Novel Palladium(II) Complexes that Influence Prominin-1/CD133 Expression and Stem Cell Factor Release in Tumor Cells
Abstract
:1. Introduction
2. Results and Discussion
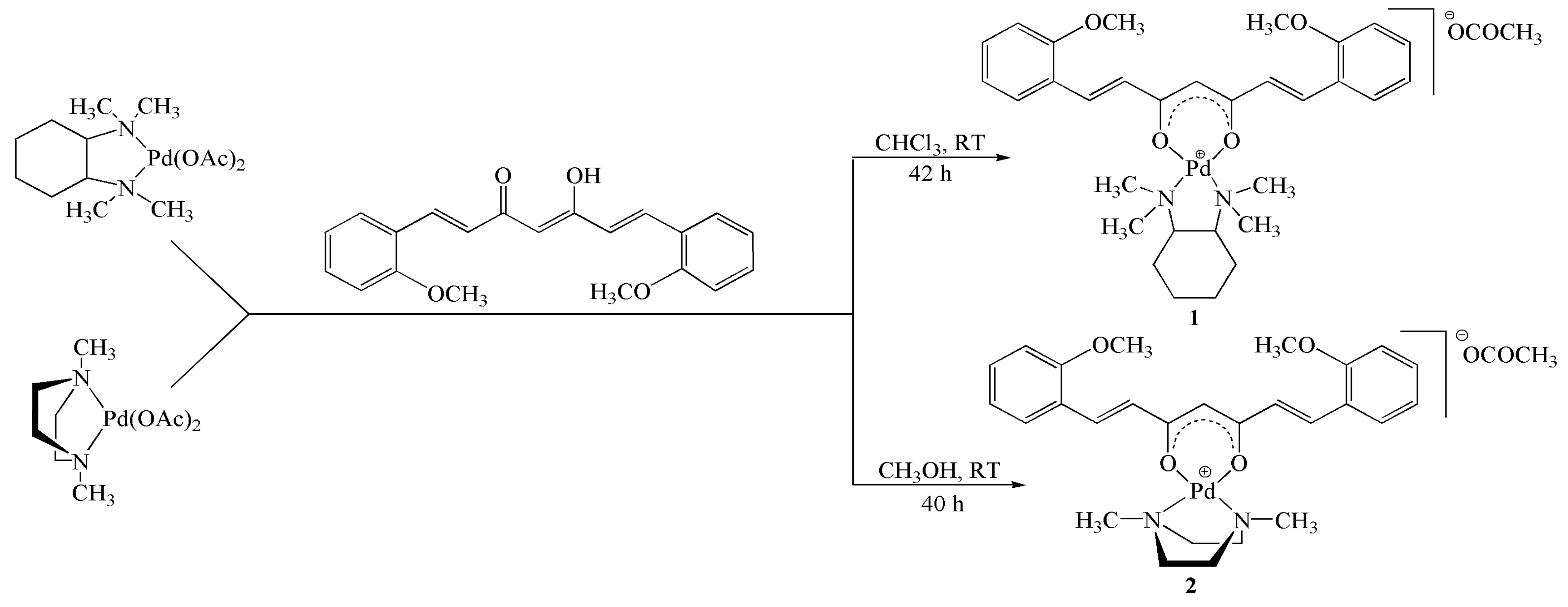
2.1. Synthesis and Characterization
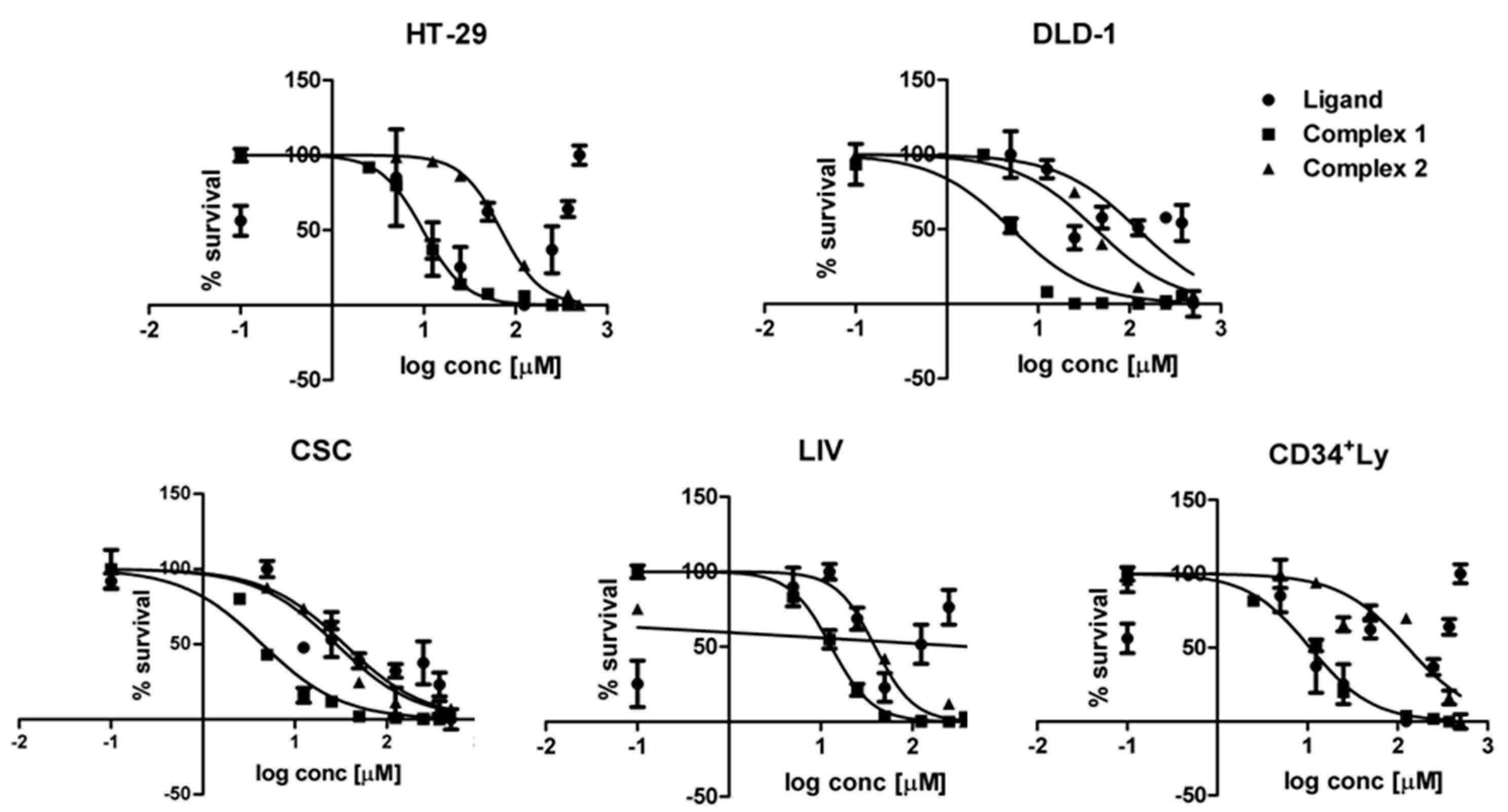
2.2. Cytotoxicity Assessments
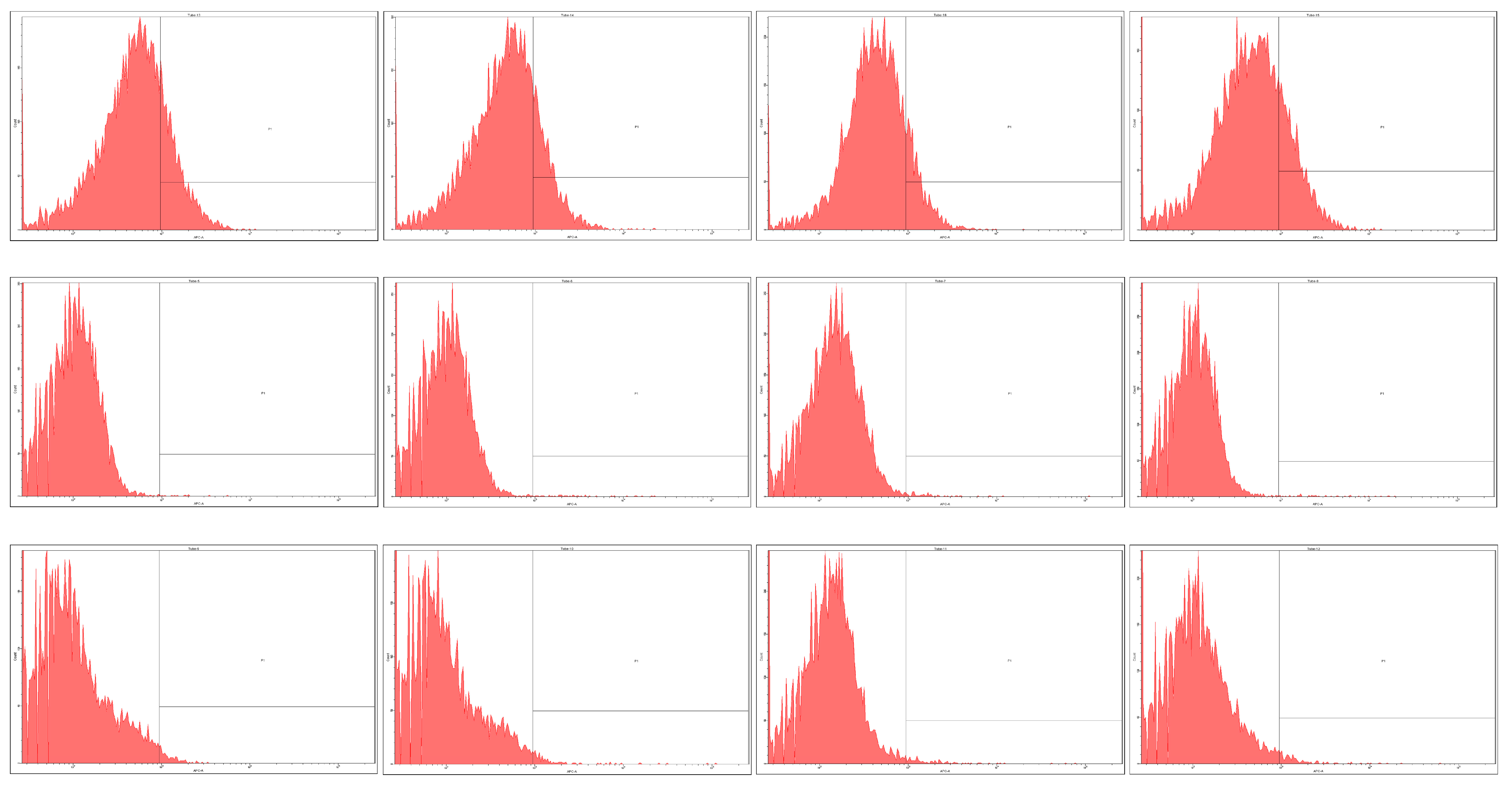
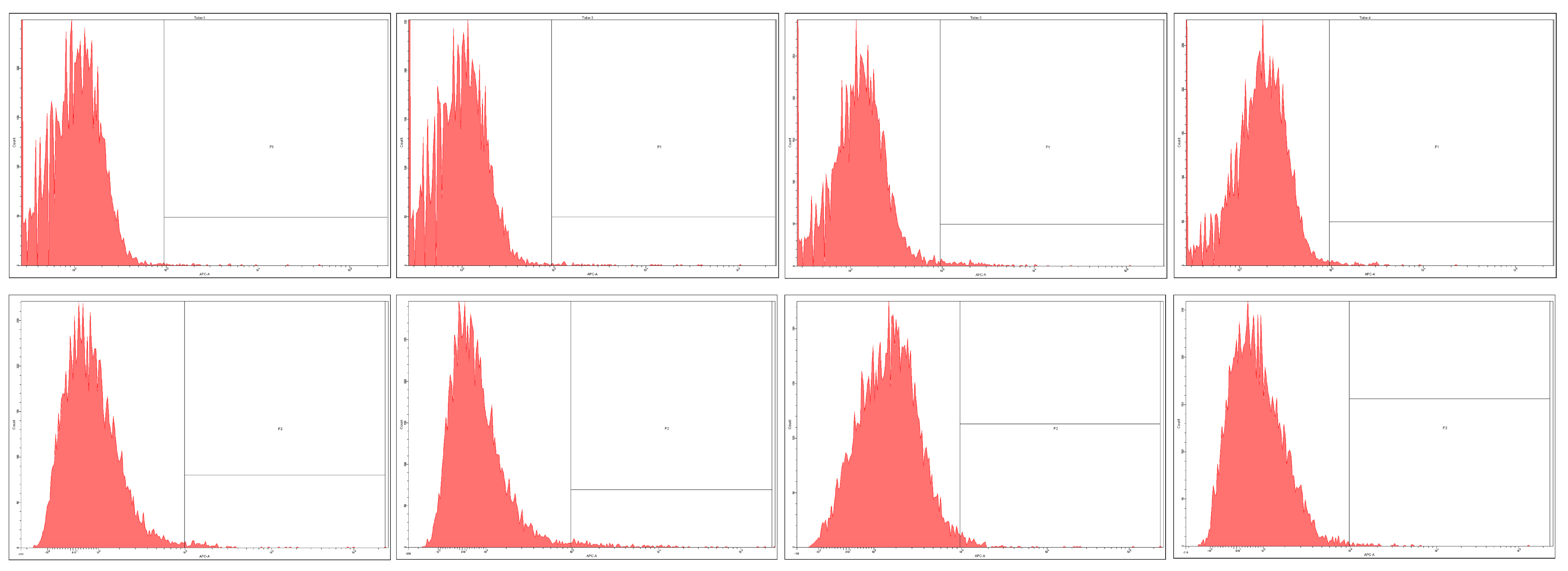
2.3. Prominin-1 (CD133) Expression
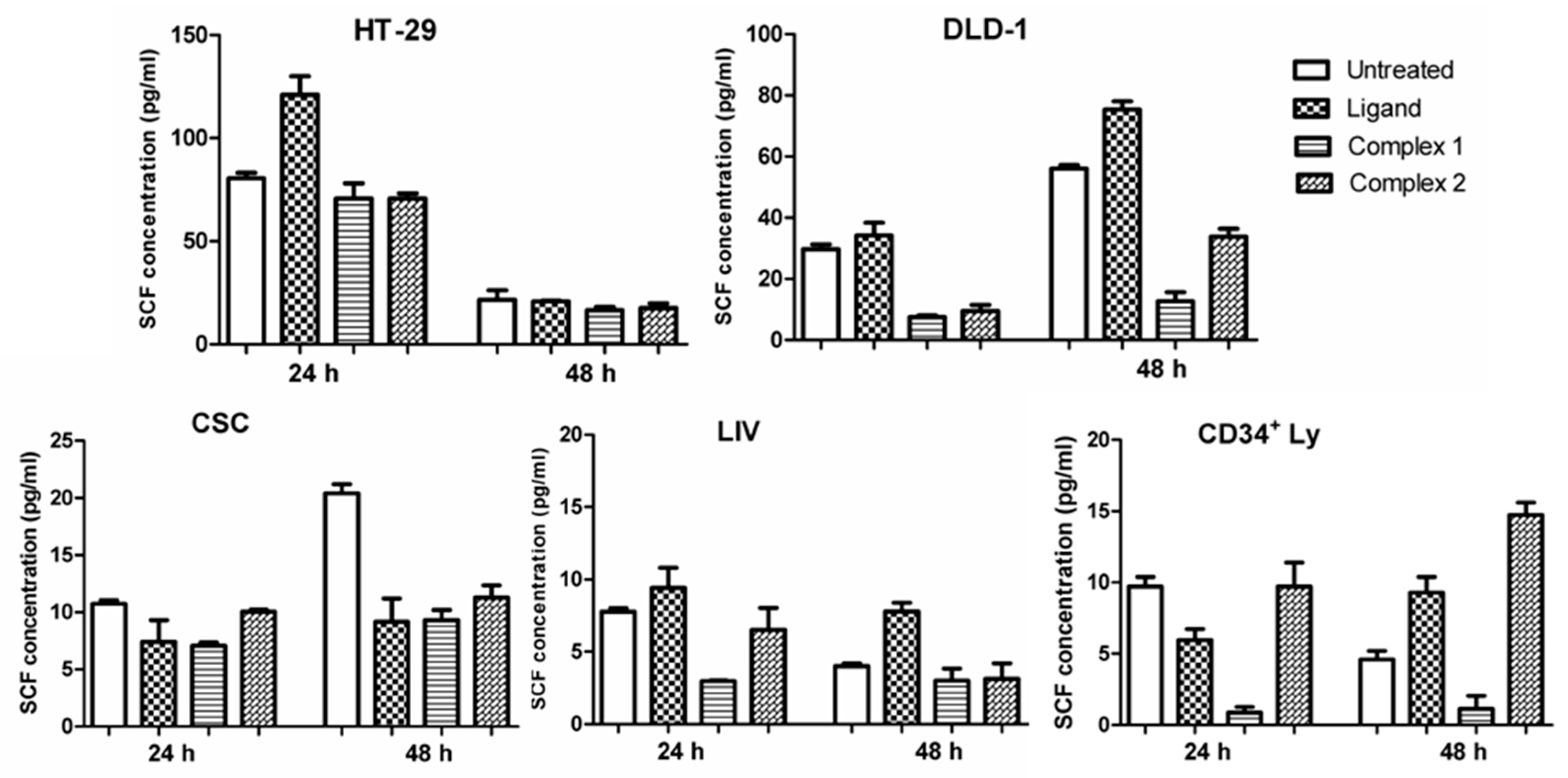
2.4. Stem Cell Factor
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of Complex 1
3.2.2. Synthesis of Complex 2
3.3. Cell Cultivation and Cytotoxicity
3.4. Quantitative Determination of Human Stem Cell Factor/c-Kit Ligand with ELISA
3.5. Immunofluorescent Staining and Flow-Cytometry Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures Atlanta: American Cancer Society. 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf (accessed on 29 January 2016).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Fabian, A.; Barok, M.; Vereb, G.; Szollosi, J. Die hard: Are cancer stem cells the Bruce Willises of tumor biology? Cytom. A 2009, 75, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Iwatsuki, M.; Ieta, K.; Ohta, D.; Haraguchi, N.; Mimori, K.; Mori, M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008, 99, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Sahlberg, S.H.; Spiegelberg, D.; Glimelius, B.; Stenerloew, B.; Nestor, M. Evaluation of Cancer Stem Cell Markers CD133, CD44, CD24: Association with AKT Isoforms and Radiation Resistance in Colon Cancer Cells. PLoS ONE 2014, 9, e94621. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Deutsch, E.; Soria, J.C. Tumour stem cell-targeted treatment: Elimination or differentiation. Ann. Oncol. 2006, 17, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Boyanapalli, S.S.; Tony Kong, A.N. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.S.; Ayob, A.Z.; Myint, H.H.; Thiagarajah, S.; Amini, F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: Insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P.N. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Abcouwer, S.F.; Deck, L.M.; Vander Jagt, D.L. Anti-oxidant activities of curcumin and related enones. Bioorg. Med. Chem. 2005, 13, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Roybal, C.N.; Bobrovnikova-Marjon, E.V.; Abcouwer, S.F.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. Synthesis of novel curcumin analogues and their evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Bioorg. Med. Chem. 2006, 14, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Van Baar, B.L.M.; Rozendal, J.; van der Goot, H. Electron ionization mass spectrometry of curcumin analogues: An olefin metathesis reaction in the fragmentation of radical cations. J. Mass Spectrom. 1998, 33, 319–327. [Google Scholar] [CrossRef]
- Akram Khan, M.; El-Khatib, R.; Rainsford, K.D.; Whitehouse, M.W. Synthesis and anti-inflammatory properties of some aromatic and heterocyclic aromatic curcuminoids. Bioorg. Chem. 2012, 40, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, Y.; Yan, L.-N.; Sun, X.; Gong, T.; Zhang, Z.-R. Synthesis and preliminary evaluation of curcumin analogues as cytotoxic agents. Bioorg. Med. Chem. Lett. 2011, 21, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Batie, S.; Lee, J.H.; Jama, R.A.; Browder, D.O.; Montano, L.A.; Huynh, C.C.; Marcus, L.M.; Tsosie, D.G.; Mohammed, Z.; Trang, V.; et al. Synthesis and biological evaluation of halogenated curcumin analogs as potential nuclear receptor selective agonists. Bioorg. Med. Chem. 2013, 21, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhanb, A.; Edelmann, F.T. Metal complexes of curcumin-synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, B.H.; Cainap, C.; Van Cutsem, E.; Gorbunova, V.; Karapetis, C.S.; Berlin, J.; Goldberg, R.M.; Qin, Q.; Qian, J.; Ricker, J.L.; et al. Randomized phase II open-label study of mFOLFOX6 in combination with linifanib or bevacizumab for metastatic colorectal cancer. Clin. Colorectal Cancer 2014, 13, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B.; Raffa, D.; Raimondi, M.V.; Cusimano, M.G.; Plescia, F.; Cascioferro, S.; Cancemi, G.; Lauricella, M.; Carlisi, D.; Daidone, G. Synthesis and antiproliferative activity of new derivatives containing the polycyclic system 5,7:7,13-dimethanopyrazolo[3,4-b]pyrazolo[3′,4′:2,3]azepino[4,5-f]azocine. Eur. J. Med. Chem. 2014, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Bosque, R.; Crespo, M.; García, G.; Granell, J.; López, C.; Lovelle, M.V.; Qadir, R.; González, A.; Jayaraman, A.; et al. Cyclopalladated primary amines: A preliminary study of antiproliferative activity through apoptosis induction. Eur. J. Med. Chem. 2014, 84, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Kaya, Y.; Samli, H.; Harrison, W.T.; Buyukgungor, O. New Palladium(II) and platinum(II) 5,5-diethylbarbiturate complexes with 2-phenylpyridine, 2,2′-bipyridine and 2,2′-dipyridylamine: Synthesis, structures, DNA binding, molecular docking, cellular uptake, antioxidant activity and cytotoxicity. Dalton Trans. 2015, 44, 6880–6895. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, T.A.; Rashan, L.J. Cis- and trans-platinum and palladium complexes: A comparative study review as antitumour agents. Boll. Chem. Farm. 2001, 140, 205–210. [Google Scholar]
- Kapdi, A.R.; Fairlamb, I.J. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef] [PubMed]
- Pucci, D.; Bloise, R.; Bellusci, A.; Bernardini, S.; Ghedini, M.; Pirillo, S.; Valentini, A.; Crispini, A. Curcumin and cyclopalladated complexes: A recipe for bifunctional biomaterials. J. Inorg. Biochem. 2007, 101, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Conforti, F.; Crispini, A.; De Martino, A.; Condello, R.; Stellitano, C.; Rotilio, G.; Ghedini, M.; Federici, G.; Bernardini, S.; et al. Synthesis, oxidant properties, and antitumoral effects of a heteroleptic Palladium(II) complex of curcumin on human prostate cancer cells. J. Med. Chem. 2009, 52, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Nunes Fernandes, J.; Ruggiero, R.; Guerra, W. Palladium complex containing curcumin as ligand: Thermal and spectral characterization. Am. J. Chem. 2012, 2, 157–159. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, X.; Jiang, B.; Lu, C.; Tian, Y.; Jiang, M. Synthesis and Antitumor Activities of Platinum(II) Complexes of Curcumin Analog. Acta Chim. Sin. 2011, 69, 2335–2340. [Google Scholar]
- Miklášová, N.; Mikláš, R.; Devínsky, F. Palladium Complexes of Curcumin and Its Analogues and Methods of Preparation of the Same. WO 2014175841 A1, 30 October 2014. [Google Scholar]
- Miklášová, N.; Fischer-Fodor, E.; Mikláš, R.; Kucková, L.; Kožíšek, J.; Liptaj, T.; Soritau, O.; Valentová, J.; Devínsky, F. Synthesis and characterization of new biologically active Palladium(II) complexes with (1E,6E)-1,7-bis(3,4-diethoxyphenyl)1,6-heptadiene-3,5-dione. Inorg. Chem. Commun. 2014, 46, 229–233. [Google Scholar] [CrossRef]
- Fischer-Fodor, E.; Mikláš, R.; Krausz, L.T.; Virag, P.; Moldovan, D.C.; Perde Schrepler, M.; Berindan-Neagoe, I.; Devínsky, F.; Miklášová, N. Immunomodulatory potential of Palladium(II) complexes with (1E,6E)-1,7-bis(3,4-dimethoxyphenyl)hepta-1,6-diene-3,5-dione. Stud. Univ. Babes-Bolyai Chem. 2015, 60, 93–100. [Google Scholar]
- Insan, M.B.; Jaitak, V. New approaches to target cancer stem cells: Current scenario. Mini Rev. Med. Chem. 2014, 14, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Helson, L. Curcumin and Cancer Stem Cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer Res. 2015, 35, 599–614. [Google Scholar] [PubMed]
- Liang, J.F.; Wang, H.K.; Xiao, H.; Li, N.; Cheng, C.X.; Zhao, Y.Z.; Ma, Y.B.; Gao, J.Z.; Bai, R.B.; Zheng, H.X. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J. Exp. Clin. Cancer Res. 2010, 29, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.A.; Tesori, V.; Lattanzi, W.; Gasbarrini, G.B.; Gasbarrini, A. Colon cancer stem cells: Controversies and perspectives. World J. Gastroenterol. 2013, 19, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Ng, R.K.; Ming, X.; Zhang, W.; Chen, L.; Chu, A.C.; Pang, R.; Lo, C.M.; Tsao, S.W.; Liu, X.; et al. Epigenetic regulation of pluripotent genes mediates stem cell features in human hepatocellular carcinoma and cancer cell lines. PLoS ONE 2013, 8, e72435. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Grandela, C.; Medema, J.P. Molecular identification and targeting of colorectal cancer stem cells. Oncotarget 2010, 1, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Elsaba, T.M.; Martinez-Pomares, L.; Robins, A.R.; Crook, S.; Seth, R.; Jackson, D.; McCart, A.; Silver, A.R.; Tomlinson, I.P.; Ilyas, M. The stem cell marker CD133 associates with enhanced colony formation and cell motility in colorectal cancer. PLoS ONE 2010, 5, e10714. [Google Scholar] [CrossRef] [PubMed]
- Irollo, E.; Pirozzi, G. CD133: To be or not to be, is this the real question? Am. J. Transl. Res. 2013, 5, 563–581. [Google Scholar] [PubMed]
- Prosperi, D.; Polito, L.; Morasso, C.; Monti, D. Biofunctionalization of spherical and anisotropic bimetallic nanomaterials. In Mixed Metal Nanomaterials; Kumar, C.R., Ed.; Wiley WCH Verlag GmbH: Weinheim, Germany, 2009; pp. 197–240. [Google Scholar]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Szmitkowski, M.; Wereszczyńska-Siemiatkowska, U.; Okulczyk, B. Stem cell factor (SCF) and interleukin 3 (IL-3) in the sera of patients with colorectal cancer. Dig. Dis. Sci. 2005, 50, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, J.; Rönnstrand, L. The stem cell factor receptor/c-Kit as a drug target in cancer. Curr. Cancer Drug Targets 2006, 6, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Matsuyama, T.; Kodera, Y. Plasma levels and production of soluble stem cell factor by marrow stromal cells in patients with aplastic anaemia. Br. J. Haematol. 1997, 99, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Carbone, A.; Sibona, N.; Bosco, O.; Tibaudi, D.; Smirne, C.; Martone, T.; Gramigni, C.; Camandona, M.; Emanuelli, G.; et al. Aberrant activation of c-kit protects colon carcinoma cells against apoptosis and enhances their invasive potential. Cancer Res. 2001, 61, 2200–2206. [Google Scholar] [PubMed]
- Cabello, N.; Kizirian, J.-C.; Gille, S.; Alexakis, A.; Bernardinelli, G.; Pinchard, L.; Caille, J.-C. Simple 1,2-Diamine Ligands for Asymmetric Addition of Aryllithium Reagents to Imines. Eur. J. Org. Chem. 2005, 22, 4835–4842. [Google Scholar] [CrossRef]
- Devínsky, F.; Lacko, I.; Mlynarčík, D.; Krasnec, L. Synthesis, IR spectra and antimicrobial activity of 1,4-dialkylpiperazine dioxides. Collect. Czechoslov. Chem. Commun. 1982, 47, 1130–1138. [Google Scholar] [CrossRef]
- Roughley, P.J.; Whiting, D.A. Experiments in the biosynthesis of curcumin. J. Chem. Soc. Perkin Trans. 1 1973, 2379–2388. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Soritau, O.; Rus-Ciuca, D.; Pop, T.; Todea, D.; Mosteanu, O.; Pintea, B.; Foris, V.; Susman, S.; Kacsó, G.; et al. Isolation and characterization of hepatic cancer cells with stem-like properties from hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2010, 19, 61–67. [Google Scholar]
- Virag, P.; Fischer-Fodor, E.; Perde-Schrepler, M.; Brie, I.; Tatomir, C.; Bălăcescu, L.; Berindan-Neagoe, I.; Victor, B.; Bălăcescu, O. Oxaliplatin induces different cellular and molecular chemoresistance patterns in colorectal cancer cell lines of identical origins. BMC Genom. 2013, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Bühring, H.J.; Seiffert, M.; Bock, T.A.; Scheding, S.; Thiel, A.; Scheffold, A.; Kanz, L.; Brugger, W. Expression of novel surface antigens on early hematopoietic cells. Ann. N. Y. Acad. Sci. 1999, 872, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Cenariu, D.; Fischer-Fodor, E.; Virag, P.; Tatomir, C.; Cenariu, M.; Pall, E.; Pintea, A.; Mocan, A.; Crisan, G. In vitro antitumour activity of tomato-extracted carotenoids on human colorectal carcinoma. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 293–301. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Cell Type | Curcumin Analogue | Complex 1 | Complex 2 | |||
|---|---|---|---|---|---|---|
| IC50 (μM) | SEM | IC50 (μM) | SEM | IC50 (μM) | SEM | |
| HT-29 | >500 | ----- | 9.78 | 0.3 | 69.77 | 5.6 |
| DLD-1 | 123.3 | 8.5 | 5.14 | 0.4 | 42.79 | 4.7 |
| CSC | 34.5 | 4.6 | 4.41 | 0.3 | 28.18 | 0.4 |
| LIV | 427.2 | 51.5 | 13.01 | 1.3 | 39.17 | 0.5 |
| LyCD34+ | 223.8 | 20.3 | 10.65 | 1.5 | 129.00 | 14.5 |
| Cell Type | CD133 Positive Cells/10,000 Total Cells | |||||||
|---|---|---|---|---|---|---|---|---|
| Untreated Cells | Curcumin Analogue | Complex 1 | Complex 2 | |||||
| Mean | CV | Mean | CV | Mean | CV | Mean | CV | |
| HT-29 | 2172 | 0.039 | 1946 | 0.029 | 1404 | 0.041 | 2120 | 0.015 |
| DLD-1 | 12 | 0.018 | 39 | 0.072 | 58 | 0.057 | 41 | 0.098 |
| CSC | 113 | 0.015 | 118 | 0.071 | 104 | 0.020 | 150 | 0.037 |
| LIV | 28 | 0.030 | 47 | 0.013 | 142 | 0.049 | 76 | 0.074 |
| LyCD34 | 100 | 0.028 | 182 | 0.062 | 116 | 0.085 | 47 | 0.020 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer-Fodor, E.; Mikláš, R.; Rišiaňová, L.; Cenariu, M.; Grosu, I.G.; Virag, P.; Perde-Schrepler, M.; Tomuleasa, C.; Berindan-Neagoe, I.; Devínsky, F.; et al. Novel Palladium(II) Complexes that Influence Prominin-1/CD133 Expression and Stem Cell Factor Release in Tumor Cells. Molecules 2017, 22, 561. https://doi.org/10.3390/molecules22040561
Fischer-Fodor E, Mikláš R, Rišiaňová L, Cenariu M, Grosu IG, Virag P, Perde-Schrepler M, Tomuleasa C, Berindan-Neagoe I, Devínsky F, et al. Novel Palladium(II) Complexes that Influence Prominin-1/CD133 Expression and Stem Cell Factor Release in Tumor Cells. Molecules. 2017; 22(4):561. https://doi.org/10.3390/molecules22040561
Chicago/Turabian StyleFischer-Fodor, Eva, Roman Mikláš, Lucia Rišiaňová, Mihai Cenariu, Ioana Georgeta Grosu, Piroska Virag, Maria Perde-Schrepler, Ciprian Tomuleasa, Ioana Berindan-Neagoe, Ferdinand Devínsky, and et al. 2017. "Novel Palladium(II) Complexes that Influence Prominin-1/CD133 Expression and Stem Cell Factor Release in Tumor Cells" Molecules 22, no. 4: 561. https://doi.org/10.3390/molecules22040561