Ergosteryl 2-naphthoate, An Ergosterol Derivative, Exhibits Antidepressant Effects Mediated by the Modification of GABAergic and Glutamatergic Systems
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials and Agents
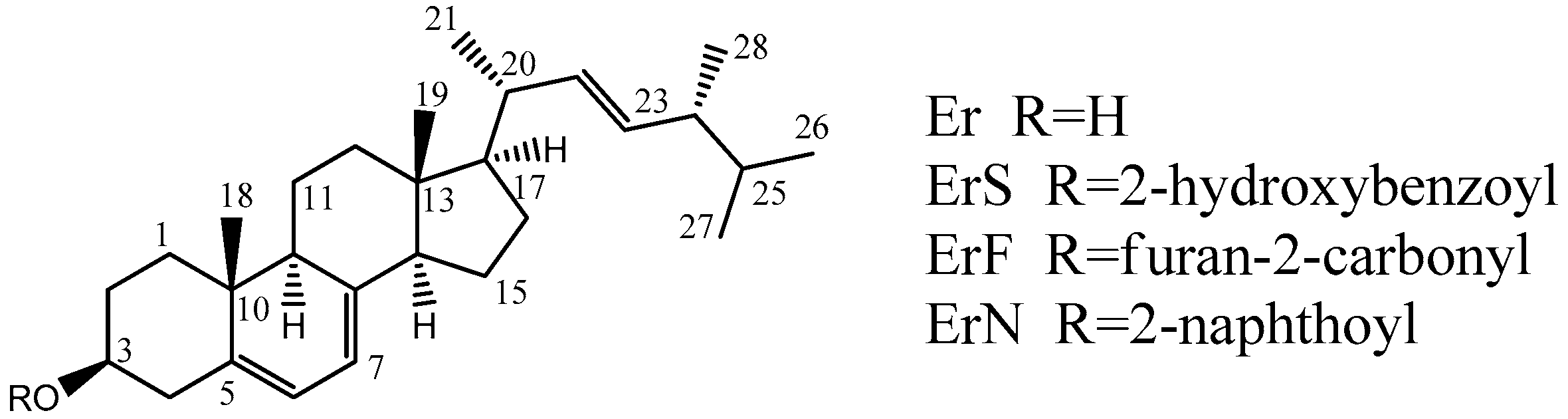
2.2. Synthesis of Compounds
2.3. Structural Determination
2.4. Spontaneous Locomotor Activity Test (SLT)
2.5. FST
2.6. Drug Treatment
2.6.1. The Antidepressant Effect of Er and Its Derivatives in the FST
2.6.2. The Effective and Sub-Effective Doses of ErN in the FST
2.6.3. The Effect of ErN on Locomotor Activity
2.6.4. The Roles of Different CNS Functions Including Noradrenergic, Dopaminergic, GABAergic and Glutamatergic Systems in the Antidepressant-Like Effect of ErN in the FST
2.7. Biochemical Measurements
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Structural Identification
3.1.1. ErN
3.1.2. ErF
3.1.3. ErS
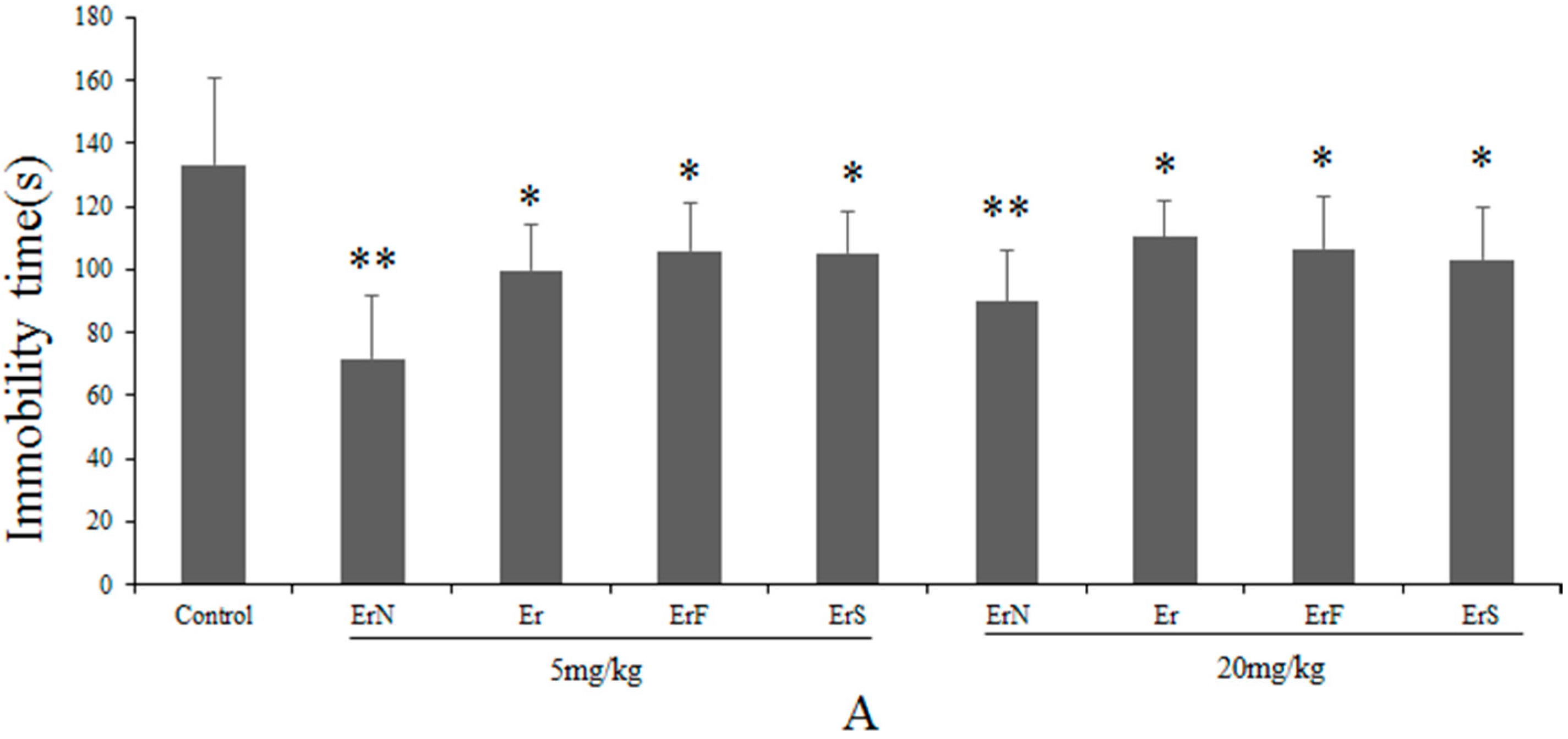
3.2. The Antidepressant Effect of Er and Its Derivatives in the FST
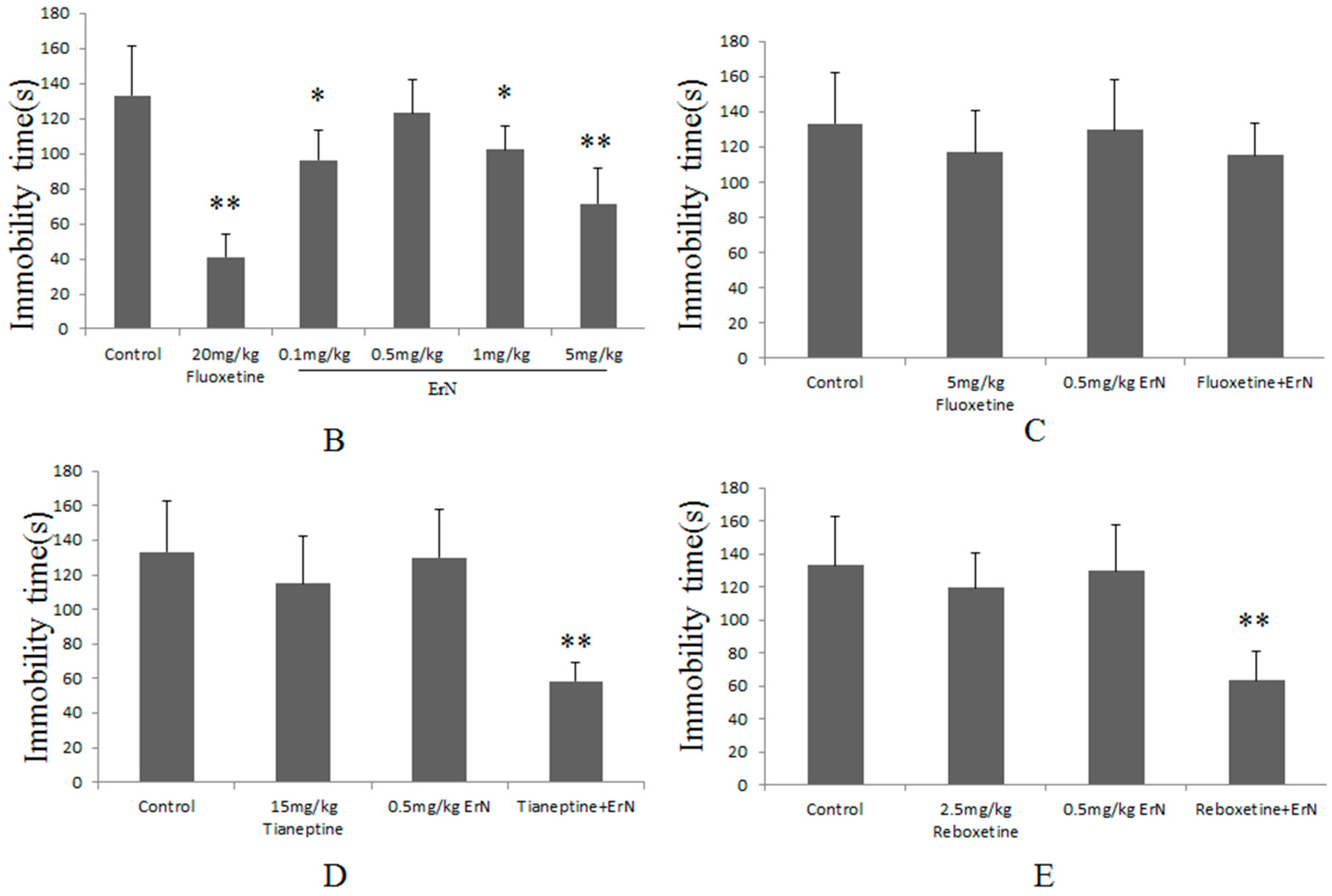
3.3. The Effective and Sub-Effective Doses of ErN in the FST
3.4. The Antidepressant Effect of Co-Administration of the Sub-Effective Doses of ErN (0.5 mg/kg) and Antidepressant Drugs in the FST
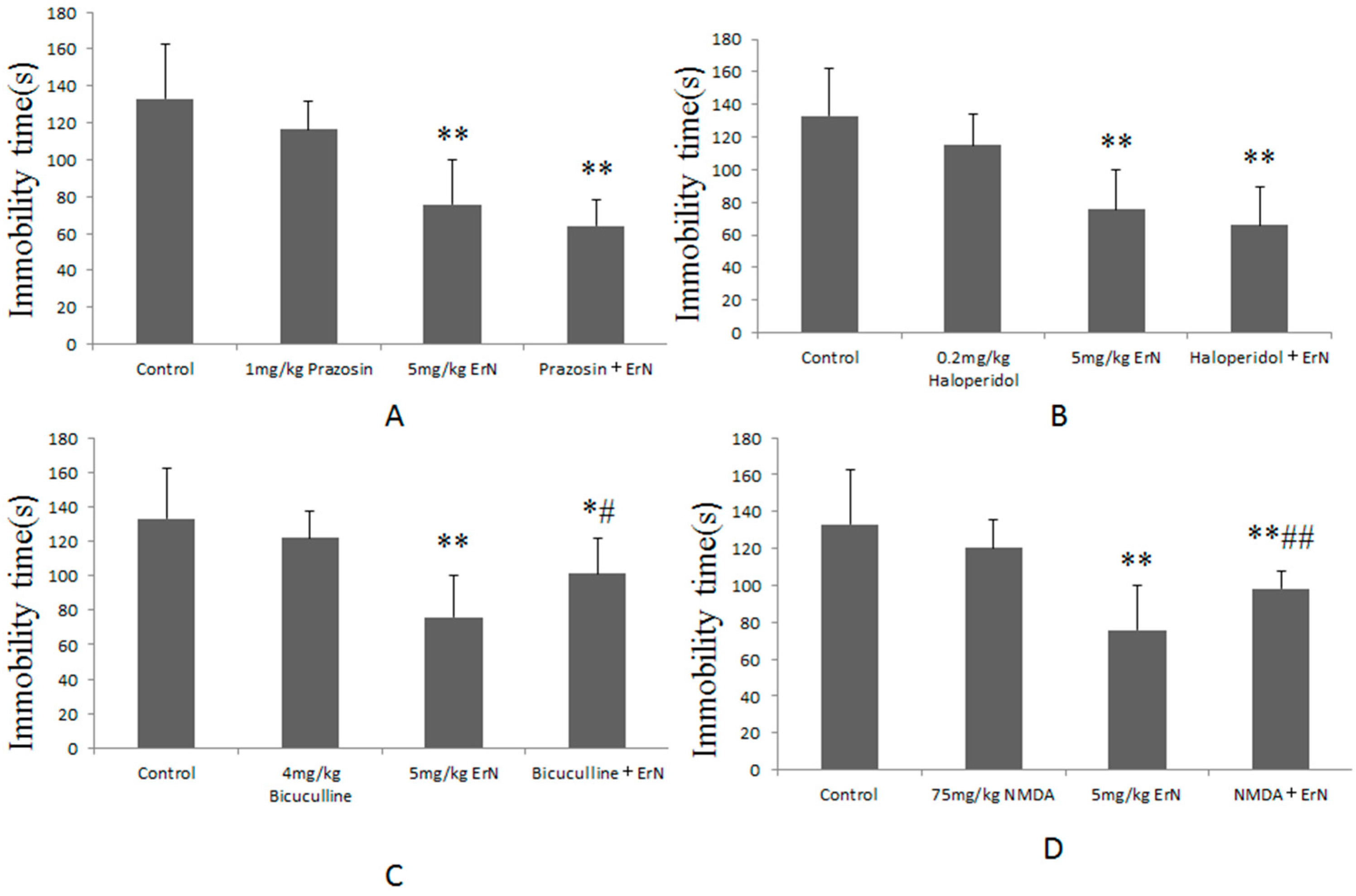
3.5. The Roles of Different the CNS Functions Including Noradrenergic, Dopaminergic, GABAergic and Glutamatergic Systems in the Antidepressant-Like Effect of ErN (5 mg/kg) in the FST
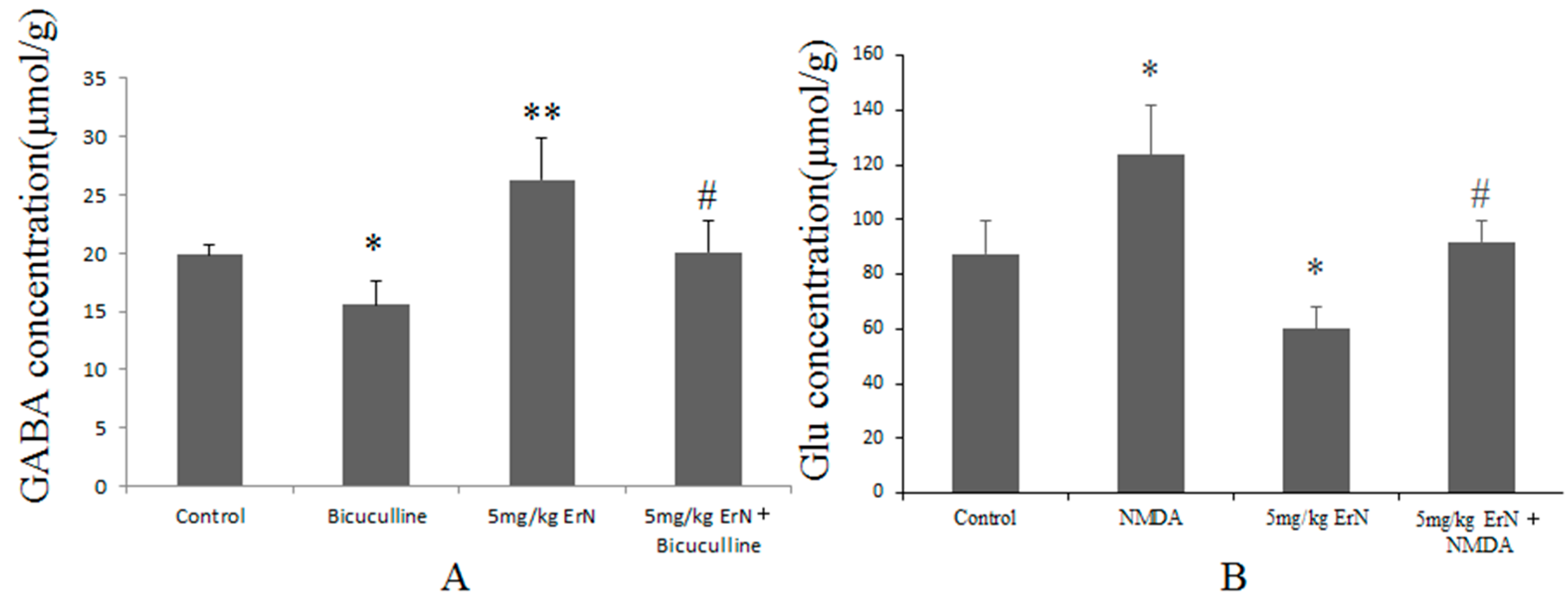
3.6. Effects of ErN on the Levels of GABA and Glu in Mice Exposed to the FST
3.7. Effect on Locomotor Activity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Er | ergosterol |
| EDCI | 1-ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride |
| DMAP | 4-dimethylaminopyridine |
| ErN | ergosteryl 2-naphthoate |
| ErF | ergosteryl furan-2-carboxylate |
| ErH | ergosteryl 2-hydroxybenzoate |
| FST | forced swim test |
| CNS | central nervous system |
| SLT | spontaneous locomotor activity test |
| GABA | γ-aminobutyric acid |
| NA | noradrenaline |
| DA | dopamine |
| Glu | glutamate |
| 5-HT | serotonin |
| MAOIs | monoamine oxidase inhibitors |
| SSRIs | selective serotonin reuptake inhibitors |
| SNRIs | selective noradrenaline reuptake inhibitors |
| TCAs | tricyclic antidepressants |
References
- Nestler, E.J.; Barrot, M.; Dileone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Browne, C.A.; Lucki, I. Antidepressant effects of ketamine: Mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; O’Hara, R.; Iacovides, A.; Camilleri, C.P.; Kprinis, S.; Kaprinis, G.; Yesavage, J. Unipolar late-onset depression: A comprehensive review. Ann. Gen. Hosp. Psychiatry 2003, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Machado-Vieira, R.; Pavlatou, M.G. Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress. Neural Plast. 2015, 2015, 581976. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Gao, W.C.; Cheng, W.M.; Lu, W.L.; Tang, J.; Peng, L.; Li, N.; Chen, F.H. Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur. Neuropsychopharmacol. 2014, 24, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Detraux, J.; de Lepeleire, J.; de Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Italiano, D.; Spina, E.; Cricelli, C.; Lapi, F.; Pecchioli, S.; Gambassi, G.; Trifirò, G. Changes in the prescribing pattern of antidepressant drugs in elderly patients: An Italian, nationwide, population-based study. Eur. J. Clin. Pharmacol. 2014, 70, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Wang, H.L.; Zhao, N.; Chen, H.X.; Li, Y.F.; Zhang, Y.Z. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of the total flavonoids extracted from Xiaobuxin-Tang. Neurosci. Lett. 2014, 575, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pesarico, A.P.; Sampaio, T.B.; Stangherlin, E.C.; Mantovani, A.C.; Zeni, G.; Nogueira, C.W. The antidepressant-like effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in the mouse forced swimming test is mediated by serotonergic and dopaminergic systems. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhat, Z.A.; Shah, M.Y. Anti-anxiety activity of successive extracts of Angelica archangelica Linn. on the elevated T-maze and forced swimming tests in rats. J. Tradit. Chin. Med. 2012, 32, 423–429. [Google Scholar] [CrossRef]
- Zhen, X.H.; Quan, Y.C.; Jiang, H.Y.; Wen, Z.S.; Qu, Y.L.; Guan, L.P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, W.; Guo, H.; Zhou, D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res. 2008, 194, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.S.; Gao, L.; Zhang, F. Determination of 4 amino acids neurotransmitters in rat hippocampi by RP-HPLC. J. Henan Agric. Sci. 2009, 38, 119–121. [Google Scholar]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Zhao, N.; Guo, W.Z.; Jin, Z.L.; Qiu, Z.K.; Chen, H.X.; Xue, R.; Zhang, Y.Z.; Yang, R.F.; Li, Y.F. Antidepressant-like and anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa). Neuropharmacology 2014, 81, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Xu, M.; Wu, B.; Liao, Z.; Liu, Z.; Zhao, X.; Bi, K.; Jia, Y. The effect of Schisandra chinensis extracts on depression by noradrenergic, dopaminergic, GABAergic and glutamatergic systems in the forced swim test in mice. Food Funct. 2016, 7, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Tardito, D.; Molteni, R.; Popoli, M.; Racagni, G. Synergistic mechanisms involved in the antidepressant effects of agomelatine. Eur. Neuropsychopharmacol. 2012, 22, S482–S486. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernandez, M.; Tellez-Alcantara, N.P.; Garcia, J.P.; Lopez, J.I.; Jaramillo, M.T. Synergistic interaction between ketoconazole and several antidepressant drugs with allopregnanolone treatments in ovariectomized Wistar rats forced to swim. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Clausius, N.; Born, C.; Grunze, H. The relevance of dopamine agonists in the treatment of depression. Neuropsychiatry 2009, 23, 15–25. [Google Scholar]
- Ma, K.; Xu, A.; Cui, S.; Sun, M.R.; Xue, Y.C.; Wang, J.H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry 2016, 6, e910. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.D.; Petty, F. Additivity of neurochemical changes in learned helplessness and imipramine. Behav. Neural Biol. 1982, 35, 344–353. [Google Scholar] [CrossRef]
- Sanacora, G.; Gueorguieva, R.; Epperson, C.N.; Wu, Y.T.; Appel, M.; Rothman, D.L.; Krystal, J.H.; Mason, G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 2004, 61, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Petty, F. GABA and mood disorders: A brief review and hypothesis. J. Affect. Disord. 1995, 34, 275–281. [Google Scholar] [CrossRef]
- Lloyd, K.G.; Zivkovic, B.; Scatton, B.; Morselli, P.L.; Bartholini, G. The GABaergic hypothesis of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 341–351. [Google Scholar] [CrossRef]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Burdick, K.E. Cognitive side effects of anticonvulsants. J. Clin. Psychiatry 2001, 62, 27–33. [Google Scholar] [PubMed]
- Sherman, A.D.; Petty, F. Neurochemical basis of the action of antidepressants on learned helplessness. Behav. Neural Biol. 1980, 30, 119–134. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist 2009, 15, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Gronli, J.; Fiske, E.; Murison, R.; Bjorvatn, B.; Sørensen, E.; Ursin, R.; Portas, C.M. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav. Brain Res. 2007, 181, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Borsini, F.; Mancinelli, A.; D’Aranno, V.; Evangelista, S.; Meli, A. On the role of endogenous GABA in the forced swimming test in rats. Pharmacol. Biochem. Behav. 1988, 29, 275–279. [Google Scholar] [CrossRef]
- Reynaert, M.L.; Marrocco, J.; Gatta, E.; Mairesse, J.; Van, C.G.; Fagioli, F.; Maccari, S.; Nicoletti, F.; Morley, F.S. A self-medication hypothesis for increased vulnerability to drug abuse in prenatally restraint stressed rats. Adv. Neurobiol. 2015, 10, 101–120. [Google Scholar] [PubMed]
- Ludka, F.K.; Zomkowski, A.D.; Cunha, M.P.; Dal, C.T.; Zeni, A.L.; Rodrigues, A.L.; Tasca, C.I. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the l-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur. Neuropsychopharmacol. 2013, 23, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Moryl, E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur. J. Pharmacol. 1994, 263, 1–7. [Google Scholar] [CrossRef]
- Maj, J.; Rogoz, Z.; Skuza, G. The effects of combined treatment with MK-801 and antidepressant drugs in the forced swimming test in rats. Pol. J. Pharmacol. Pharm. 1992, 44, 217–226. [Google Scholar] [PubMed]
- Pochwat, B.; Palucha-Poniewiera, A.; Szewczyk, B.; Pilc, A.; Nowak, G. NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin. Investig. Drugs 2014, 23, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, A.; Rappeneau, V. Sex-dependent mental illnesses and mitochondria. Schizophr Res. 2017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Gao, Y.; Liu, S.; Yang, H.; Zhang, L.; Tang, G.; et al. Ergosteryl 2-naphthoate, An Ergosterol Derivative, Exhibits Antidepressant Effects Mediated by the Modification of GABAergic and Glutamatergic Systems. Molecules 2017, 22, 565. https://doi.org/10.3390/molecules22040565
Lin M, Li H, Zhao Y, Cai E, Zhu H, Gao Y, Liu S, Yang H, Zhang L, Tang G, et al. Ergosteryl 2-naphthoate, An Ergosterol Derivative, Exhibits Antidepressant Effects Mediated by the Modification of GABAergic and Glutamatergic Systems. Molecules. 2017; 22(4):565. https://doi.org/10.3390/molecules22040565
Chicago/Turabian StyleLin, Mingzhu, Haijun Li, Yan Zhao, Enbo Cai, Hongyan Zhu, Yugang Gao, Shuangli Liu, He Yang, Lianxue Zhang, Guosheng Tang, and et al. 2017. "Ergosteryl 2-naphthoate, An Ergosterol Derivative, Exhibits Antidepressant Effects Mediated by the Modification of GABAergic and Glutamatergic Systems" Molecules 22, no. 4: 565. https://doi.org/10.3390/molecules22040565




