Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings
Abstract
:1. Introduction
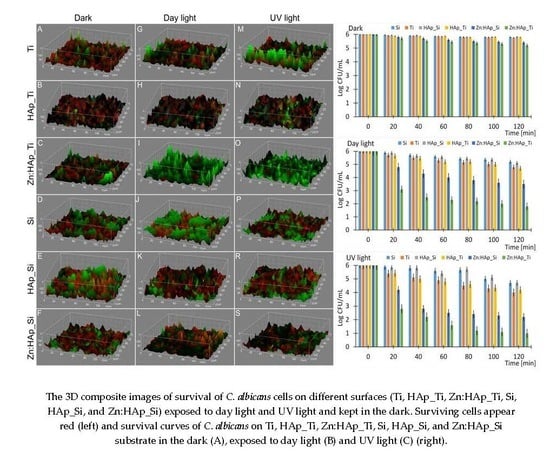
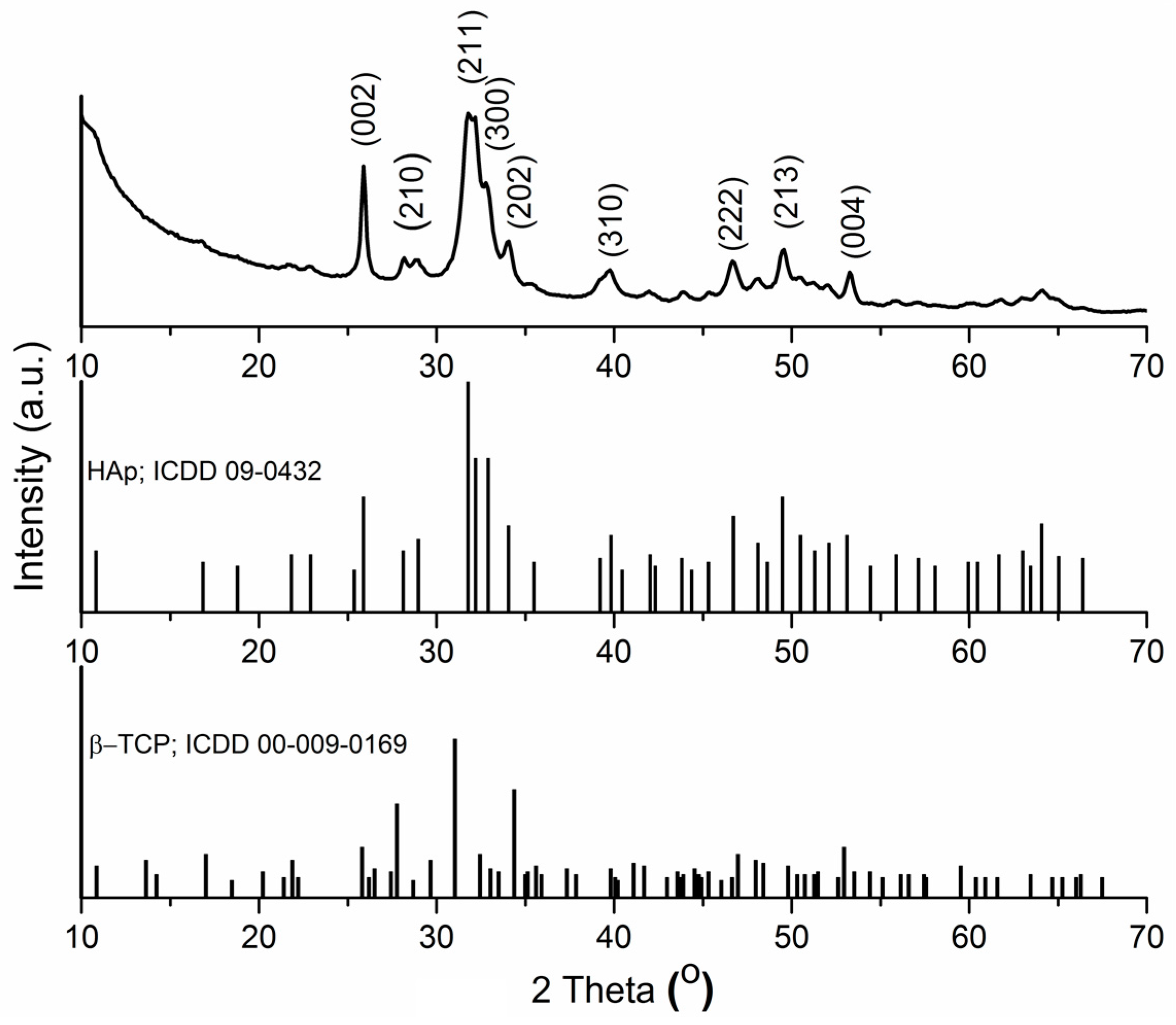
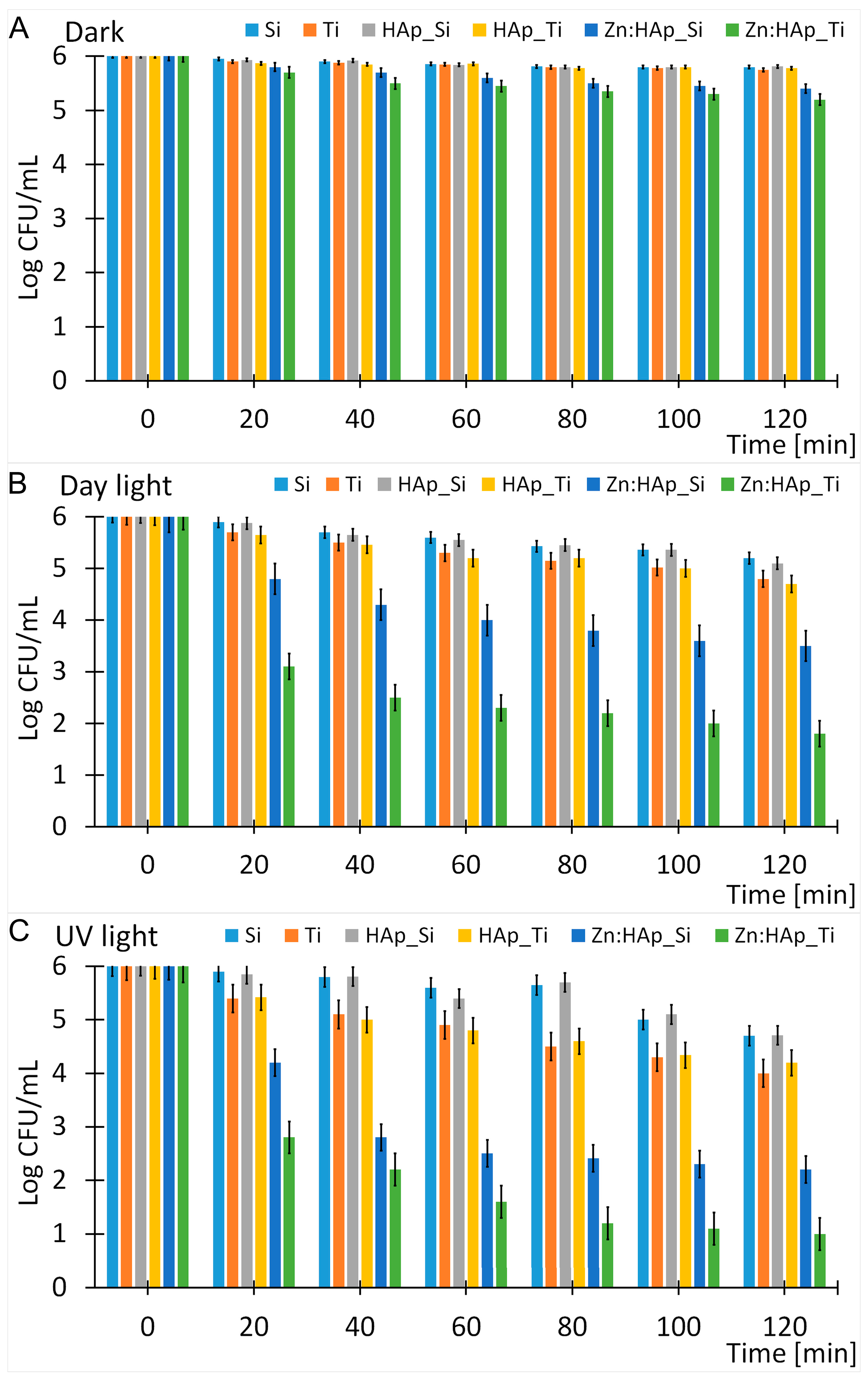
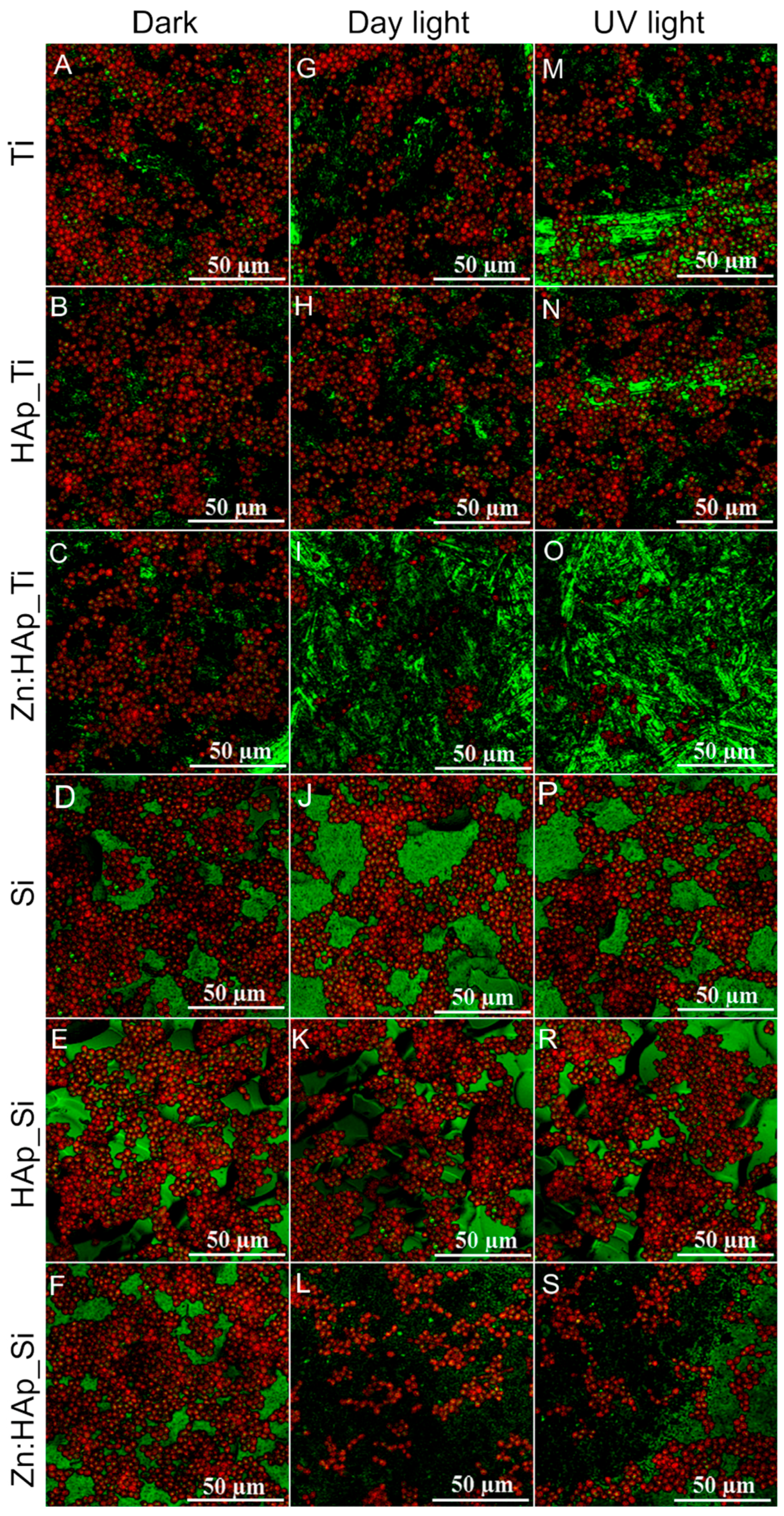
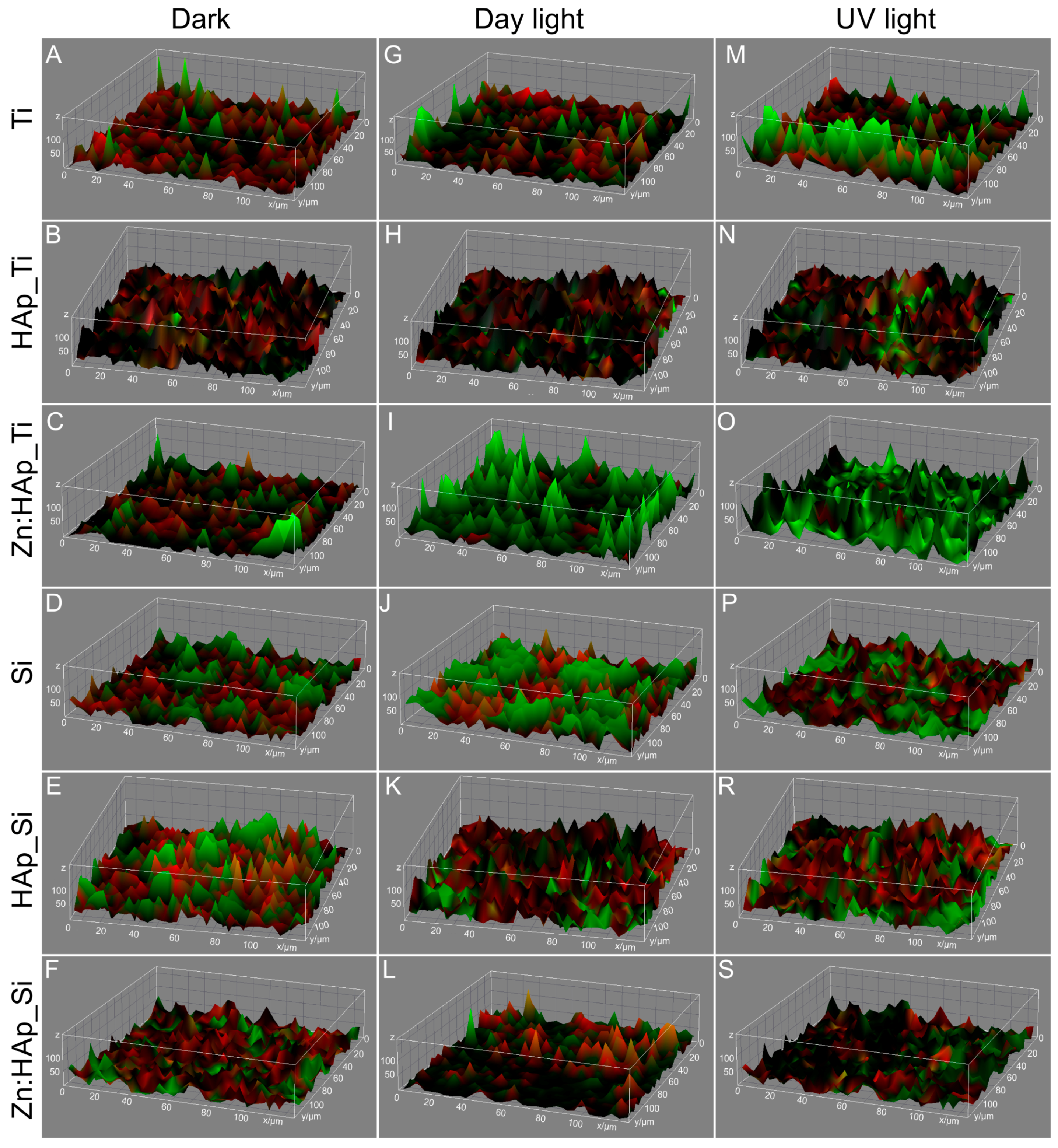
2. Results and Discussion
3. Materials and Methods
3.1. Thin Layer of Zinc-Doped Hydroxyapatite
3.2. Structural and Morphologycal Characterizations
3.3. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometer (LA-ICP-MS) Analysis
3.4. In Vitro Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, K.; Xie, Y.; Ao, H.; Huang, L.; Ji, H.; Zheng, X. The enhanced bactericidal effect of plasma sprayed zinc-modified calcium silicate coating by the addition of silver. Ceram. Int. 2013, 39, 7895–7902. [Google Scholar] [CrossRef]
- Darouiche, R.O. Current concepts—treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Samani, S.; Hossainalipour, S.M.; Tamizifar, M.; Rezaie, H.R. In vitro antibacterial evaluation of sol–gelderived Zn-, Ag-, and (Zn + Ag)-doped hydroxyapatite coatings against methicillin-resistant Staphylococcus aureus. J. Biomed. Mater. Res. A 2013, 101A, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Z.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Millar, M. Microbial biofilms and clinical implants. In Surfaces and Interfaces for Biomaterials; Vadgama, P., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 619–639. [Google Scholar]
- Higashi, J.M.; Marchant, R.E. Implant infections. In Handbook of Biomaterials Evaluation, Scientific, Technical and Clinical Testing of Implant Materials; von Recum, A.F., Ed.; Taylor & Francis: Philadelphia, PA, USA, 1998; pp. 493–507. [Google Scholar]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Sm:HAp Nanopowders Present Antibacterial Activity against Enterococcus faecalis. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Popa, C.L.; Ciobanu, C.S. Synthesis and characterization of fluorescent hydroxyapatite. Rom. Rep. Phys. 2016, 68, 1170–1177. [Google Scholar]
- Vernè, E.; Nunzio, S.D.; Bosetti, M.; Appendino, P.; Brovarone, C.V.; Maina, G.; Cannas, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Best, S.M.; Bonfield, W.; Brooks, R.A.; Rushton, N.; Jayasinghe, S.N.; Edirisinghe, M.J. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Norhidayu, D.; Sopyan, I.; Ramesh, S. Development of Zinc Doped Hydroxyapatite for Bone Implant Applications. ICCBT 2008, F 24, 257–270. [Google Scholar]
- Groza, A.; Ciobanu, C.S.; Popa, C.L.; Iconaru, S.L.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural Properties and Antifungal Activity against Candida albicans Biofilm of Different Composite Layers Based on Ag/Zn Doped Hydroxyapatite-Polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef]
- Iqbal, N.; Rafiq, M.; Kadir, A.; Mahmood, N.H.; Salim, N.; Froemming, G.R.A.; Balaji, H.R.; Kamarul, T. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bernard, S.; Xue, W.; Bose, S. Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 Dopants. J. Am. Ceram. Soc. 2006, 89, 2675–2688. [Google Scholar] [CrossRef]
- Miao, S.; Cheng, K.; Weng, W.; Du, P.; Shen, G.; Han, G.; Yan, W.; Zhang, S. Fabrication and evaluation of Zn containing fluoridated hydroxyapatite layer with Zn release ability. Acta Biomater. 2008, 4, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, J.; Møller-Madsen, B.; Thomsen, J.S.; Danscher, G.; Mosekilde, L.I. The positive effects of zinc on skeletal strength in growing rats. Bone 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Hall, S.L.; Dimai, H.P.; Farley, J.R. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif. Tissue. Int. 1999, 64, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, J.M.; Xie, Y.T.; Huang, L.P.; Ye, X.J.; Zheng, X.B. Chemical stability and antimicrobial activity of plasma sprayed bioactive Ca2Zn-Si2O7 coating. J. Mater. Sci. Mater. Med. 2011, 22, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, Y.T.; Huang, L.P.; Ji, H.; Zheng, X.B. Antibacterial mechanism of plasma sprayed Ca2ZnSi2O7 coating against Escherichia coli. J. Mater. Sci.: Mater. Med. 2013, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Knowles, J.C.; Salih, V.; Kim, H.E. Hydroxyapatite and flour-hydroxyapatite layered film on titanium processed by a sol-gel route for hard-tissue implants. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2004, 71B, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and Biological Assessment of Zinc Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Antimicrobial products—Test for antimicrobial activity and efficacy. Japanese Industrial Standard JIS Z 2801:2000. Available online: http://zibajp.com/H.G/HG_Downloads/Antimicrobial%20products-Test%20for%20antimicrobial%20activity%20and%20efficacy.pdf (assessed on 8 April 2017).
- LeGeros, R.Z.; Tung, M.S. Chemical stability of carbonate- and fluoride-containing apatites. Caries. Res. 1983, 17, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mat. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, X.; Huang, Y.; Yan, Y.; Pang, X. In vitro cytocompatibility and corrosion resistance of zinc-doped hydroxyapatite coatings on a titanium substrate. J. Mater. Sci. 2015, 50, 189–202. [Google Scholar] [CrossRef]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob. Agents. Ch. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.H.; da Silva Ferreira, M.E.; dos Reis Marques, E.; Savoldi, M.; Perlin, D.; Park, S.; Godoy Martinez, P.C.; Goldman, M.H.S.; Colombo, A.L. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV infected patients in Brazil. Diagn. Micr. Infec. Dis. 2004, 50, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Image Processing and Analysis in Java (ImageJ). Available online: http://imagej.nih.gov/ij (accessed on 2 July 2016).
- Zajicova, V.; Exnar, P.; Stanova, I. Properties of hybrid coatings based on 3-trimethoxysilylpropyl methacrylate. Ceramics–Silikáty 2011, 55, 221–227. [Google Scholar]
- Khan, M.F.; Hameedullah, M.; Ansari, A.H.; Ahmad, E.; Lohani, M.B.; Khan, R.H.; Alam, M.M.; Khan, W.; Husain, F.M.; Ahmad, I. Flower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal properties. Int. J. Nanomed. 2014, 9, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Phaechamuda, T.; Mahadlek, J.; Aroonrerk, N.; Choopun, S.; Charoenteeraboon, J. Antimicrobial activity of ZnO-doxycycline hyclate thermosensitive gel. Science Asia 2012, 38, 64–74. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles - an antimicrobial study. Sci. Tech. Adv. Mater. 2008, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Motelica-Heino, M.; Donard, O.F.X. Comparison of UV and IR laser ablation ICP-MS on silicate reference materials and implementation of normalisations factors for quantitative measurements. Geostandards Newslett. 2001, 25, 345–359. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings. Molecules 2017, 22, 604. https://doi.org/10.3390/molecules22040604
Iconaru SL, Prodan AM, Buton N, Predoi D. Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings. Molecules. 2017; 22(4):604. https://doi.org/10.3390/molecules22040604
Chicago/Turabian StyleIconaru, Simona Liliana, Alina Mihaela Prodan, Nicolas Buton, and Daniela Predoi. 2017. "Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings" Molecules 22, no. 4: 604. https://doi.org/10.3390/molecules22040604