A Study about Regioisomeric Hydroquinones with Multiple Intramolecular Hydrogen Bonding
Abstract
:1. Introduction
2. Results
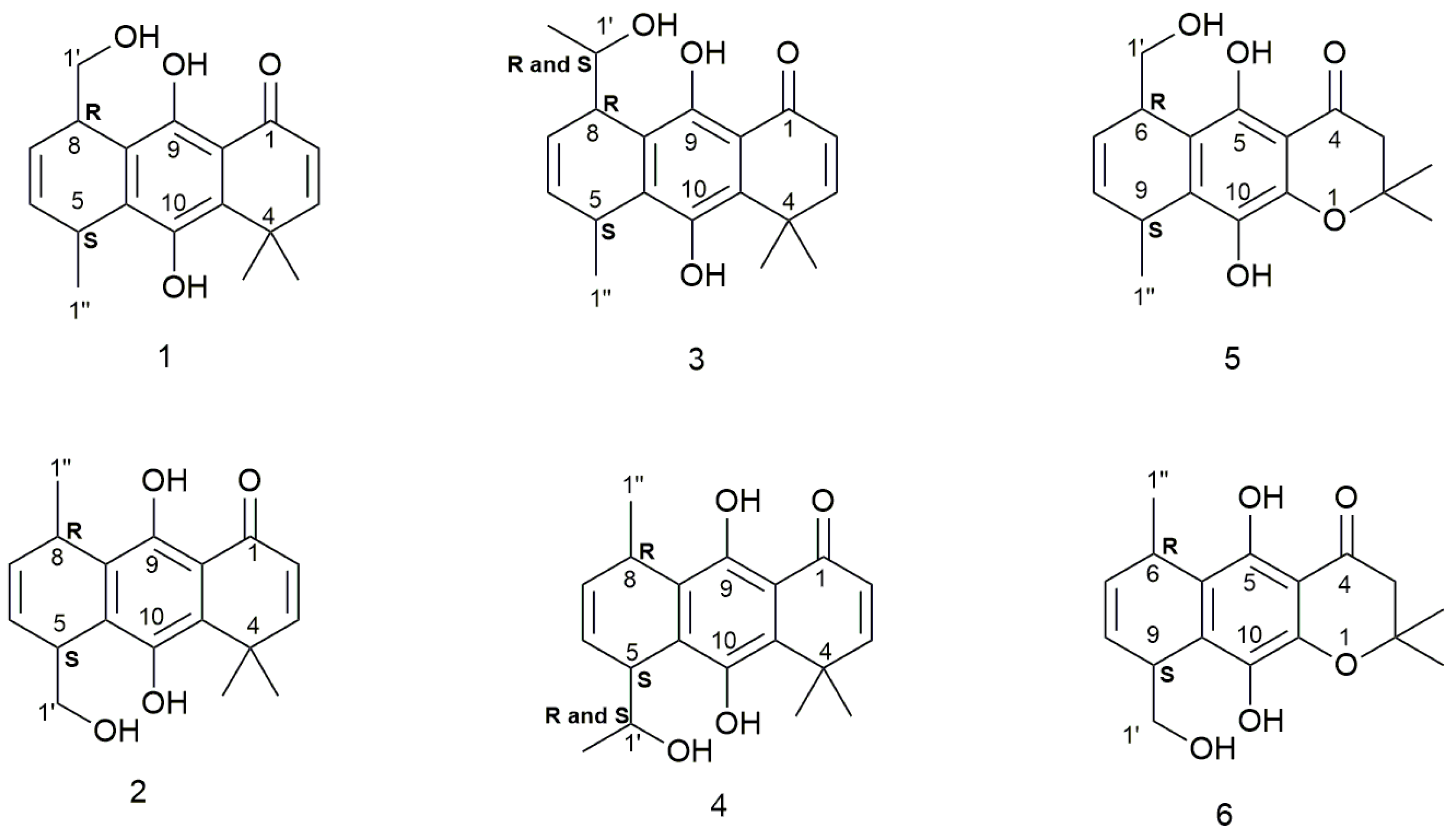
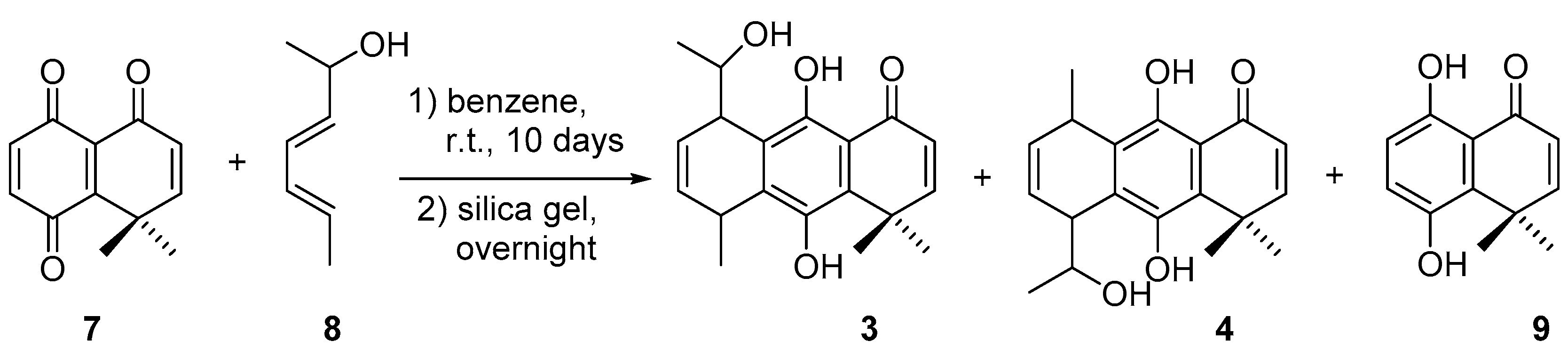
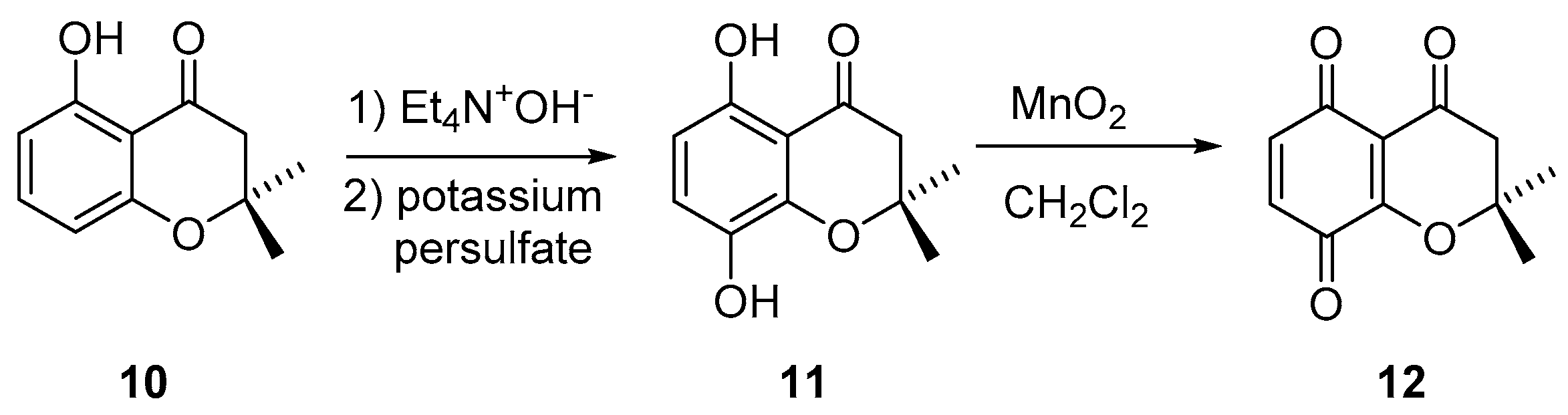
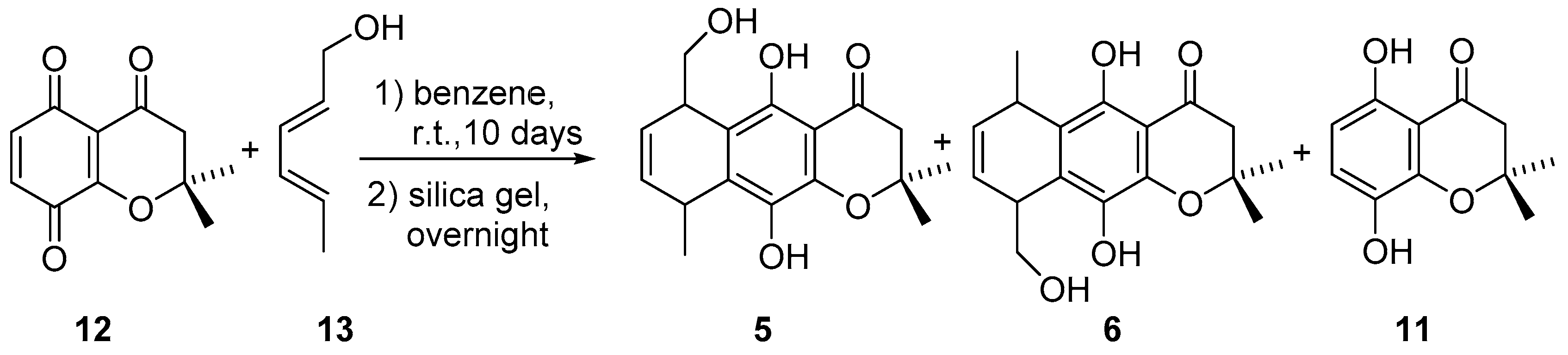
2.1. Synthesis
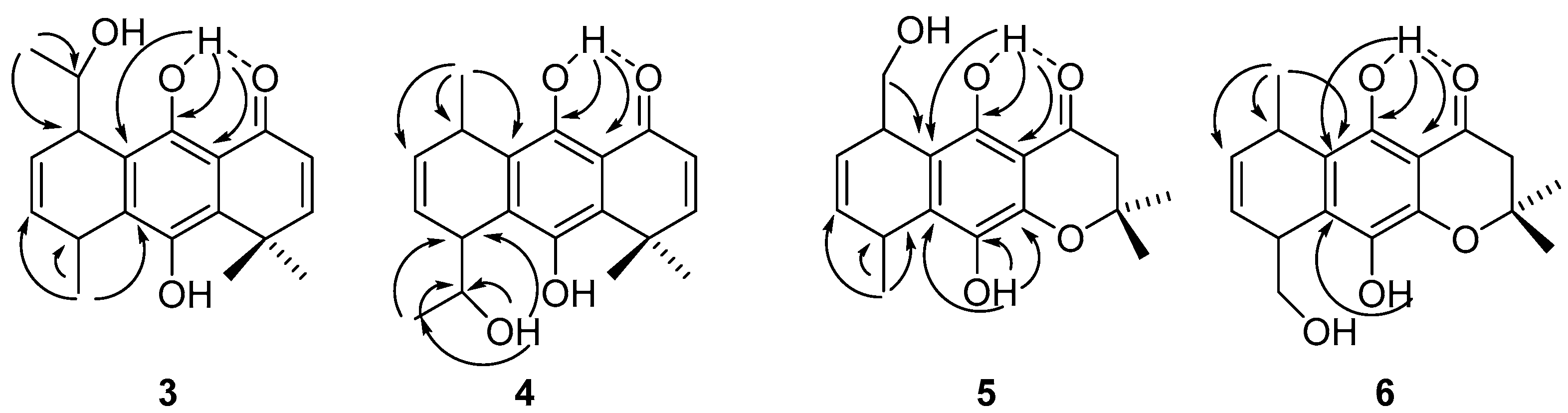
2.2. Structural Assignments
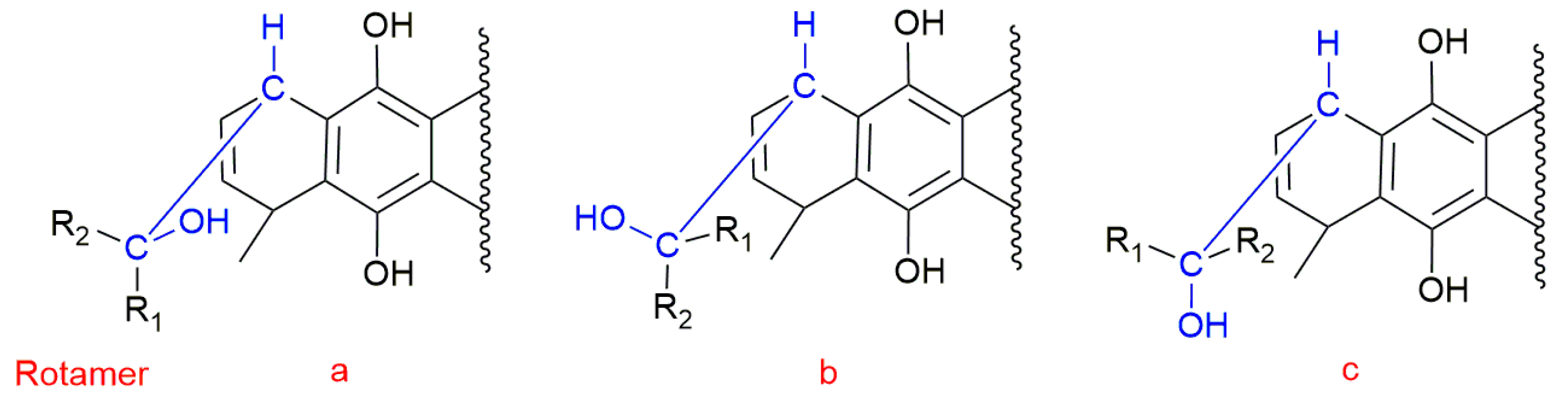
2.3. Conformational Analysis
2.4. NBO and AIM Analysis
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Computational Calculation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grabowski, S.J. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Kojic-Prodic, B.; Molcanov, K. The nature of hydrogen bond: New iinsights into old theories. Acta Chim. Slov. 2008, 55, 692–708. [Google Scholar]
- Kuhn, B.; Mohr, P.; Stahl, M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 2010, 53, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Castellano, R.K. Special issue: Intramolecular hydrogen bonding. Molecules 2014, 19, 15783–15785. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assessment of the presence and strength of H-bonds by means of corrected NMR. Molecules 2016, 21, 1426. [Google Scholar] [CrossRef] [PubMed]
- Bilonda, M.K.; Mammino, L. Intramolecular hydrogen bonds in conformers of quinine and quinidine: An HF, MP2 and DFT study. Molecules 2017, 22, 245. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Competing intramolecular vs. Intermolecular hydrogen bonds in solution. Int. J. Mol. Sci. 2014, 15, 19562–19633. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; Filarowski, A. Some brief notes on theoretical and experimental investigations of intramolecular hydrogen bonding. Molecules 2016, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Tessensohn, M.E.; Webster, R.D. Using voltammetry to measure hydrogen-bonding interactions in non-aqueous solvents: A mini-review. Electrochem. Commun. 2016, 62, 38–43. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Konig, G.M.; Fisch, K.M.; Holler, U.; Jones, P.G.; Wright, A.D. New antioxidant hydroquinone derivatives from the algicolous marine fungus Acremonium sp. J. Nat. Prod. 2002, 65, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Aknin, M.; Dayan, T.L.A.; Rudi, A.; Kashman, Y.; Gaydou, E.M. Hydroquinone antioxidants from the indian ocean tunicate aplidium savignyi. J. Agric. Food Chem. 1999, 47, 4175–4177. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chen, C.Y.; Kuo, Y.H. New sesquiterpene hydroquinones from a taiwanese marine sponge, hippospongia metachromia. J. Nat. Prod. 2001, 64, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.P.J.; Mueller-Harvey, I.; Brandt, E.V.; Ferreira, D. Polyphenols, condensed tannins, and other natural products in onobrychis viciifolia (sainfoin). J. Agric. Food Chem. 2000, 48, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Codina, C. A single extraction step in the quantitative analysis of arbutin in bearberry (arctostaphylos uva-ursi) leaves by high-performance liquid chromatography. Phytochem. Anal. 2001, 12, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone chemistry and toxicity. Toxicol. Appl. Pharm. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Buettner, G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010, 49, 919–962. [Google Scholar] [CrossRef] [PubMed]
- Okun, J.G.; Lummen, P.; Brandt, U. Three classes of inhibitors share a common binding domain in mitochondrial complex i (nadh : Ubiquinone oxidoreductase). J. Biol. Chem. 1999, 274, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Weiss-Lopez, B.; Araya-Maturana, R. Determinants of anti-cancer effect of mitochondrial electron transport chain inhibitors: Bioenergetic profile and metabolic flexibility of cancer cells. Curr. Pharm. Des. 2016, 22, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Córdova-Delgado, M.; Lapier, M.; Orellana-Manzano, A.; Acevedo-Arévalo, L.; Pessoa-Mahana, H.; González-Vivanco, J.M.; Martínez-Cifuentes, M.; Ramírez-Rodróguez, O.; Millas-Vargas, J.P.; et al. Small structural changes on a hydroquinone scaffold determine the complex i inhibition or uncoupling of tumoral oxidative phosphorylation. Toxicol. Appl. Pharmacol. 2016, 291, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using hacat keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.; Mottley, C.; Sinha, B.K.; Kalyanaraman, B.; Mason, R.P. One-electron reduction of daunomycin, daunomycinone, and 7-deoxydaunomycinone by the xanthine/xanthine oxidase system: Detection of semiquinone free radicals by electron spin resonance. J. Am. Chem. Soc. 1987, 109, 4. [Google Scholar] [CrossRef]
- Armendariz-Vidales, G.; Martinez-Gonzalez, E.; Cuevas-Fernandez, H.J.; Fernandez-Campos, D.O.; Burgos-Castillo, R.C.; Frontana, C. The stabilizing role of intramolecular hydrogen bonding in disubstituted hydroxy-quinones. Electrochim. Acta 2013, 110, 628–633. [Google Scholar] [CrossRef]
- Salazar, R.; Vidal, J.; Martínez-Cifuentes, M.; Araya-Maturana, R.; Ramírez-Rodríguez, O. Electrochemical characterization of hydroquinone derivatives with different substituents in acetonitrile. New J. Chem. 2015, 39, 1237–1246. [Google Scholar] [CrossRef]
- Foti, M.C.; Johnson, E.R.; Vinqvist, M.R.; Wright, J.S.; Barclay, L.R.C.; Ingold, K.U. Naphthalene diols: A new class of antioxidants intramolecular hydrogen bonding in catechols, naphthalene diols, and their aryloxyl radicals. J. Org. Chem. 2002, 67, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Olea-Azar, C.; Cavieres, C.; Norambuena, E.; Delgado-Castro, T.; Soto-Delgado, J.; Araya-Maturana, R. Antioxidant properties and free radical-scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones. Bioorg. Med. Chem. 2007, 15, 7058–7065. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W. Molecular mechanisms of action of anticancer agents involving free radical intermediates. Adv. Free Radic. Biol. Med. 1985, 1, 225–264. [Google Scholar] [CrossRef]
- Pedroza, D.A.; De Leon, F.; Varela-Ramirez, A.; Lema, C.; Aguilera, R.J.; Mito, S. The cytotoxic effect of 2-acylated-1,4-naphthohydroquinones on leukemia/lymphoma cells. Bioorg. Med. Chem. 2014, 22, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Li, Z.L.; Wu, J.Y.; Lu, F.J.; Chen, C.H. An oxidative stress mechanism of shikonin in human glioma cells. PLoS ONE 2014, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Loya, S.; Bakhanashvili, M.; Kashman, Y.; Hizi, A. Peyssonol-a and peyssonal-b, 2 novel inhibitors of the reverse transcriptases of human-immunodeficiency-virus type-1 and type-2. Arch. Biochem. Biophys. 1995, 316, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Araya-Maturana, R.; Cardona, W.; Cassels, B.K.; Delgado-Castro, T.; Ferreira, J.; Miranda, D.; Pavani, M.; Pessoa-Mahana, H.; Soto-Delgado, J.; Weiss-Lopez, B. Effects of 9,10-dihydroxy-4,4-dimethyl-5,8-dihydro-1(4H)-anthracenone derivatives on tumor cell respiration. Bioorg. Med. Chem. 2006, 14, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
- Araya-Maturana, R.; Delgado-Castro, T.; Garate, M.; Ferreira, J.; Pavani, M.; Pessoa-Mahana, H.; Cassels, B.K. Effects of 4,4-dimethyl-5,8-dihydroxynaphtalene-1-one and 4,4-dimethyl-5,8-dihydroxytetralone derivatives on tumor cell respiration. Bioorg. Med. Chem. 2002, 10, 3057–3060. [Google Scholar] [CrossRef]
- Urra, F.A.; Martínez-Cifuentes, M.; Pavani, M.; Lapier, M.; Jaña-Prado, F.; Parra, E.; Maya, J.D.; Pessoa-Mahana, H.; Ferreira, J.; Araya-Maturana, R. An ortho-carbonyl substituted hydroquinone derivative is an anticancer agent that acts by inhibiting mitochondrial bioenergetics and by inducing g2/m-phase arrest in mammary adenocarcinoma ta3. Toxicol. Appl. Pharmacol. 2013, 267, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Soto-Delgado, J.; Bahamonde-Padilla, V.; Araya-Maturana, R.; Weiss-Lopez, B.E. On the mechanism of biological activity of hydroquinone derivatives that inhibit tumor cell respiration. A theoretical study. Comp. Theor. Chem. 2013, 1013, 97–101. [Google Scholar] [CrossRef]
- Dobado, J.A.; Gomez-Tamayo, J.C.; Calvo-Flores, F.G.; Martinez-Garcia, H.; Cardona, W.; Weiss-Lopez, B.; Ramirez-Rodriguez, O.; Pessoa-Mahana, H.; Araya-Maturana, R. Nmr assignment in regioisomeric hydroquinones. Magn. Reson. Chem. 2011, 49, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Araya-Maturana, R.; Cassels, B.K.; Delgado-Castro, T.; Valderrama, J.A.; Weiss-Lopez, B.E. Regioselectivity in the diels-alder reaction of 8,8-dimethylnaphthalene-1,4,5(8H)-trione with 2,4-hexadien-1-ol. Tetrahedron 1999, 55, 637–648. [Google Scholar] [CrossRef]
- Sethna, S.M. The elbs persulfate oxidation. Chem. Rev. 1951, 49, 91–101. [Google Scholar] [CrossRef]
- Araya-Maturana, R.; Cassels, B.K.; Delgado-Castro, T.; Hurtado-Guzmán, C.; Jullian, C. Complete assignment of the 13c nmr spectra of a series of 5,8-disubstituted 4,4-dimethylanthracene-1,9,10(4H)-triones. Magn. Reson. Chem. 1999, 37, 312–316. [Google Scholar] [CrossRef]
- Martínez-Cifuentes, M.; Weiss-López, B.E.; Santos, L.S.; Araya-Maturana, R. Intramolecular hydrogen bond in biologically active o-carbonyl hydroquinones. Molecules 2014, 19, 9354–9368. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision a.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. Nbo 6.0; University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Biegler-Konig, F.; Schonbohm, J. Update of the aim 2000-program for atoms in molecules. J. Comp. Chem. 2002, 23, 1489–1494. [Google Scholar]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |
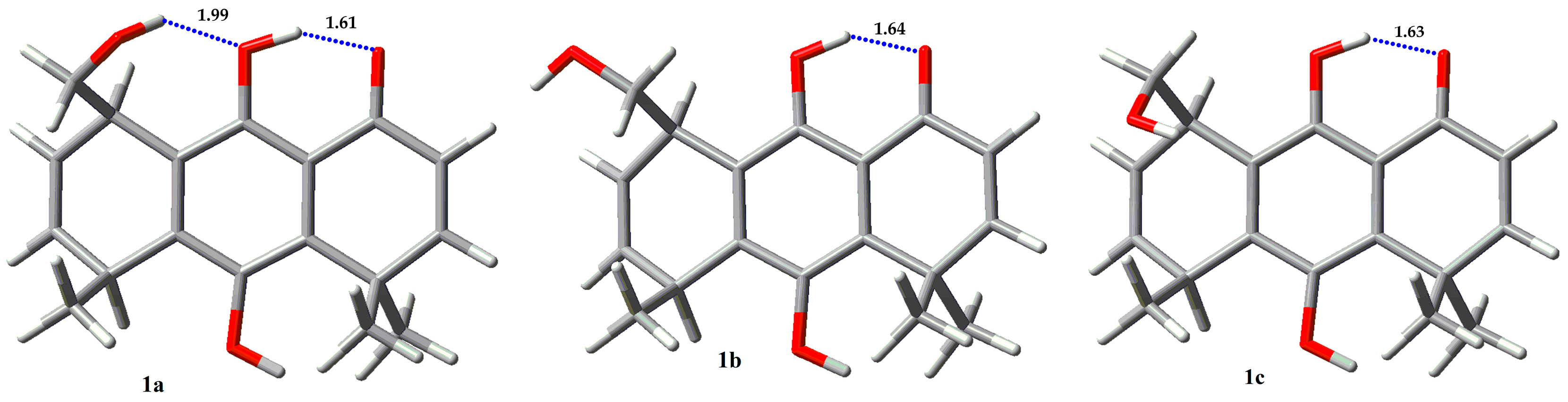
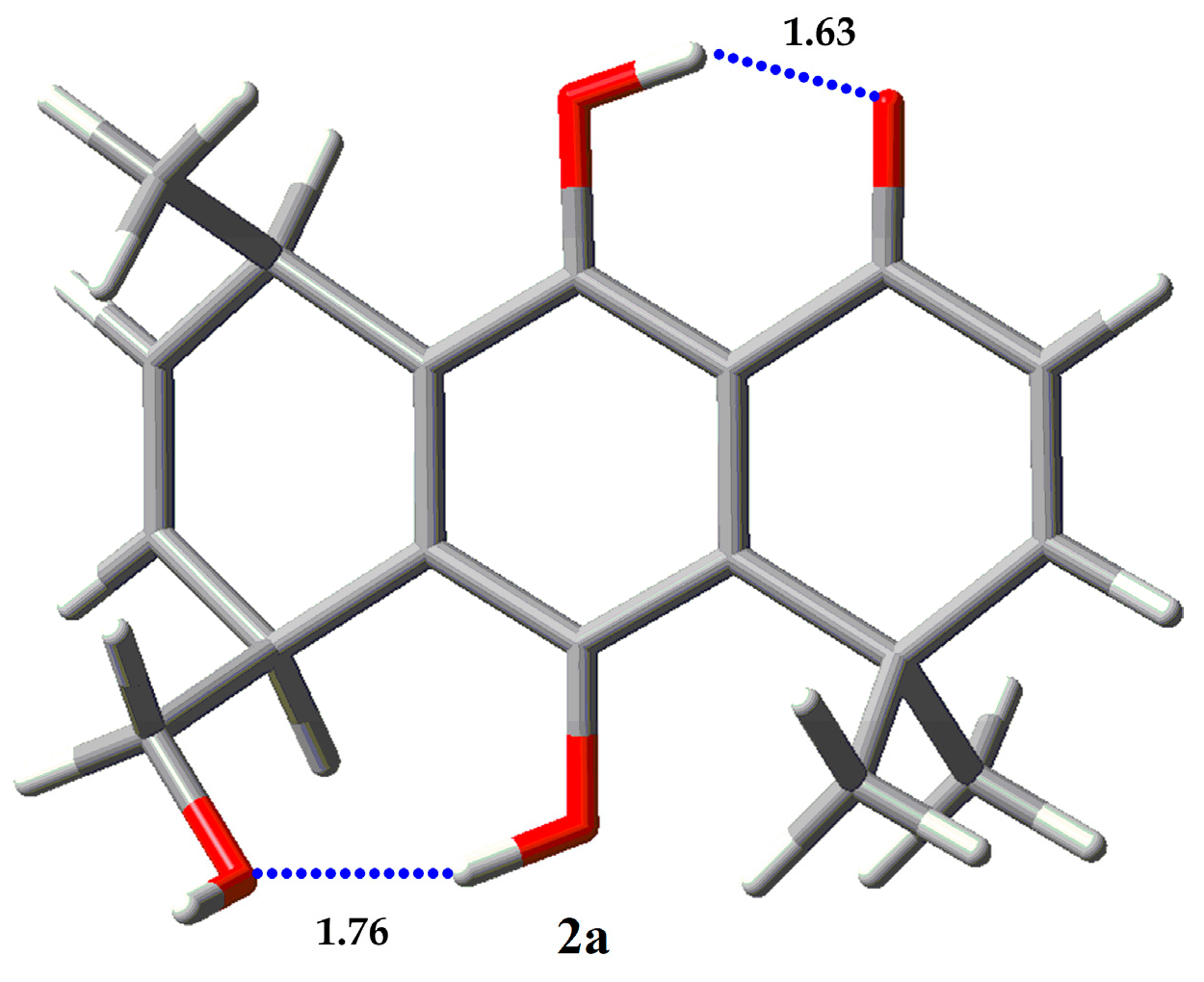
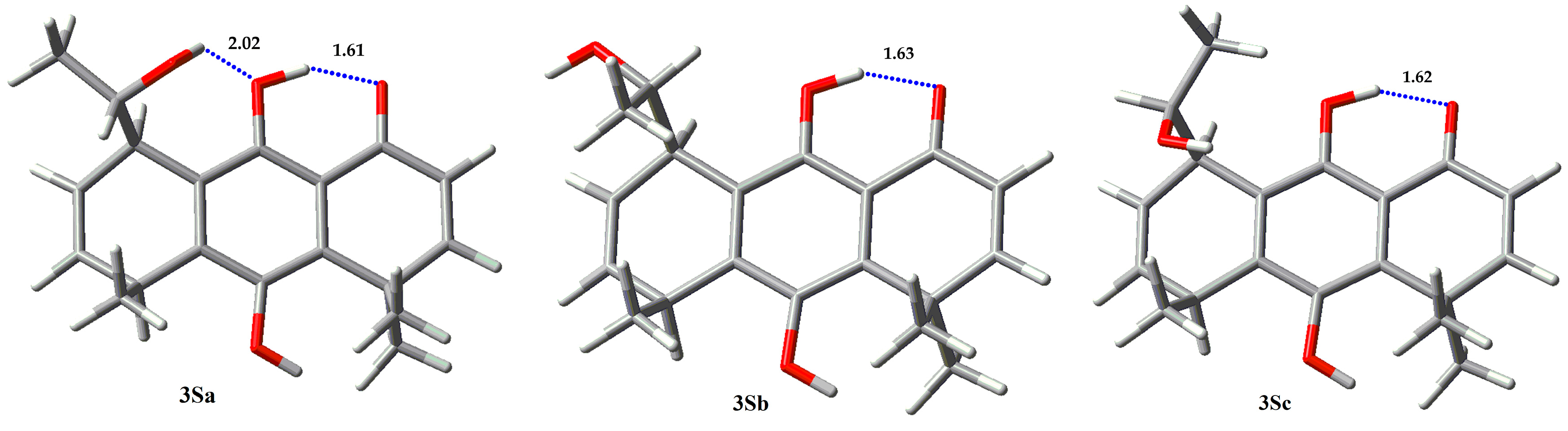
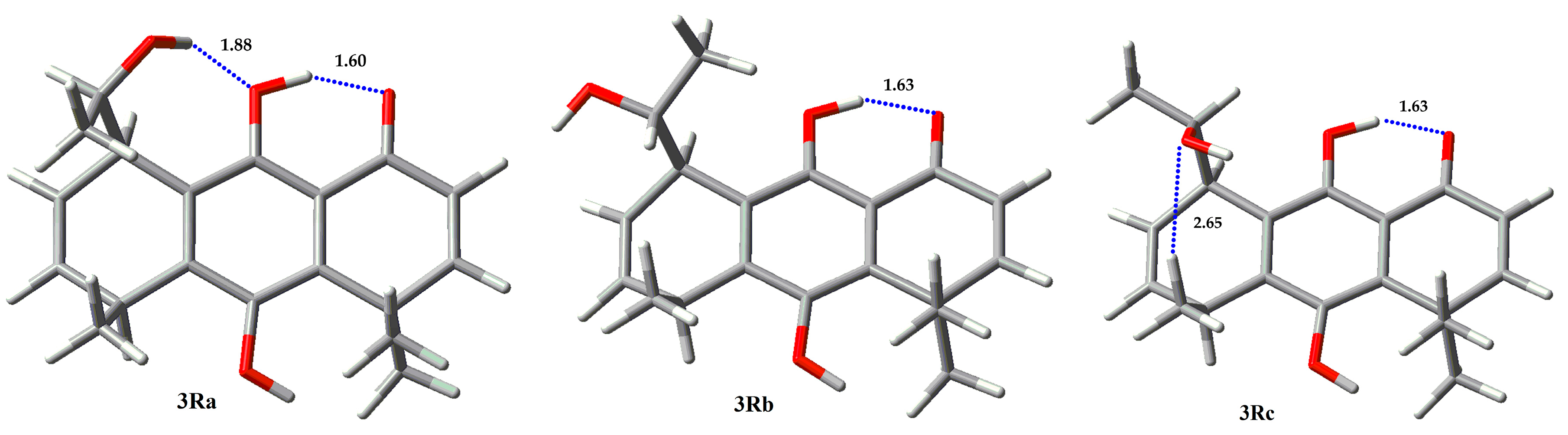
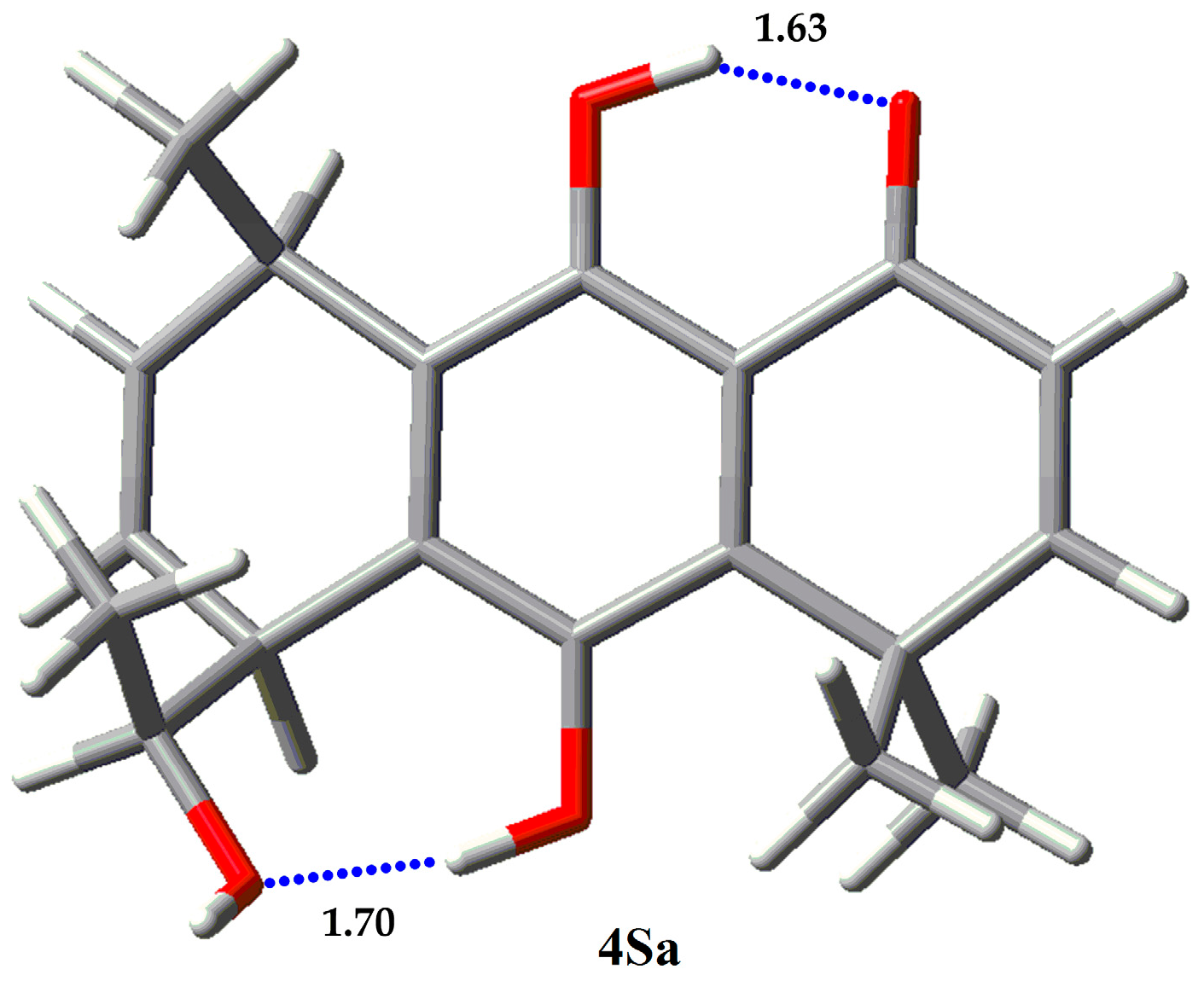


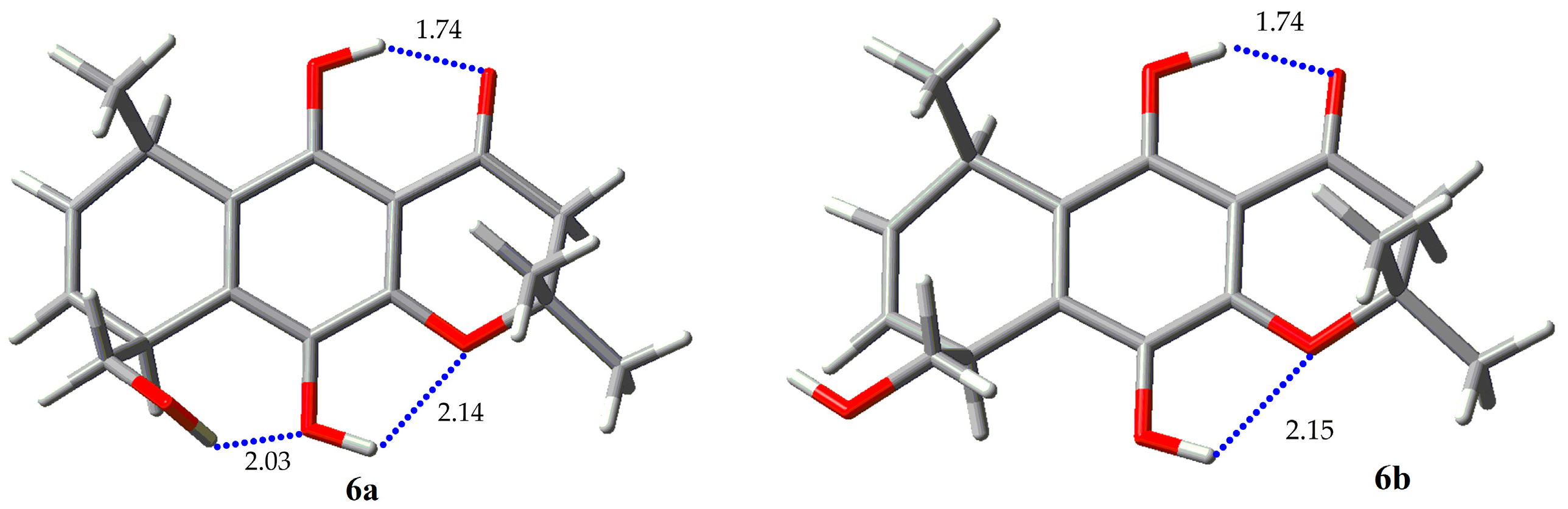
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 1a | 0.0 | 59.41 |
| 1b | 0.70 | 18.12 |
| 1c | 0.57 | 22.48 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 2a | 0 | 99.99 |
| 2b | 7.46 | 0.00 |
| 2c | 5.31 | 0.01 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 3Sa | 0.0 | 95.33 |
| 3Sb | 1.88 | 4.00 |
| 3Sc | 2.88 | 0.67 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 3Ra | 0.87 | 17.85 |
| 3Rb | 1.64 | 4.87 |
| 3Rc | 0 | 77.28 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 4Sa | 0 | 100.00 |
| 4Sb | 6.65 | 0.00 |
| 4Sc | 6.97 | 0.00 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 4Ra | 0 | 99.99 |
| 4Rb | 5.42 | 0.01 |
| 4Rc | 9.19 | 0.00 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 5a | 0.0 | 48.44 |
| 5b | 0.44 | 23.30 |
| 5c | 0.32 | 27.88 |
| Compound | Erel (Kcal/mol) | % |
|---|---|---|
| 6a | 0.0 | 57.18 |
| 6b | 0.24 | 38.14 |
| 6c | 1.48 | 4.69 |
| Molecule | LP O → σ* H-O | ||
|---|---|---|---|
| O9…H-O1′ | O1′…H-O10 | O1…H-O9 | |
| 1a | 5.38 | 33.08 | |
| 1b | 29.64 | ||
| 1c | 30.69 | ||
| 2a | 16.26 | 30.59 | |
| Molecule | Bond | ρBCP | ∇2ρ | G | V | H | EHB |
|---|---|---|---|---|---|---|---|
| 1a | O9…H-O1′ | 0.0232 | −0.0217 | 0.0198 | −0.0450 | −0.0252 | 5.62 |
| O1…H-O9 | 0.0600 | −0.0392 | 0.0510 | −0.1118 | −0.0608 | 19.67 | |
| 1b | O1…H-O9 | 0.0564 | −0.0386 | 0.0483 | −0.1063 | −0.0580 | 18.17 |
| 1c | O1…H-O9 | 0.0576 | −0.0389 | 0.0492 | −0.1081 | −0.0589 | 18.67 |
| 2a | O1′…H-O10 | 0.0217 | −0.0211 | 0.0190 | −0.0433 | −0.0243 | 5.27 |
| O1…H-O9 | 0.0568 | −0.0387 | 0.0486 | −0.1069 | −0.0583 | 18.32 |
| Molecule | LP O → σ* H-O | ||
|---|---|---|---|
| O9…H-O1′ | O1′…H-O10 | O1…H-O9 | |
| 3Sa | 4.61 | 33.24 | |
| 3Ra | 8.50 | 34.92 | |
| 3Rc | 30.73 | ||
| 4Sa | 20.61 | 31.04 | |
| 4Ra | 15.67 | 30.45 | |
| Molecule | Bond | ρBCP | ∇2ρ | G | V | H | E |
|---|---|---|---|---|---|---|---|
| 3Sa | O9…H-O1′ | 0.0221 | −0.0205 | 0.0187 | −0.0425 | −0.0238 | 5.30 |
| O1…H-O9 | 0.0602 | −0.0393 | 0.0512 | −0.1122 | −0.0610 | 19.80 | |
| 3Ra | O9…H-O1′ | 0.0292 | −0.0274 | 0.0259 | −0.0587 | −0.0328 | 7.69 |
| O1…H-O9 | 0.0620 | −0.0396 | 0.0526 | −0.1151 | −0.0625 | 20.55 | |
| 3Rc | O1…H-O9 | 0.0576 | −0.0389 | 0.0492 | −0.1081 | −0.0589 | 18.67 |
| 4Sa | O1′…H-O10 | 0.0440 | −0.0368 | 0.0398 | −0.0888 | −0.0490 | 13.43 |
| O1…H-O9 | 0.0578 | −0.0389 | 0.0494 | −0.1085 | −0.0591 | 18.76 | |
| 4Ra | O1′…H-O10 | 0.0385 | −0.0344 | 0.0352 | −0.0790 | −0.0438 | 11.33 |
| O1…H-O9 | 0.0571 | −0.0386 | 0.0487 | −0.1071 | −0.0584 | 18.45 |
| Molecule | LP O → σ* H-O | |||
|---|---|---|---|---|
| O5…H-O1′ | O4…H-O5 | O1…H-O10 | O10…H-O1′ | |
| 5a | 4.41 | 21.57 | 1.07 | |
| 5b | 19.53 | 1.09 | ||
| 5c | 19.93 | 1.10 | ||
| 6a | 19.86 | 1.16 | 4.40 | |
| 6b | 19.88 | 1.06 | ||
| Molecule | Bond | ρBCP | ∇2ρ | G | V | H | E |
|---|---|---|---|---|---|---|---|
| 5a | O5…H-O1′ | 0.0216 | −0.0202 | 0.0183 | −0.0417 | −0.0234 | 5.16 |
| O4…H-O5 | 0.0460 | −0.0338 | 0.0385 | −0.0855 | −0.0470 | 13.56 | |
| O1…H-O10 | - | - | - | - | - | - | |
| 5b | O4…H-O5 | 0.0436 | −0.0331 | 0.0367 | −0.0817 | −0.0450 | 12.65 |
| O1…H-O10 | - | - | - | - | - | - | |
| 5c | O4…H-O5 | 0.0441 | −0.0332 | 0.0371 | −0.0825 | −0.0454 | 12.83 |
| O1…H-O10 | - | - | - | - | - | - | |
| 6a | O4…H-O5 | 0.0470 | −0.0346 | 0.0397 | −0.0881 | −0.0484 | 14.03 |
| O1…H-O10 | - | - | - | - | - | - | |
| O10…H-O1′ | 0.0350 | −0.0316 | 0.0314 | −0.0707 | −0.0393 | 9.82 | |
| 6b | O4…H-O5 | 0.0441 | −0.0333 | 0.0371 | −0.0825 | −0.0454 | 12.83 |
| O1…H-O10 | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cifuentes, M.; Cardona, W.; Saitz, C.; Weiss-López, B.; Araya-Maturana, R. A Study about Regioisomeric Hydroquinones with Multiple Intramolecular Hydrogen Bonding. Molecules 2017, 22, 593. https://doi.org/10.3390/molecules22040593
Martínez-Cifuentes M, Cardona W, Saitz C, Weiss-López B, Araya-Maturana R. A Study about Regioisomeric Hydroquinones with Multiple Intramolecular Hydrogen Bonding. Molecules. 2017; 22(4):593. https://doi.org/10.3390/molecules22040593
Chicago/Turabian StyleMartínez-Cifuentes, Maximiliano, Wilson Cardona, Claudio Saitz, Boris Weiss-López, and Ramiro Araya-Maturana. 2017. "A Study about Regioisomeric Hydroquinones with Multiple Intramolecular Hydrogen Bonding" Molecules 22, no. 4: 593. https://doi.org/10.3390/molecules22040593






