Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways
Abstract
:1. Introduction
2. Results
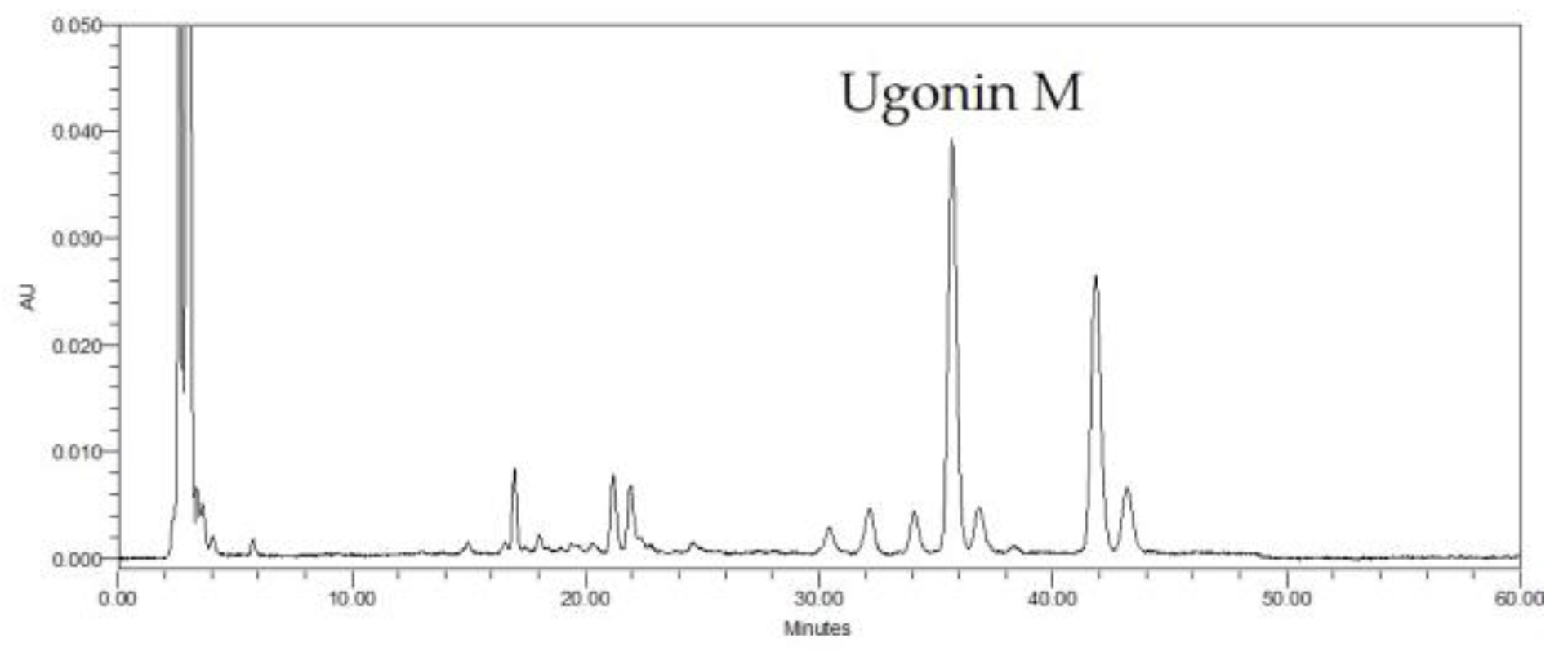
2.1. HPLC Analysis of H. zeylanica
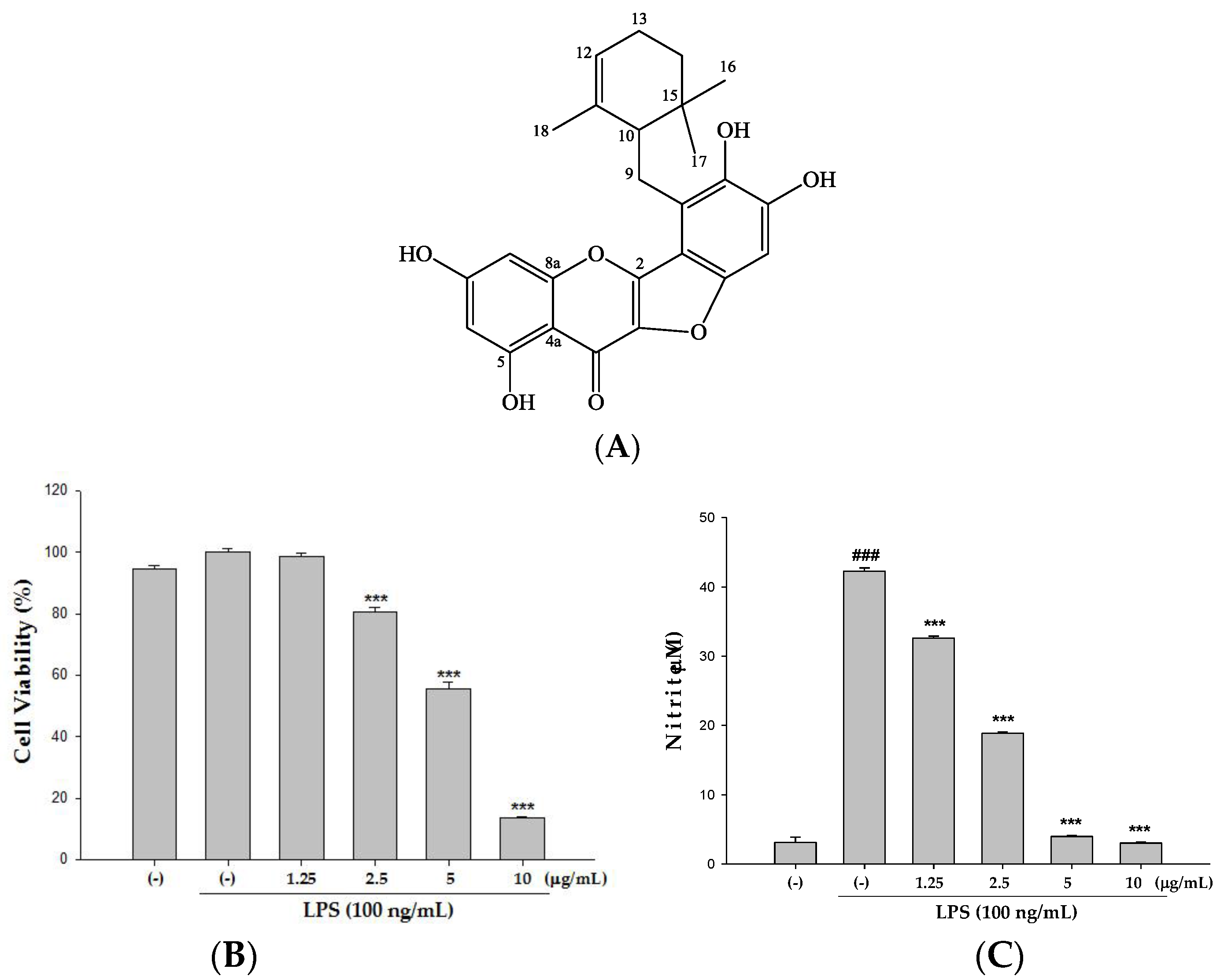
2.2. Cytotoxicity and the Effect(s) of Ugonin M on NO Production in Raw 264.7 Cell
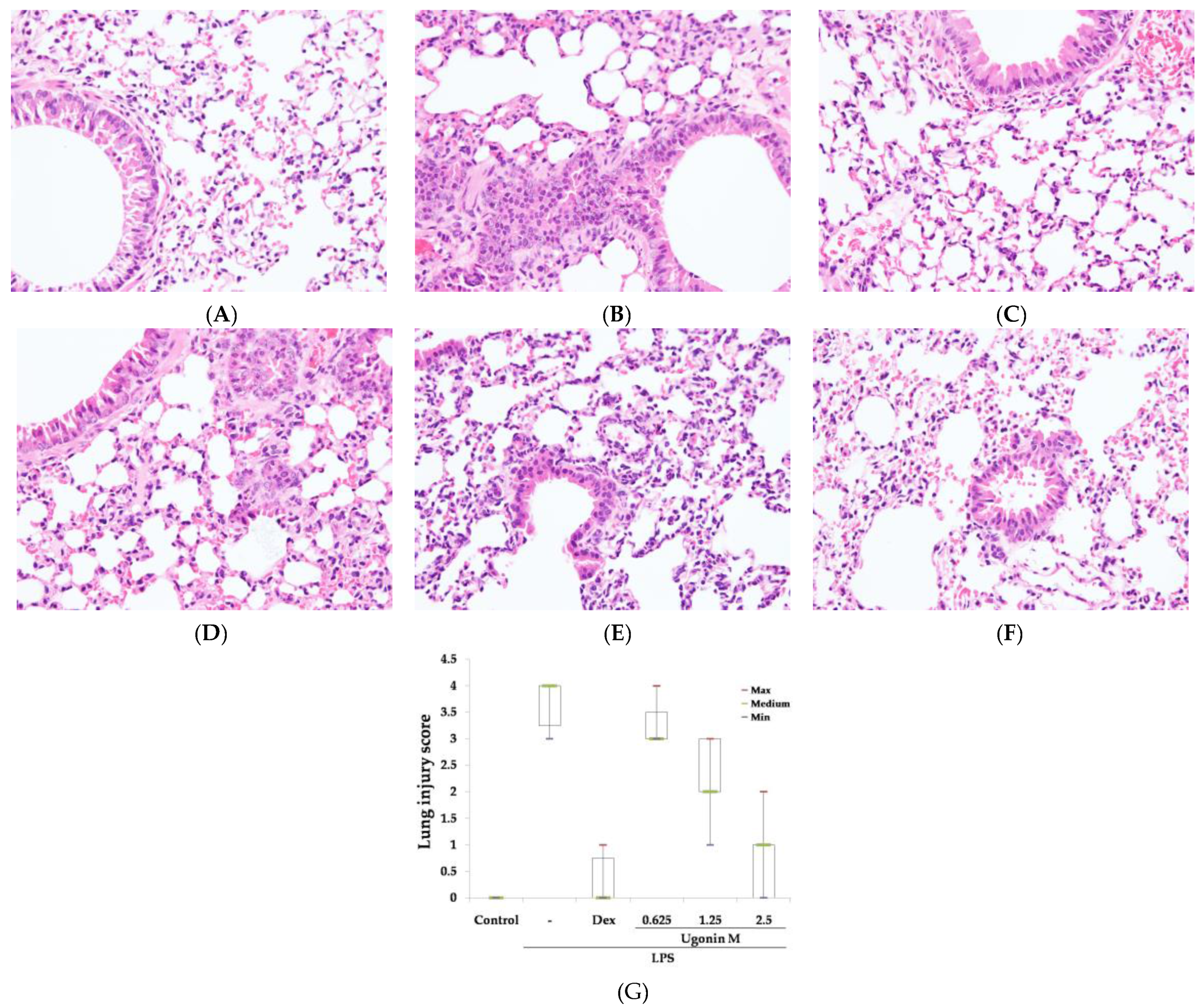
2.3. Effect(s) of Ugonin M on LPS-Induced Acute Lung Injury
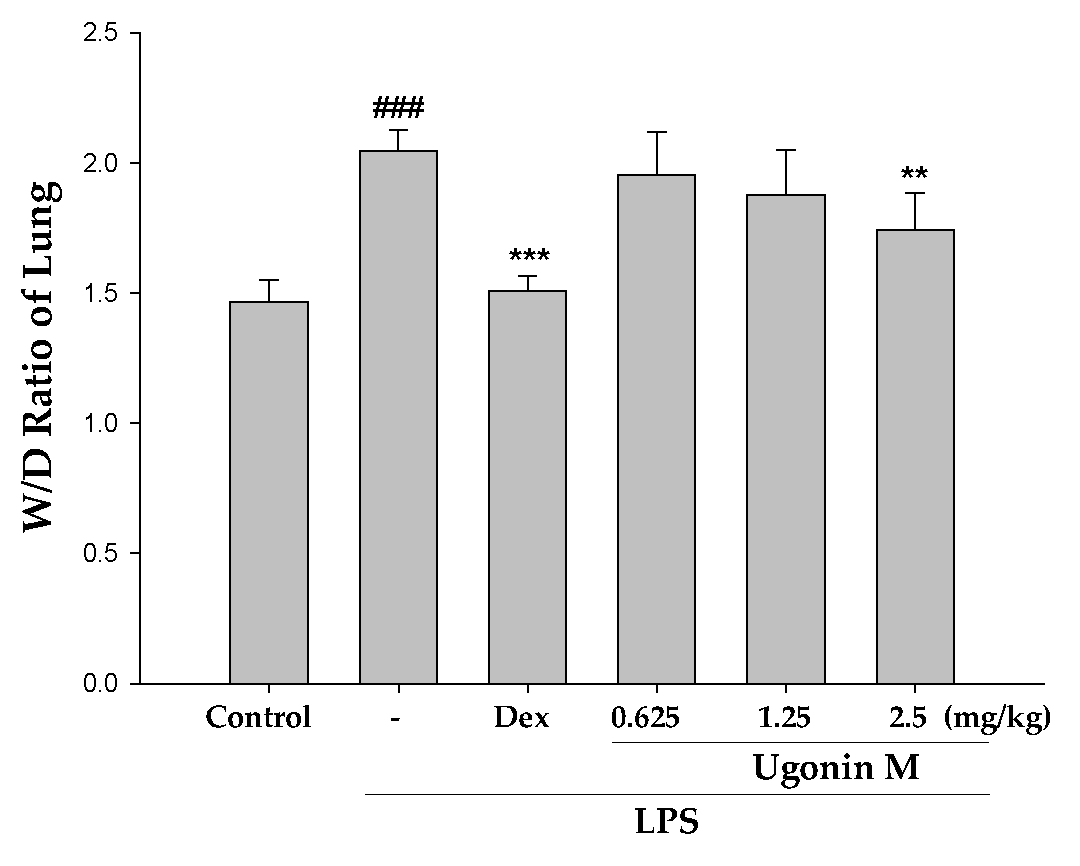
2.4. Effect(s) of Ugonin M on Pulmonary Edema in Lung Tissue
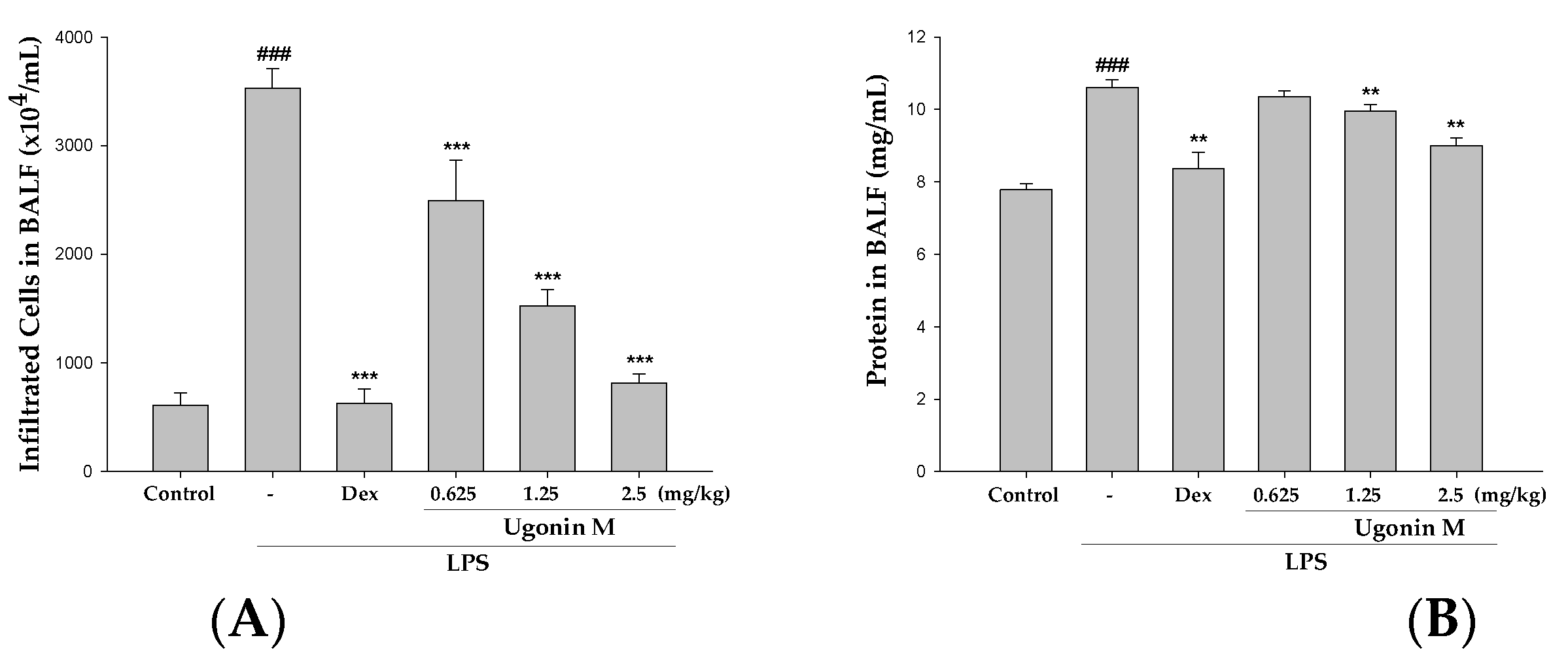
2.5. Effect(s) of Ugonin M on Infiltrated Cellular Counts and Proteins Levels in BALF
2.6. Effect(s) of Ugonin M on NO, TNF-α, IL-6, and IL-1β Levels in BALF
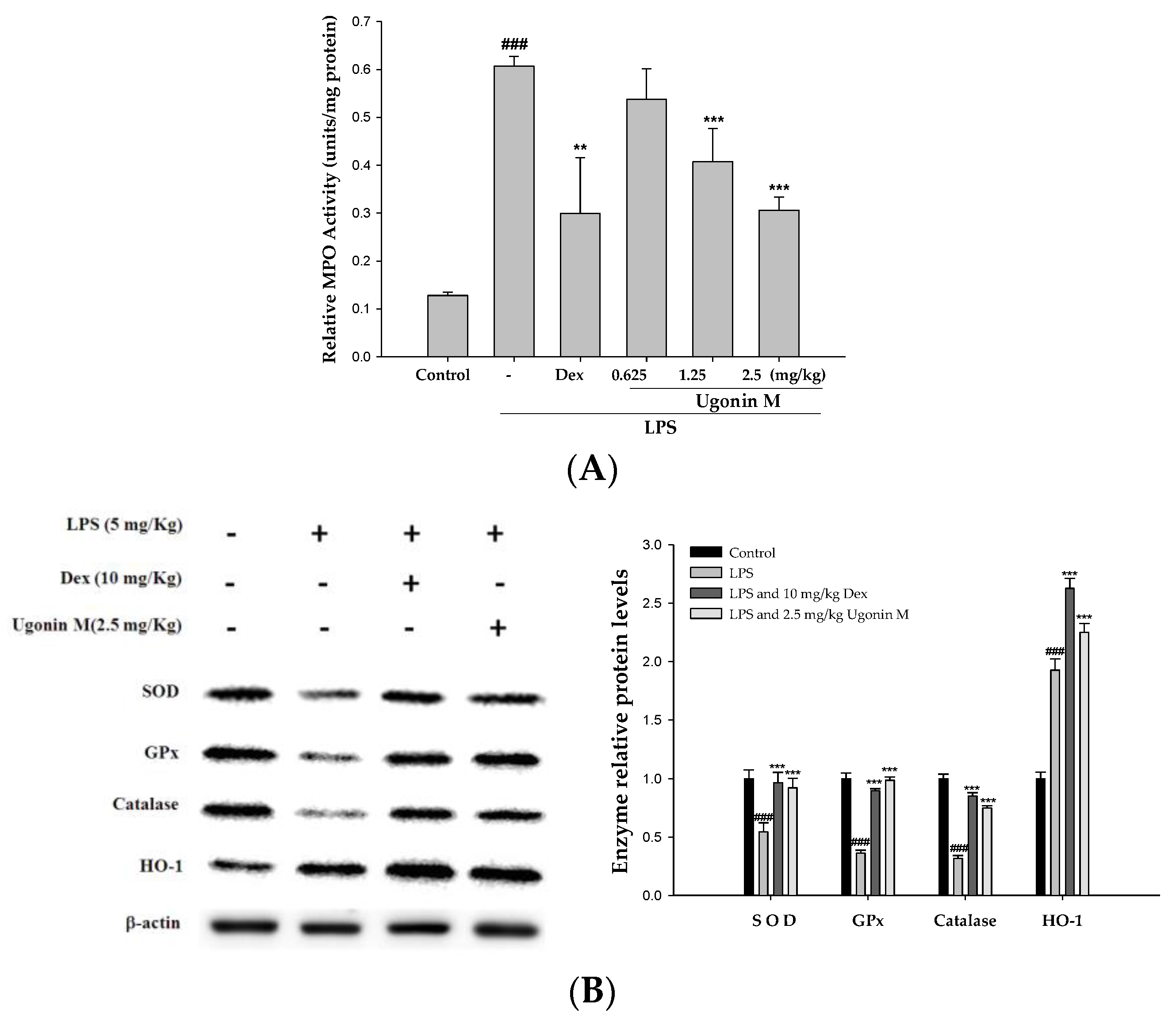
2.7. Effect(s) of Ugonin M on the Activity of MPO and Antioxidative Enzymes in Lung Tissue
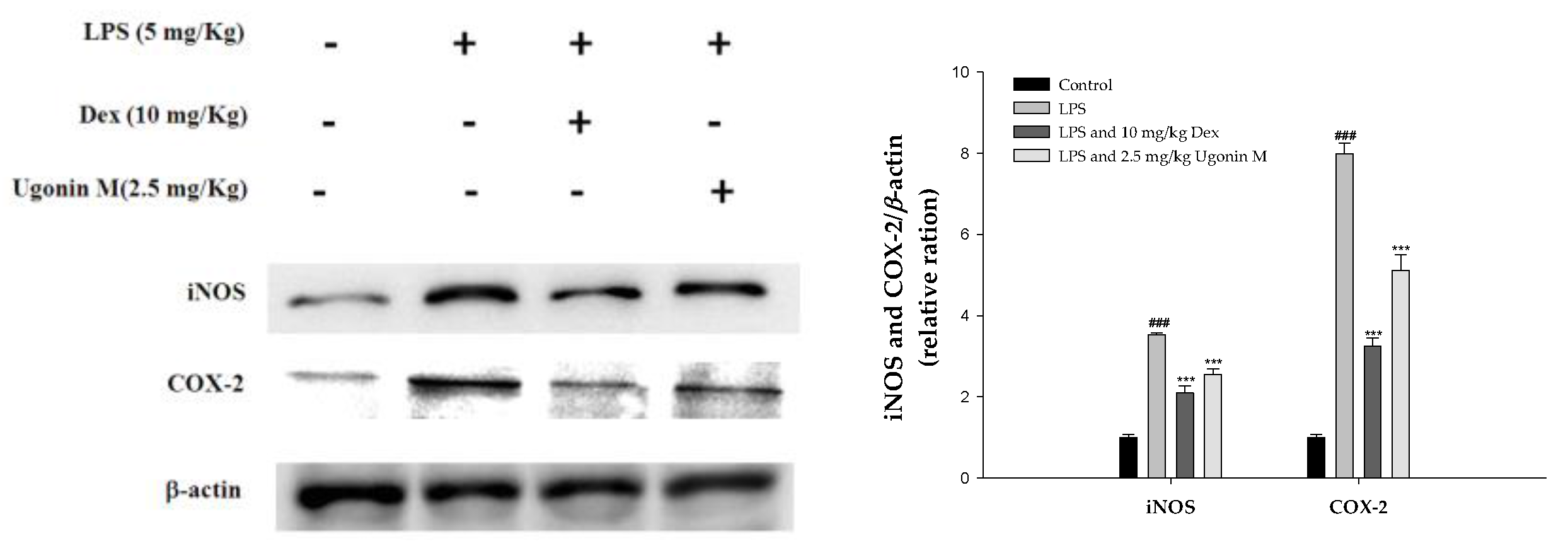
2.8. Effect(s) of Ugonin M on iNOS and COX-2 Proteins Expression in Lung Tissue
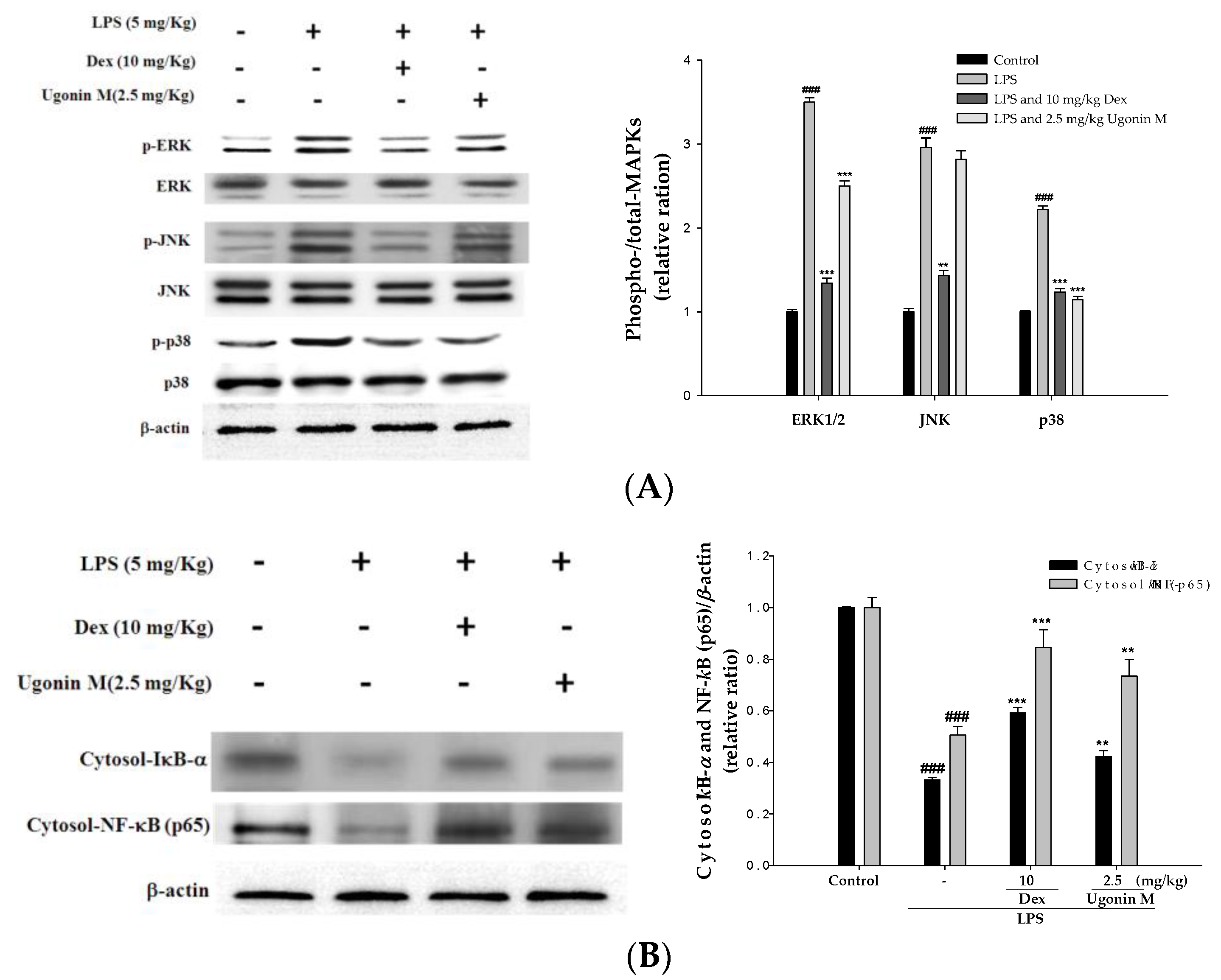
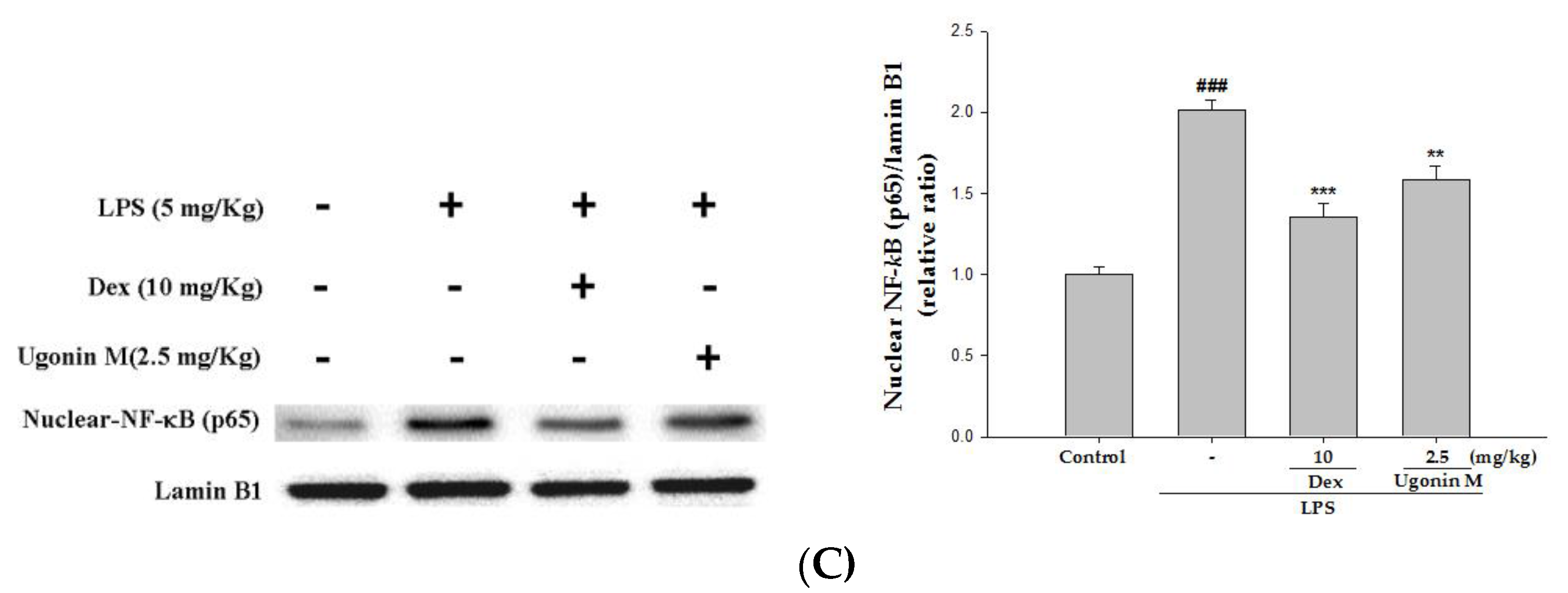
2.9. Effect(s) of Ugonin M on Activities of MAPK and NF-κB in Lung Tissue
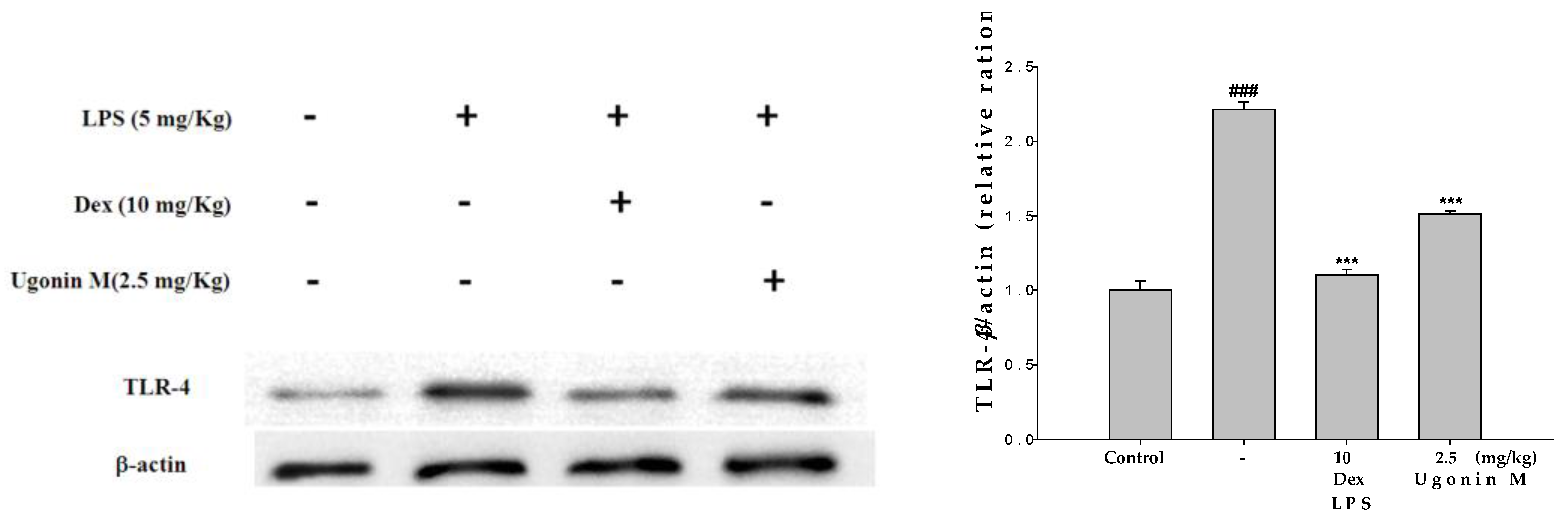
2.10. Effect(s) of Ugonin M on TLR4 Expression in Lung Tissues
3. Discussion
4. Materials and Methods
4.1. Isolation and Determination of Ugonin M from H. zeylanica
4.2. HPLC Analysis of H. zeylanica
4.3. Cell Culture
4.4. Cytotoxicity and the Measurement of Nitric Oxide
4.5. Animals
4.6. Model of LPS Induced ALI
4.7. Histological Examination
4.8. Lung Wet to Dry (W/D) Weight Ratio
4.9. Bronchoalveolar Lavage Fluid (BALF), Total Cell Count and Protein Analysis
4.10. TNF-α, IL-6, and IL-1β Cytokines in BALF
4.11. Myeloperoxidase (MPO) Activity Assay
4.12. Western Blot Analysis of the Lung Tissues
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Al Hoshani, A.R.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-kappaB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, T.; Zhang, W.; Wang, T.; Shen, Y.C.; Wan, Q.F.; Wen, F.Q. Emodin ameliorates LPS-induced acute lung injury, involving the inactivation of NF-kappaB in mice. Int. J. Mol. Sci. 2014, 15, 19355–19368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Ying, S.; Chen, G.; Wu, B.; Xu, T.; Liu, Z.; Liu, X.; Huang, L.; Shan, X.; Dai, Y.; Liang, G. Discovery of new MD2 inhibitor from chalcone derivatives with anti-inflammatory effects in LPS-induced acute lung injury. Sci. Rep. 2016, 6, 25130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, Y.; Mi, X. The protective effects of bone marrow-derived mesenchymal stem cell (BMSC) on LPS-induced acute lung injury via TLR3-mediated IFNs, MAPK and NF-kappaB signaling pathways. Biomed. Pharmacother. 2016, 79, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Jiang, K.; Chen, X.; Zhu, Z.; Qiu, C.; Li, C.; Deng, G. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kappaB and MAPK activation. Int. Immunopharmacol. 2016, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Lim, H.; Kwon, Y.S. Therapeutic potential of medicinal plants and their constituents on lung inflammatory disorders. Biomol. Ther. (Seoul) 2017, 25, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Huang, L.H.; Chang, Y.S.; Lin, I.S. The Illustration of Common Medicinal Plants in Taiwan; Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan: Taipei, Taiwan, 2009; Volume I, p. 37. [Google Scholar]
- Suja, S.R.; Latha, P.G.; Pushpangadan, P.; Rajasekharan, S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J. Ethnopharmacol. 2004, 92, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Huang, Y.L.; Yeh, P.Y.; Ou, J.C. Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica. Planta Med. 2003, 69, 964–967. [Google Scholar] [PubMed]
- Huang, Y.L.; Yeh, P.Y.; Shen, C.C.; Chen, C.C. Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica. Phytochemistry 2003, 64, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hwang, T.L.; Chang, C.S.; Yang, Y.L.; Shen, C.N.; Liao, W.Y.; Chen, S.C.; Liaw, C.C. Anti-inflammatory flavonoids from the rhizomes of Helminthostachys zeylanica. J. Nat. Prod. 2009, 72, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K promotes osteoblastic differentiation and mineralization by activation of p38 MAPK- and ERK-mediated expression of Runx2 and osterix. Eur. J. Pharmacol. 2011, 668, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur. J. Pharmacol. 2012, 676, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, S.H.; Lee, T.H.; Fang, J.Y.; Lin, C.F. Evaluation of anti-inflammatory effects of Helminthostachys zeylanica extracts via inhibiting bradykinin-induced MMP-9 expression in brain astrocytes. Mol. Neurobiol. 2016, 53, 5995–6005. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hwang, T.L.; Yang, Y.L.; Wu, S.H.; Hsu, M.H.; Wang, J.P.; Chen, S.C.; Huang, L.J.; Liaw, C.C. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med. 2010, 76, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Huang, Y.L.; Huang, W.C.; Yeh, K.W.; Huang, T.Y.; Lin, C.F. Water extract of Helminthostachys zeylanica attenuates LPS-induced acute lung injury in mice by modulating NF-kappaB and MAPK pathways. J. Ethnopharmacol. 2017, 199, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Feng, X.X.; Li, C.W.; Zhang, X.J.; Chen, Z.W.; Chen, J.N.; Lai, X.P.; Zhang, S.X.; Li, Y.C.; Su, Z.R. The protective effects of the supercritical-carbon dioxide fluid extract of Chrysanthemum indicum against lipopolysaccharide-induced acute lung injury in mice via modulating Toll-like receptor 4 signaling pathway. Mediat. Inflamm. 2014, 2014, 246407. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Barquero, L.; Villegas, I.; Sanchez-Calvo, J.M.; Talero, E.; Sanchez-Fidalgo, S.; Motilva, V.; Alarcon de la Lastra, C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Cornélio Favarin, D.; Martins Teixeira, M.; Lemos de Andrade, E.; de Freitas Alves, C.; Lazo Chica, J.E.; Artério Sorgi, C.; Faccioli, L.H.; Paula Rogerio, A. Anti-Inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediat. Inflamm. 2013, 2013, 164202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Huang, S.S.; Lee, M.M.; Deng, J.S.; Huang, G.J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-kappaB and MAPK signaling pathway in the carrageenan-induced paw edema. Int. Immunopharmacol. 2015, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ozment, T.R. Pulmonary myeloperoxidase activity. Bio-protocol 2015, 3, e851. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Joh, E.H.; Gu, W.; Kim, D.H. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-kappaB and MAPK pathways. Biochem. Pharmacol. 2012, 84, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhu, C.; Liao, Y.; Gao, Y.; Lu, G.; Zhong, W.; Zheng, Y.; Chen, W.; Ci, X. Tenuigenin ameliorates acute lung injury by inhibiting NF-kappaB and MAPK signalling pathways. Respir. Physiol. Neurobiol. 2015, 216, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, R.Q.; Wei, X.G.; Ren, X.M.; Gao, X.Q. Mechanism of TLR-4/NF-kappaB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac. J. Trop. Med. 2016, 9, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xiao, H.T.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Han, X.Q.; Ko, C.H.; Lau, C.B.; Wong, C.K.; Fung, K.P.; Leung, P.C.; Bian, Z.X.; Han, Q.B. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharmacol. 2016, 179, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, G.; Yang, W.; Xu, Y.; Xu, Y.; Huang, X.; Liu, J.; Feng, Y.; Xu, Y.; Liu, B. Hyaluronan ameliorates LPS-induced acute lung injury in mice via Toll-like receptor (TLR) 4-dependent signaling pathways. Int. Immunopharmacol. 2015, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Deng, J.S.; Lin, J.G.; Lee, C.Y.; Huang, G.J. Anti-inflammatory effects of trilinolein from Panax notoginseng through the suppression of NF-kappaB and MAPK expression and proinflammatory cytokine expression. Am. J. Chin. Med. 2014, 42, 1485–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Ho, Y.L.; Hsieh, W.T.; Huang, S.S.; Chang, Y.S.; Huang, G.J. Apigenin-7-glycoside prevents LPS-induced acute lung injury via downregulation of oxidative enzyme expression and protein activation through inhibition of MAPK phosphorylation. Int. J. Mol. Sci. 2015, 16, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Ho, Y.L.; Huang, G.J.; Chang, Y.S. Anti-oxidative and anti-inflammatory effects of Lobelia chinensis in vitro and in vivo. Am. J. Chin. Med. 2015, 43, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chen, C.C.; Huang, C.J.; Sung, P.J.; Huang, S.S.; Kuo, Y.H. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-kappaB and MAPK pathways in mice. J. Agric. Food. Chem. 2014, 62, 5321–5329. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-C.; Huang, S.-S.; Kuo, Y.-H.; Ho, Y.-L.; Yang, C.-S.; Chang, Y.-S.; Huang, G.-J. Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways. Molecules 2017, 22, 573. https://doi.org/10.3390/molecules22040573
Wu K-C, Huang S-S, Kuo Y-H, Ho Y-L, Yang C-S, Chang Y-S, Huang G-J. Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways. Molecules. 2017; 22(4):573. https://doi.org/10.3390/molecules22040573
Chicago/Turabian StyleWu, Kun-Chang, Shyh-Shyun Huang, Yueh-Hsiung Kuo, Yu-Ling Ho, Chang-Syun Yang, Yuan-Shiun Chang, and Guan-Jhong Huang. 2017. "Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways" Molecules 22, no. 4: 573. https://doi.org/10.3390/molecules22040573





