0.02 ± 0.00 | 0.74 ± 0.13 | 0.64 ± 0.13 | 0.53 ± 0.13 | 0.22 ± 0.05 |
| Humulene | 1469 | 0.01 ± 0.00 | 0.41 ± 0.09 | 0.43 ± 0.05 | 0.33 ± 0.13 | 0.18 ± 0.05 |
| (E,E)-α-Farnesene | 1494 | 0.61 ± 0.02 | 12.08 ± 1.66 | 53.86 ± 3.49 | 39.03 ± 1.89 | 20.71 ± 4.20 |
| Muurolene | 1502 | 0.08 ± 0.00 | 1.10 ± 0.18 | 1.57 ± 0.25 | 0.93 ± 0.22 | 1.23 ± 0.20 |
| (Z)-3-Hexenyl benzoate | 1585 | 0.08 ± 0.05 | 0.45 ± 0.06 | 2.07 ± 0.91 | 1.90 ± 0.80 | 0.98 ± 0.13 |
| Chemical Compound | Retention Index | 0 h | 2 h | 4 h | 8 h | 16 h |
|---|---|---|---|---|---|---|
| (mg/kg FW) | ||||||
| Methyl acetate | 986 | 0.30 ± 0.07 z | 0.86 ± 0.09 | 1.02 ± 0.06 | 1.73 ± 0.05 | 1.06 ± 0.31 |
| (Z)-3-Hexenyl acetate | 1008 | 0.07 ± 0.01 | 0.97 ± 0.10 | 1.88 ± 0.10 | 0.40 ± 0.04 | 0.60 ± 0.12 |
| α-Ocimene | 1016 | 0.00 ± 0.00 | 0.15 ± 0.02 | 0.10 ± 0.01 | 1.13 ± 0.02 | 1.04 ± 0.17 |
| Methyl benzoate | 1095 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.22 ± 0.01 | 2.25 ± 0.06 | 0.66 ± 0.15 |
| Linalool | 1107 | 0.68 ± 0.05 | 15.76 ± 1.66 | 19.52 ± 2.69 | 22.03 ± 1.98 | 19.31 ± 4.34 |
| Benzyl acetate | 1167 | 0.05 ± 0.01 z | 0.09 ± 0.05 | 3.69 ± 0.23 | 16.68 ± 2.40 | 12.66 ± 1.76 |
| Methyl salicylate | 1193 | 0.01 ± 0.00 | 0.11 ± 0.02 | 0.41 ± 0.10 | 0.77 ± 0.19 | 0.57 ± 0.13 |
| Elemene | 1336 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.13 ± 0.05 | 0.25 ± 0.03 | 1.15 ± 0.21 |
| Ethyl decanoate y | 1395 | 1.00 ±0.01 | 1.00 ±0.00 | 1.00 ±0.01 | 1.01 ±0.00 | 0.99 ±0.00 |
| Caryophyllene | 1420 | 0.00 ± 0.00 | 0.05 ± 0.00 | 0.19 ± 0.06 | 0.27 ± 0.03 | 0.23 ± 0.04 |
| Humulene | 1469 | 0.00 ± 0.00 | 0.04 ± 0.00 | 0.16 ± 0.02 | 0.28 ± 0.02 | 0.26 ± 0.02 |
| (E,E)-α-Farnesene | 1494 | 0.66 ± 0.24 | 1.45 ± 0.37 | 12.20 ± 3.02 | 30.66 ± 4.42 | 30.71 ± 0.55 |
| Muurolene | 1502 | 0.26 ± 0.01 | 0.23 ± 0.03 | 0.38 ± 0.11 | 0.47 ± 0.04 | 1.22 ± 0.12 |
| (Z)-3-Hexenyl benzoate | 1585 | 0.18 ± 0.01 | 0.63 ± 0.13 | 0.83 ± 0.06 | 1.82 ± 0.44 | 2.78 ± 0.34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to α-Farnesene Biosynthesis. Molecules 2017, 22, 546. https://doi.org/10.3390/molecules22040546
Yu Y, Lyu S, Chen D, Lin Y, Chen J, Chen G, Ye N. Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to α-Farnesene Biosynthesis. Molecules. 2017; 22(4):546. https://doi.org/10.3390/molecules22040546
Chicago/Turabian StyleYu, Ying, Shiheng Lyu, Dan Chen, Yi Lin, Jianjun Chen, Guixin Chen, and Naixing Ye. 2017. "Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to α-Farnesene Biosynthesis" Molecules 22, no. 4: 546. https://doi.org/10.3390/molecules22040546







