Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors
Abstract
:1. Introduction
2. Results and Discussion
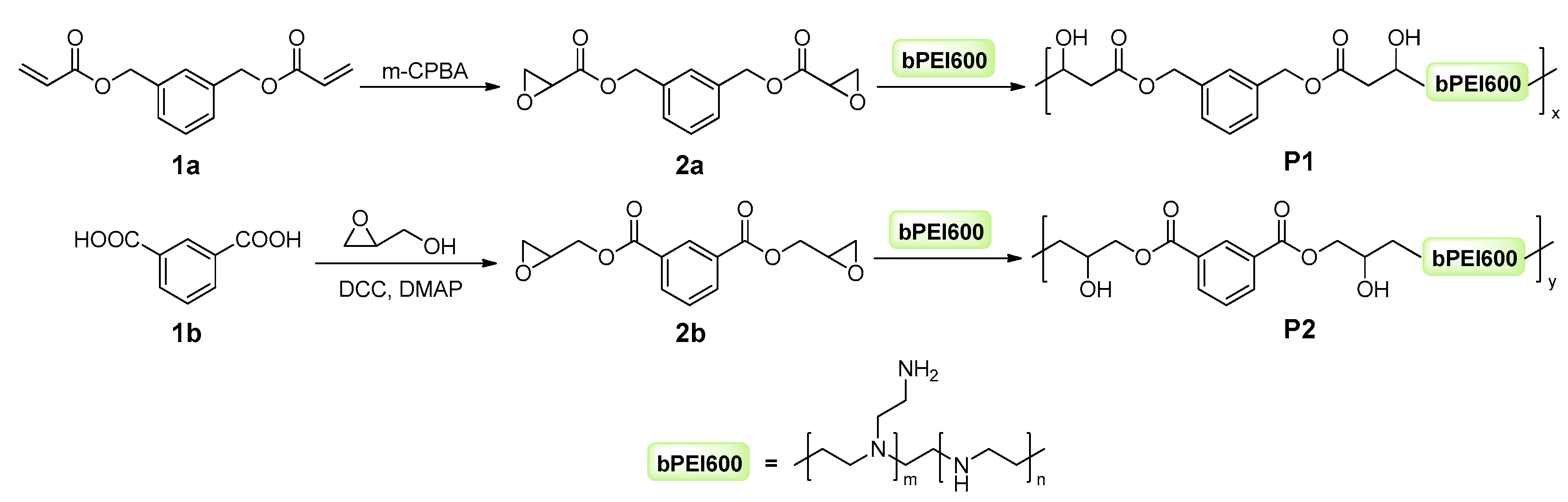
2.1. Synthesis and Characterization of Target Polymers
2.2. Buffer Capacity
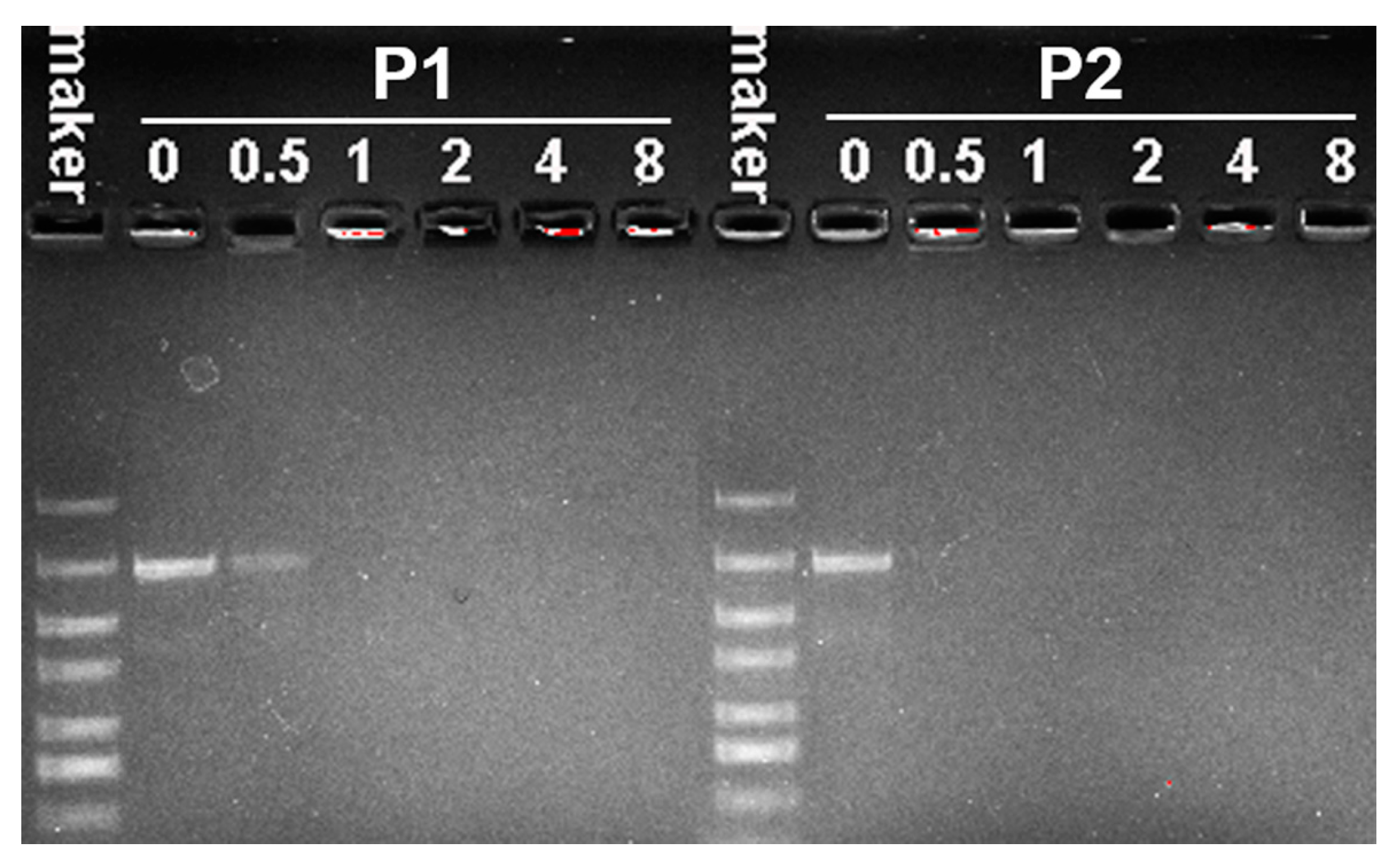
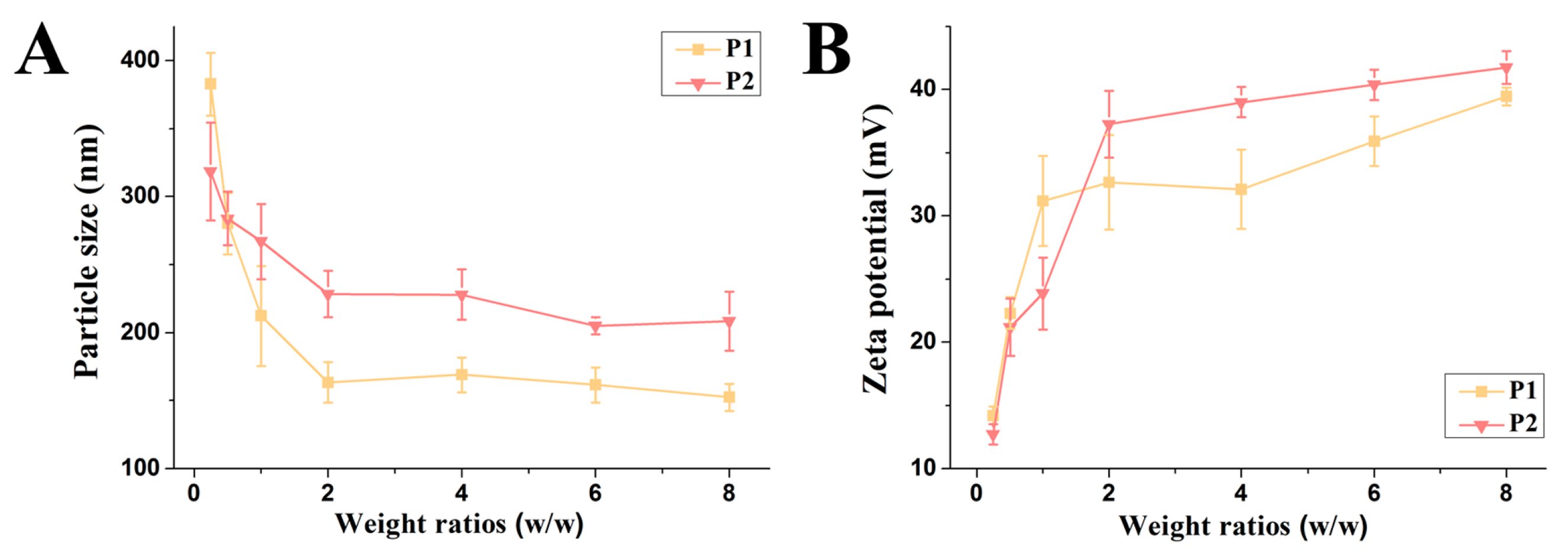
2.3. The Formation and Properties of Polymer/DNA Polyplexes
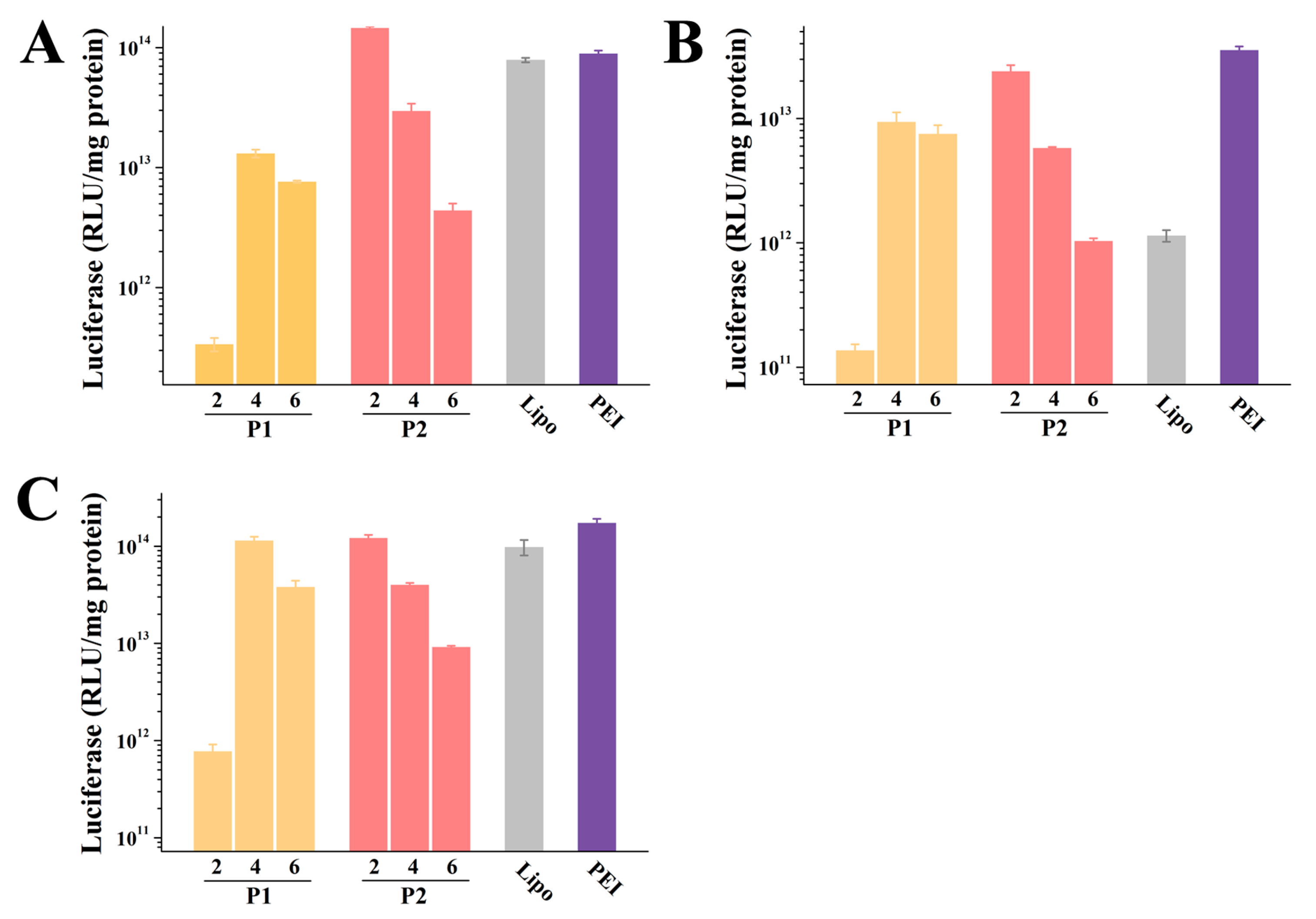
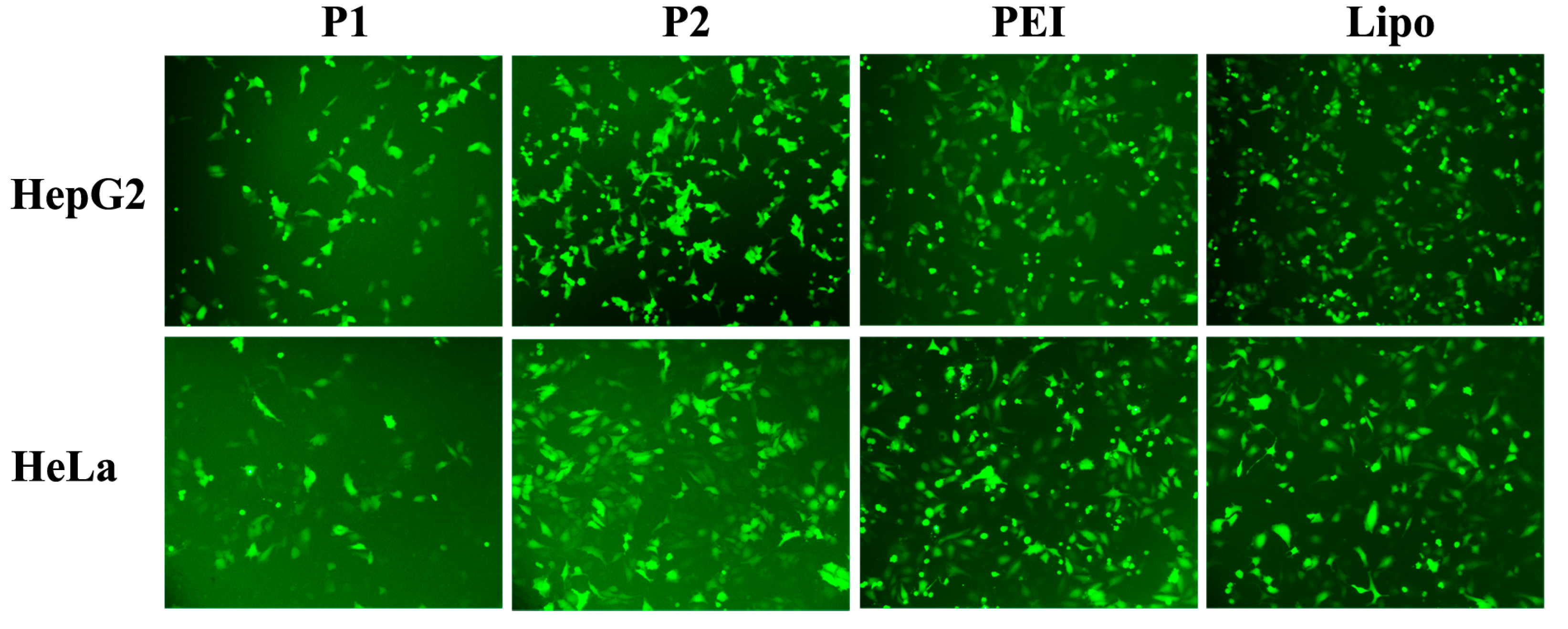
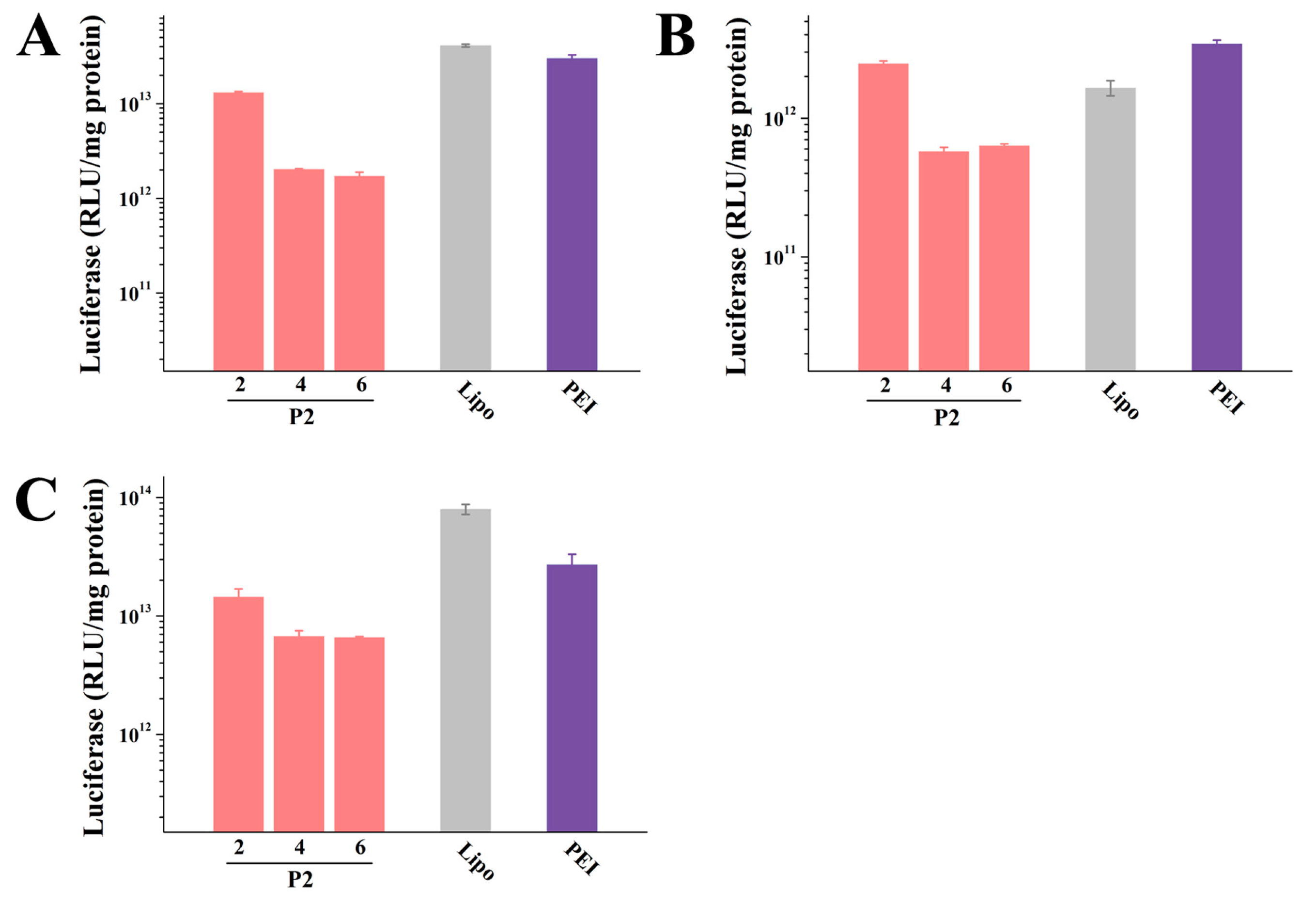
2.4. In Vitro Transfection
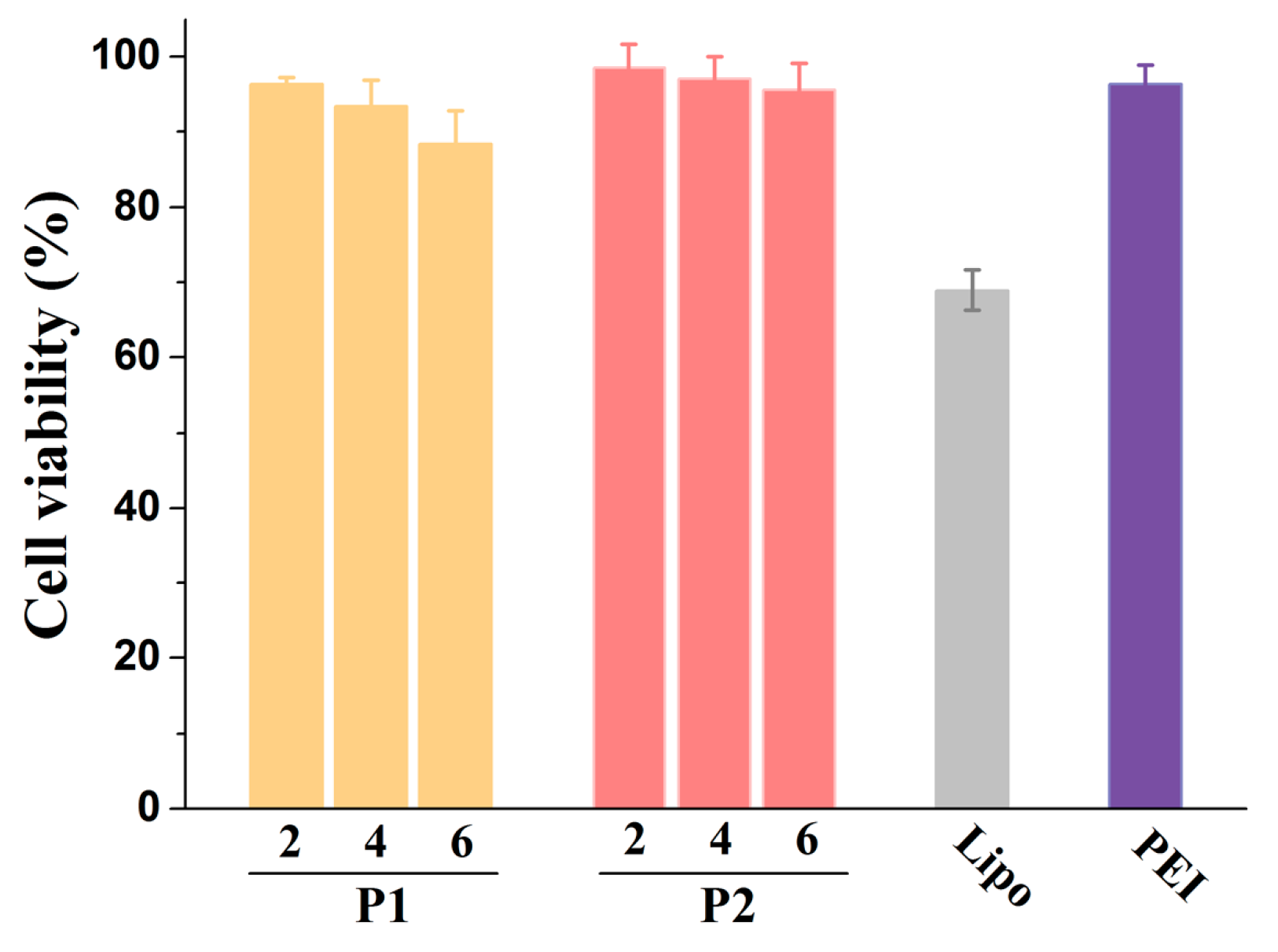
3. Cytotoxicity
4. Experimental Section
4.1. Materials and Methods
4.2. General Method for Preparation of Biodegradable Poly(Amino Ester) with Aromatic Backbone
4.2.1. Synthesis and Characterization Linker of 2a
4.2.2. Synthesis and Characterization Linker of 2b
4.2.3. Synthesis and Characterization of Target Polymers P1, P2
4.3. Amplification and Purification of Plasmid DNA
4.4. Acid-Base Titration
4.5. Polymer Degradation Study
4.6. Agarose Gel Retardation
4.7. Particle Size and Zeta Potential Measurements
4.8. Cell Culture
4.9. In Vitro Transfection Experiments
4.10. Cytotoxicity Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, H.; Bai, Y.; Wang, J.; Deng, Q.; Zhu, L.; Meng, F.; Zhong, Z.; Yin, L. Reversibly cross-linked polyplexes enable cancer-targeted gene delivery via self-promoted DNA release and self-diminished toxicity. Biomacromolecules 2015, 16, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.H.; Li, S.; Howard, O.M.Z.; Dunlap, M.; Trivett, A.; Schneider, J.P.; Oppenheim, J.J. Enhanced immunostimulatory effects of DNA-encapsulated peptide hydrogels. Biomaterials 2015, 53, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, L.; Wang, J.; Tang, R.; Yan, G.; Cao, Z.; Wang, X. Acid-labile poly(ortho ester amino alcohols) by ring-opening polymerization for controlled DNA release and improved serum tolerance. Polymer 2016, 96, 146–155. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Gao, J.; Cao, Z.; Jiang, Q.; Liu, J.; Jiang, Z. Enzymatic PEGylated Poly(lactone-co-β-amino ester) Nanoparticles as Biodegradable, Biocompatible and Stable Vectors for Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Okuda, T.; Okamoto, H. Development of New Formulation Dry Powder for Pulmonary Delivery Using Amino Acids to Improve Stability. Biol. Pharm. Bull. 2016, 39, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Gupta, K.C.; Kumar, P. Biodegradable and versatile polyethylenimine derivatives efficiently transfer DNA and siRNA into mammalian cells. Colloids Surf. B 2015, 135, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wu, C. Progress and perspectives in developing polymeric vectors for in vitro gene delivery. Biomater. Sci. 2013, 1, 152–170. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, M.; Liang, D.; Yang, J.; Tang, X. Vitamin E-Labeled Polyethylenimine for in vitro and in vivo Gene Delivery. Biomacromolecules 2016, 17, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.H.; Lee, J.H.; Kim, K.S.; Lee, J.Y.; Kim, M.S.; Khang, G.; Lee, I.W.; Lee, H.B. Polyethyleneimine-mediated gene delivery into human adipose derived stem cells. Biomaterials 2008, 29, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Hu, J.; Na, K.; Bae, Y.H. Role of polymeric endosomolytic agents in gene transfection: A comparative study of poly(l-lysine) grafted with monomeric l-histidine analogue and poly(L-histidine). Biomacromolecules 2014, 15, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, C.; Hu, Y.; Zhou, H.; Chen, J.; Zhang, Z.; Guo, T. The effects of a multifunctional oligomer and its incorporation strategies on the gene delivery efficiency of poly(l-lysine). Chem. Commun. 2012, 48, 4594–4596. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconj. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Orr, B.G.; Holl, M.M.B. Role of cell membrane–vector interactions in successful gene delivery, Acc. Chem. Res. 2016, 49, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Taranejoo, S.; Chandrasekaran, R.; Cheng, W.; Hourigan, K. Bioreducible PEI-functionalized glycol chitosan: A novel gene vector with reduced cytotoxicity and improved transfection efficiency. Carbohydr. Polym. 2016, 153, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ferro, V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.; Fischer, D.; Kissel, T.J. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005, 7, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Lungwitz, U.; Breunig, M.; Blunk, T.; Gopferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005, 60, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Saltik, M.; Lehrmann, H.; Killisch, I.; Mautner, V.; Lamm, G.; Christofori, G.; Cotton, M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997, 4, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualch, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Wightman, L.; Kircheis, R.; Rössler, V.; Carotta, S.; Ruzicka, R.; Kursa, M.; Wagner, E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001, 3, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Venkiteswaran, S.; Thomas, T.; Thomas, T.J. Selectivity of polyethyleneimines on DNA nanoparticle preparation and gene transport. ChemistrySelect 2016, 6, 1144–1150. [Google Scholar] [CrossRef]
- Li, H.; Jiang, H.; Zhao, M.; Fu, Y.; Sun, X. Intracellular redox potential-responsive micelles based on polyethylenimine-cystamine-poly(ε-caprolactone) block copolymer for enhanced miR-34a delivery. Polym. Chem. 2015, 6, 1952–1960. [Google Scholar] [CrossRef]
- Luo, X.H.; Huang, F.W.; Qin, S.Y.; Wang, H.F.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. A strategy to improve serum-tolerant transfection activity of polycation vectors by surface hydroxylation. Biomaterials 2011, 32, 9925–9939. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.Y.; Yang, C.; Hedrick, J.L.; Engler, A.C.; Coady, D.J.; Ghaem-Maghami, S.; George, A.J.; Yang, Y.Y. Hydrophobic modification of low molecular weight polyethylenimine for improved gene transfection. Biomaterials 2013, 34, 7971–7979. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Bieber, T.; Li, Y.; Elsasser, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, W.; Jin, M.-J.; Fan, B.; Xia, G.-M.; Gao, Z.-G. Inhibition of murine breast cancer growth and metastasis by survivin-targeted siRNA using disulfide cross-linked linear PEI. Eur. J. Pharm. Sci. 2016, 82, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, C.; Meng, F.; Peng, R.; Zhong, Z. pH-sensitive degradable hydrophobe modified 1.8 kDa branched polyethylenimine as “artificial viruses” for safe and efficient intracellular gene transfection. Macromol. Res. 2012, 20, 327–334. [Google Scholar]
- Zhao, D.; Gong, T.; Zhu, D.; Zhang, Z.; Sun, X. Comprehensive comparison of two new biodegradable gene carriers. Int. J. Pharm. 2011, 413, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.P.; Forrest, M.L.; Ton, G.; Zhao, A.; Davies, N.M.; Kwon, G.S. Poly(aspartate-g-PEI800), a polyethylenimine analogue of low toxicity and high transfection efficiency for gene delivery. Biomaterials 2007, 28, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.Q.; Du, J.Z.; Sun, T.M.; Yao, Y.D.; Zhang, P.Z.; Song, E.W.; Wang, J. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. Biomaterials 2011, 32, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.-J.; Yu, X.-C.; Wang, B.; Zhang, J.; Yu, Q.-Y.; Zhou, X.-D.; Yu, X.-Q. TACN-based oligomers with aromatic backbones for efficient nucleic acid delivery. Chem. Commun. 2014, 50, 6454–6457. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.-R.; Liu, Y.-H.; Zhang, J.; Yu, Q.-Y.; Huang, Z.; Wang, B.; Yu, X.-Q. Low Molecular Weight Oligomers with Aromatic Backbone as Efficient Nonviral Gene Vectors. ACS Appl. Mater. Interfaces 2016, 8, 10743–10751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-F.; Yu, Q.-Y.; Geng, Y.; Zhang, J.; Wu, W.-X.; Wang, G.; Gu, Z.; Yu, X.-Q. Ring-Opening Polymerization for Hyperbranched Polycationic Gene Delivery Vectors with Excellent Serum Tolerance. ACS Appl. Mater. Interfaces 2014, 6, 15733–15742. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.-J.; Zhang, Q.-F.; Zhang, J.; Liu, Q.; Ren, L.; Chen, Q.-M.; Guo, L.; Yu, X.-Q. Cyclen-based lipidic oligomers as potential gene delivery vehicles. Acta Biomater. 2014, 10, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-F.; Yi, W.-J.; Wang, B.; Zhang, J.; Ren, L.; Chen, Q.-M.; Guo, L.; Yu, X.-Q. Linear polycations by ring-opening polymerization as non-viral gene delivery vectors. Biomaterials 2013, 34, 5391–5401. [Google Scholar] [CrossRef] [PubMed]
- Xun, M.-M.; Liu, Y.-H.; Guo, Q.; Zhang, J.; Zhang, Q.-F.; Wu, W.-X.; Yu, X.-Q. Low molecular weight PEI-appended polyesters as non-viral gene delivery vectors. Eur. J. Med. Chem. 2014, 78, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Morille, M.; Passirani, C.; Vonarbourg, A.; Clavreul, A.; Benoit, J.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.A.; Thomas, T. Polyamine–DNA interactions and development of gene delivery vehicles. Amino Acids 2016, 48, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Vijayanathan, V.; Thomas, T.J.; Thomas, T. DNA Nanoparticles and Development of DNA Delivery Vehicles for Gene Therapy. Biochemistry 2002, 41, 14085–14094. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tang, T.; Uludag, H. Molecular Dynamics Simulations of PEI Mediated DNA Aggregation. Biomacromolecules 2011, 12, 3698–3707. [Google Scholar] [CrossRef] [PubMed]
- Farber, I.Y.; Domb, A.J. Cationic polysaccharides for gene delivery. Mater. Sci. Eng. C 2007, 27, 595–598. [Google Scholar] [CrossRef]
- Liu, Y.M.; Reineke, T.M. Hydroxyl Stereochemistry and Amine Number within Poly(glycoamidoamine)s Affect Intracellular DNA Delivery. J. Am. Chem. Soc. 2005, 127, 3004–3015. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, W.J. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomaterials 2010, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Chen, C.-K.; Jiang, M.; Fang, L.; Cheng, C.; Pfeife, B.A. Synthesis of Cationic Polylactides with Tunable Charge Densities as Nanocarriers for Effective Gene Delivery. Mol. Pharm. 2013, 10, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.X.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, N.; Song, Z.; Yin, L.; Cheng, J. Trigger-responsive, fast-degradable poly(β-amino ester)s for enhanced DNA unpackaging and reduced toxicity. Biomaterials 2014, 35, 5006–5015. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a and 2b are available from the authors. |
| Polymer | PEI 600 | PEI 25K | P1 | P2 |
|---|---|---|---|---|
| Buffering capacity (%) | 11.20 | 18.09 | 25.85 | 28.37 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Su, R.-C.; Yi, W.-J.; Zhao, Z.-G. Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors. Molecules 2017, 22, 566. https://doi.org/10.3390/molecules22040566
Liu Q, Su R-C, Yi W-J, Zhao Z-G. Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors. Molecules. 2017; 22(4):566. https://doi.org/10.3390/molecules22040566
Chicago/Turabian StyleLiu, Qiang, Rong-Chuan Su, Wen-Jing Yi, and Zhi-Gang Zhao. 2017. "Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors" Molecules 22, no. 4: 566. https://doi.org/10.3390/molecules22040566






