Computational Identification of Antibody Epitopes on the Dengue Virus NS1 Protein
Abstract
:1. Introduction
2. Results
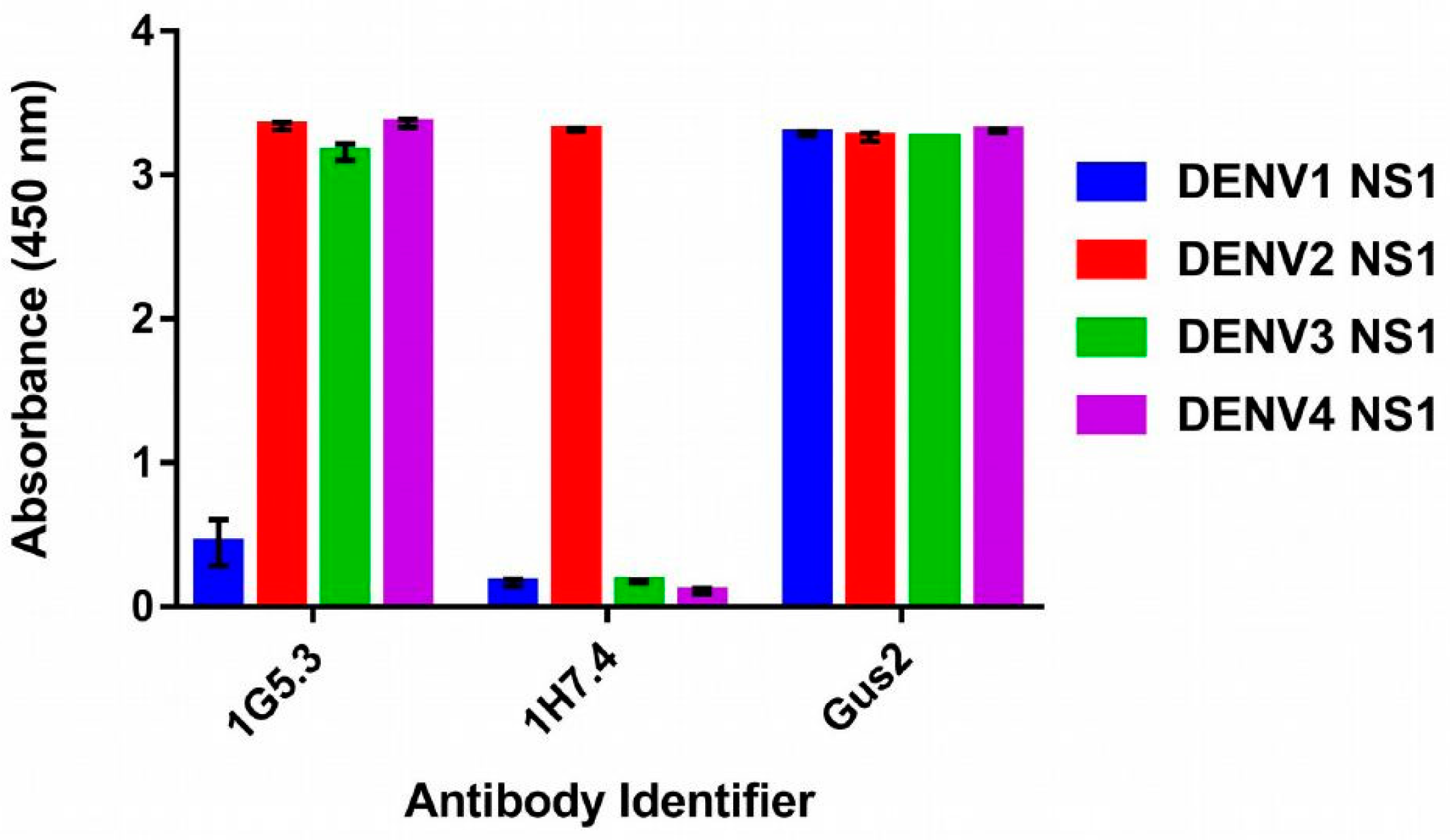
2.1. Specificity of Mabs against DENV
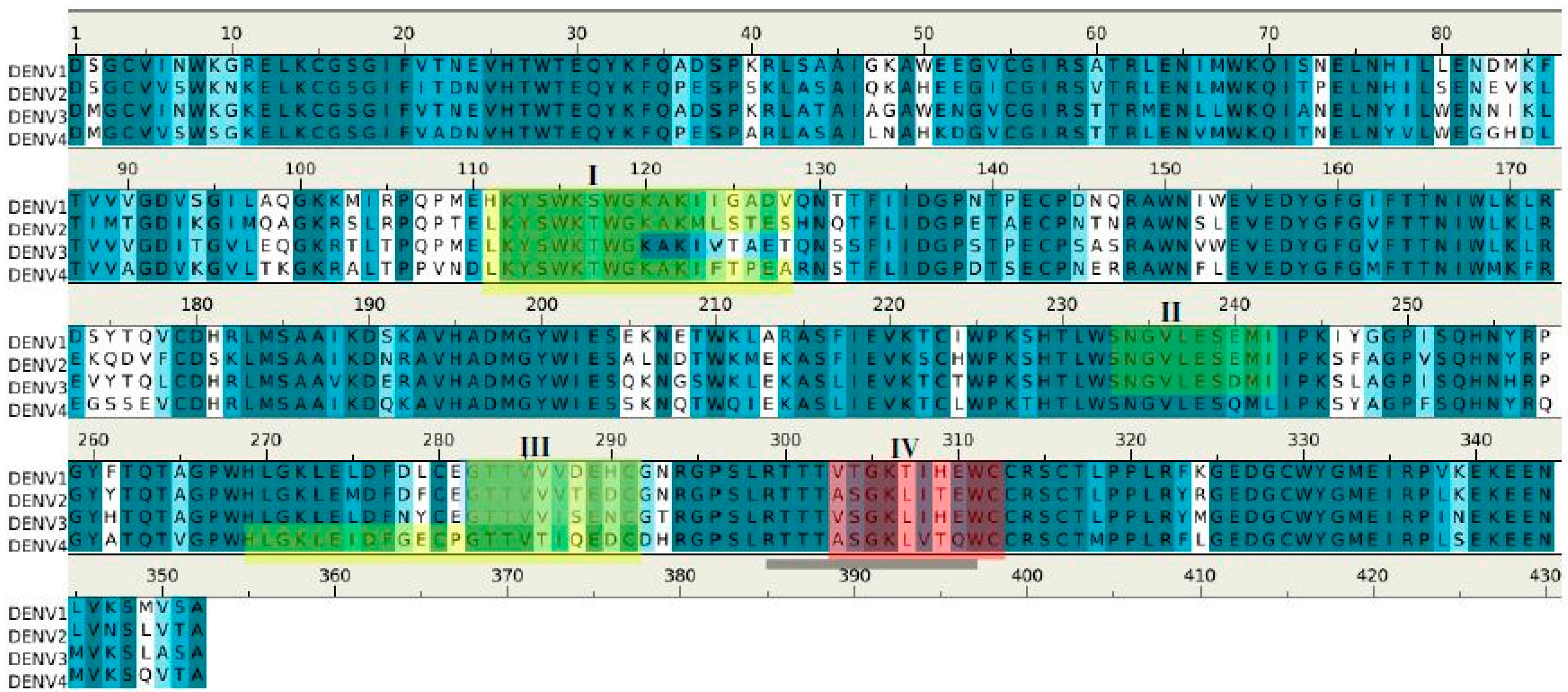
2.2. Recognition of DENV1–4 NS1 by Antibody 1H7.4
2.3. Recognition of DENV NS1 to Antibody 1G5.3
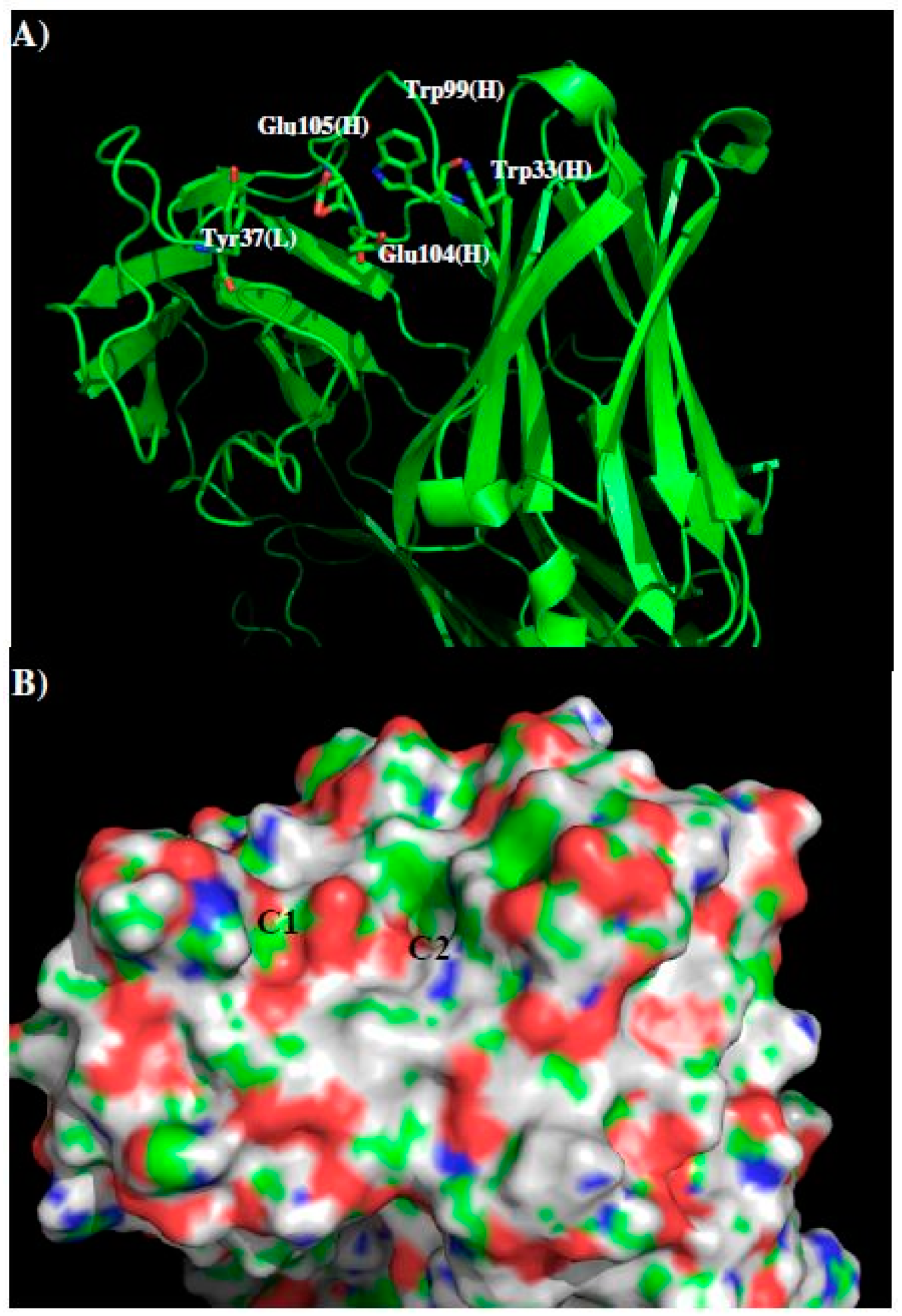
2.4. Prediction of Epitopes of DENVs to Antibody GUS2
3. Materials and Methods
3.1. Antibodies
3.2. Enzyme Linked Immunosorbent Assay
3.3. Homology Modeling of the Antibodies
3.4. MCSS of Functional Groups
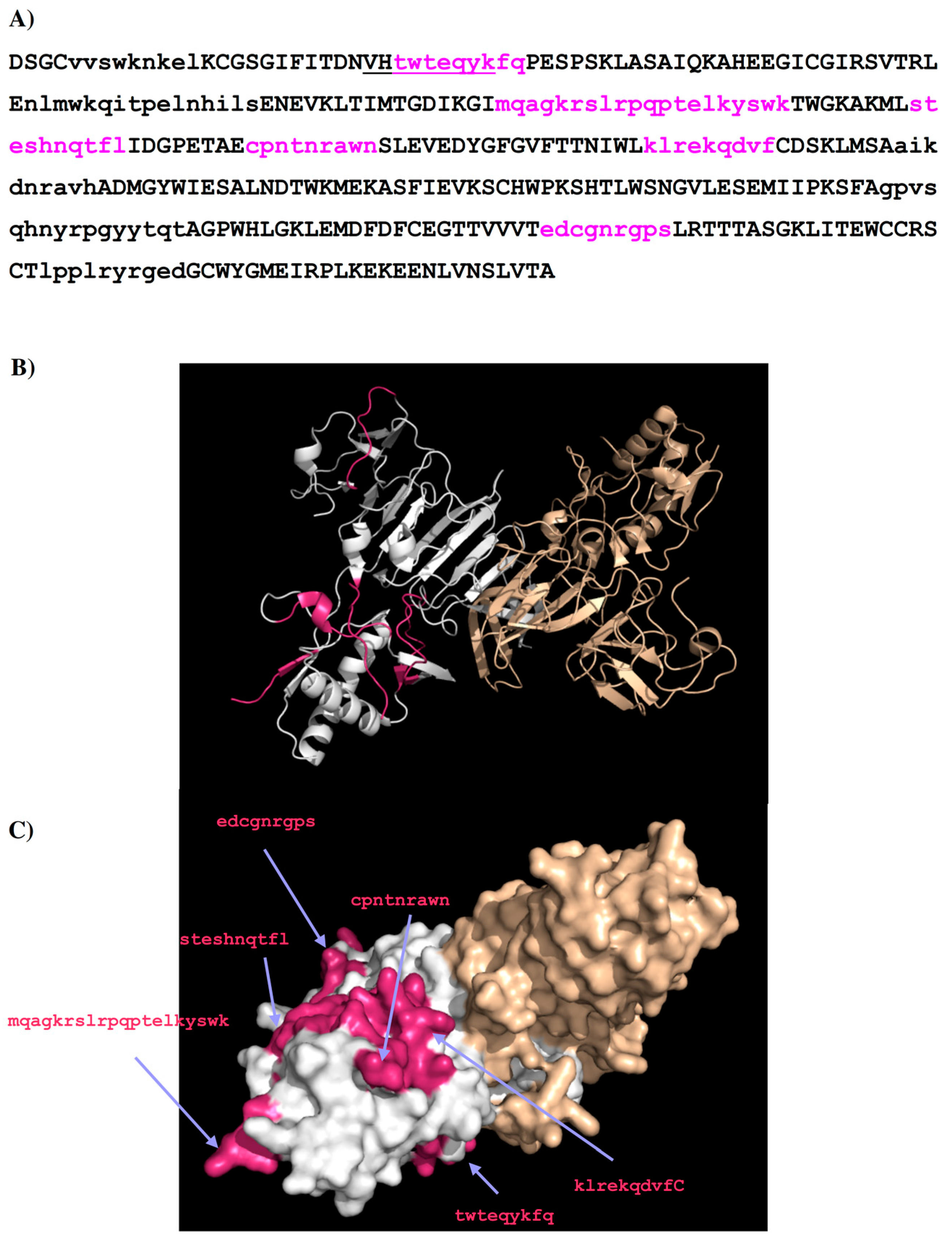
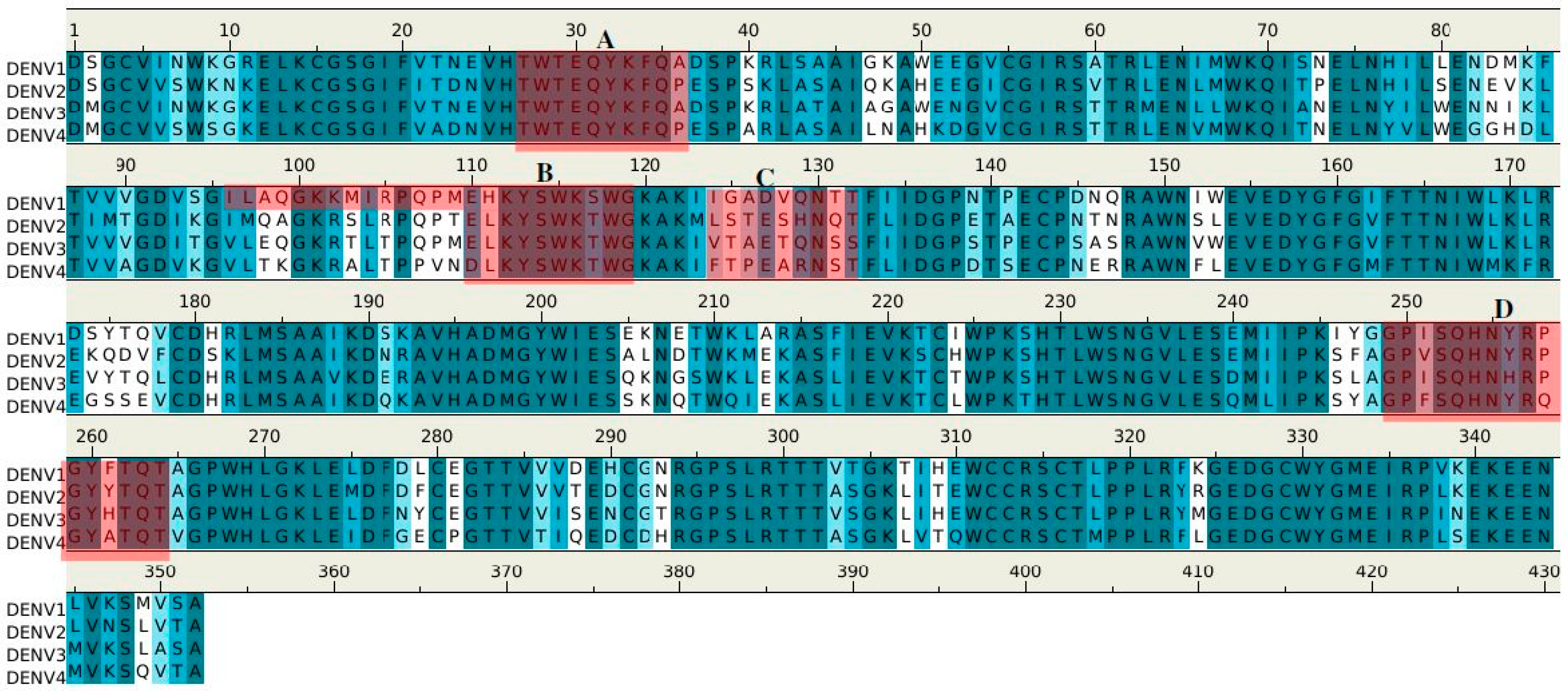
3.5. Identification of Sequence Pattern
3.6. Search for Epitopes Based on the Sequence Pattern
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue; Imperial College Press: London, UK, 2008. [Google Scholar]
- Peeling, R.W.; Smith, P.G.; Bossuyt, P.M. A guide for diagnostic evaluations. Nat. Rev. Microbiol. 2008, 8, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; Diniz, M.O.; Cariri, F.A.; Rodrigues, J.F.; Bizerra, R.S.P.; Goncalves, A.J.S.; Alves, A.M.d. Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine 2012, 30, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Crooks, A.J.; Lee, J.M.; Easterbrook, L.M.; Timofeev, A.V.; Stephenson, J.R. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J. Gen. Virol. 1994, 75 Pt. 12, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K. Monoclonal antibodies that bind to common epitopes on the dengue virus type 2 nonstructural-1 and envelope glycoproteins display weak neutralizing activity and differentiated responses to virulent strains: implications for pathogenesis and vaccines. Clin. Vaccine Immunol. 2008, 15, 549. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, H.; Sadeghizadeh, M.; Mohammadipour, M. In silico analysis of an envelope domain III-based multivalent fusion protein as a potential dengue vaccine candidate. Clin. Exp. Vaccine Res. 2016, 5, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Legge, F.S.; Zeng, X.; Treutlein, H.R.; Zeng, J. Antibody Recognition of Shiga Toxins Stxs, Computational Identification of the Epitopes of Stx2 Subunit A to the Antibodies 11E10 and S2C4. PLoS ONE 2014, 9, e88191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, X.; Zhang, L.; Peng, H.; Jiao, Y.; Zeng, J.; Treutlein, H.R. Computational identification of epitopes in the glycoproteins of novel bunyavirus SFTS virus, recognized by a human monoclonal antibody MAb 4–5. J. Comput. Aided Mol. Des. 2013, 27, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, A. Computational combinatorial ligand design application to human alpha-thrombin. J. Comput. Aided Mol. Des. 1996, 10, 372–396. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, A.; Karplus, M. Acid and thermal denaturation of barnase investigated by molecular dynamics simulations. J. Mol. Biol. 1995, 252, 672–708. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J. Mini-review computational structure-based design of inhibitors that target protein surfaces. Comb. Chem. High Throughput Screen. 2000, 3, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Nheu, T.; Zorzet, A.; Catimel, B.; Nice, E.; Maruta, H.; Burgess, A.W.; Treutlein, H.R. Design of inhibitors of Ras–Raf interaction using a computational combinatorial algorithm. Protein Eng. 2001, 14, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Treutlein, H.R. A method for computational combinatorial peptide design of inhibitors of Ras protein. Protein Eng. 1999, 12, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Treutlein, H.R.; Rudy, G.B. Predicting sequences and structures of MHC-binding peptides a computational combinatorial approach. J. Comput. Aided Mol. Des. 2001, 15, 573–586. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, Y.; Fang, D.-Y.; Tang, Y.-X.; Jiang, L.F.; Zhou, J.-M. Selection and identification of B-cell epitope on NS1 protein of dengue virus type 2. Virus Res. 2010, 150, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.; Young, P.; Miles, M. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch. Virol. 1994, 137, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.K.; DelProposto, J.; Ogata, C.W.; Skiiotis, G.; Kuhn, R.J.; Smith, J.L.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.; Young, P. Production of dimer-specific and dengue virus group cross-reactive mouse monoclonal antibodies to the dengue 2 virus non-structural glycoprotein NS1. J. Gen. Virol. 1991, 72, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Brocks, B.; Garin-Chesa, P.; Behrle, E.; Park, J.E.; Rettig, W.J.; Pfizenmaier, K.; Moosmayer, D. Species-crossreactive scFv against the tumor stroma marker “fibroblast activation protein” selected by phage display from an immunized FAP-/- knock-out mouse. Mol. Med. 2001, 7, 461–469. [Google Scholar] [PubMed]
- Kjer-Nielsen, L.; Dunstone, M.A.; Kostenko, L.; Ely, L.K.; Beddoe, T.; Mifsud, N.A.; Purcell, A.W.; Brooks, A.G.; McCluskey, J.; Rossjohn, J. Crystal structure of the human T cell receptor CD3 epsilon gamma heterodimer complexed to the therapeutic mAb OKT3. Proc. Natl. Acad. Sci. USA 2004, 101, 7675–7680. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Singh, K.; Su, H.P.; Klein, M.M.; Stowers, A.W.; Saul, A.J.; Long, A.C.; Garboczi, D.N. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 2006, 13, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A. Structure of Fab fragment of malaria transmission blocking antibody 2A8 against P. vivax P25 protein. Int. J. Biol. Macromol. 2012, 50, 153. [Google Scholar] [CrossRef] [PubMed]
- Barbey-Martin, C.; Gigant, B.; Bizebard, T.; Calder, L.; Wharton, S.; Skehell, J.J.; Knossow, M. An antibody that prevents the hemagglutinin low pH fusogenic transition. Virology 2002, 294, 70–74. [Google Scholar] [CrossRef] [PubMed]
- McCraw, D.M.; O’Donnell, J.K.; Taylor, K.A.; Stagg, S.M.; Chapman, M.S. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology 2012, 431, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhu, J.; Huang, P.S.; Wang, J.H.; Pullen, N.; Springer, T.A. Domain 1 of mucosal addressing cell adhesion molecule has an I1-set fold and a flexible integrin-binding loop. J. Biol. Chem. 2013, 288, 6284–6294. [Google Scholar] [CrossRef] [PubMed]
- Raposo, B.; Dobritzsch, D.; Ge, C.; Ekman, D.; Xu, B.; Lindh, I.; Forster, M.; Usysal, H.; Nandakumar, K.S.; Schneider, G.; et al. Epitope-specific antibody response is controlled by immunoglobulin VH, polymorphisms. J. Exp. Med. 2014, 211, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Hernandez-Guzman, F.G.; Luo, W.; Wang, X.; Ferrone, S.; Ghosh, D. Structural basis of antigen mimicry in a clinically relevant melanoma antigen system. J. Biol. Chem. 2005, 280, 41546–41552. [Google Scholar] [CrossRef] [PubMed]
- Lescar, J.; Stouracova, R.; Riottot, M.-M.; Chitarra, V.; Brynda, J.; Fabry, M.; Sedlacek, J.; Bentley, G.A.; Horejsi, M. Three-dimensional structure of an Fab-peptide complex structural basis of HIV-1 protease inhibition by a monoclonal antibody. J. Mol. Biol. 1997, 267, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Van Roon, A.-M.M.; Pannu, N.S.; de Vrind, J.P.; van der Marel, G.A.; van Boom, J.H.; Hokke, C.H.; Deelder, A.M.; Abrahams, J.P. Structure of an anti-Lewis X Fab fragment in complex with its Lewis X antigen. Structure 2004, 12, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Debler, E.W.; Kaufmann, G.F.; Meijler, M.M.; Heine, A.; Mee, J.M.; Pljevaljcic, G.; Di Bilio, A.J.; Schultz, P.G.; Millar, D.P.; Janda, K.D.; et al. Deeply Inverted Electron-Hole Recombination in a Luminescent Antibody-Stilbene Complex. Science 2008, 319, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Chase, E.S.; Schmidt, T.J.; Olson, N.H.; Baker, T.S. Neutralizing antibody to human rhinovirus 14 penetrates the receptor-binding canyon. Nature 1996, 383, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Smith, T.J.; Lee, W.-M.; Mosser, A.G.; Rueckert, R.R.; Olson, N.H.; Cheng, R.H.; Baker, T.S. Structure determination of an Fab fragment that neutralizes human rhinovirus 14 and analysis of the Fab-virus complex. J. Mol. Biol. 1994, 240, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Nettleship, J.E.; Ren, J.; Rahman, N.; Berrow, N.S.; Hatherley, D.; Barclay, A.N.; Owens, R.J. A pipeline for the production of antibody fragments for structural studies using transient expression in HEK 293T cells. Protein Expr. Purif. 2008, 62, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, A.; Miranker, A.; Karplus, M. Multiple copy simultaneous search and construction of ligands in binding sites application to inhibitors of HIV-1 aspartic proteinase. J. Med. Chem. 1993, 36, 2142–2167. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Simonson, T.; Bruenger, A.T. Solvation free energies estimated from macroscopic continuum theory an accuracy assessment. J. Phys. Chem. 1994, 98, 4683–4694. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System; Science and Education Publishing Co. Ltd.: Newark, DE, USA.
Sample Availability: Samples of the antibodies used in this study are available to researchers from author P.R.Y. |
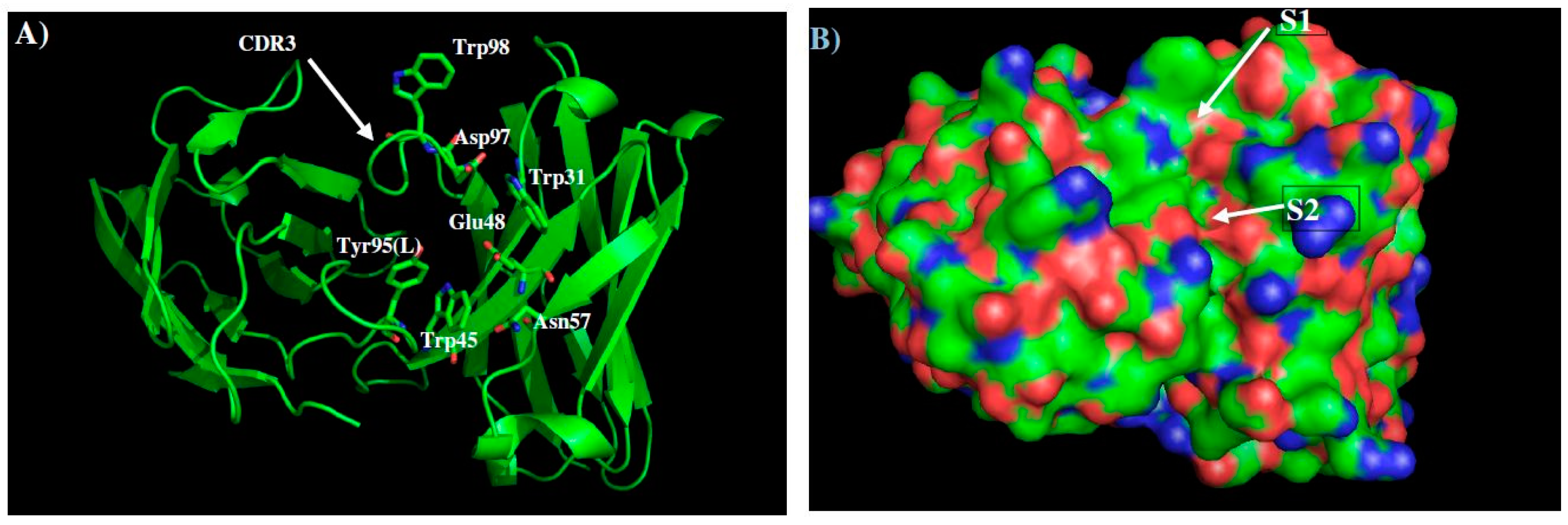
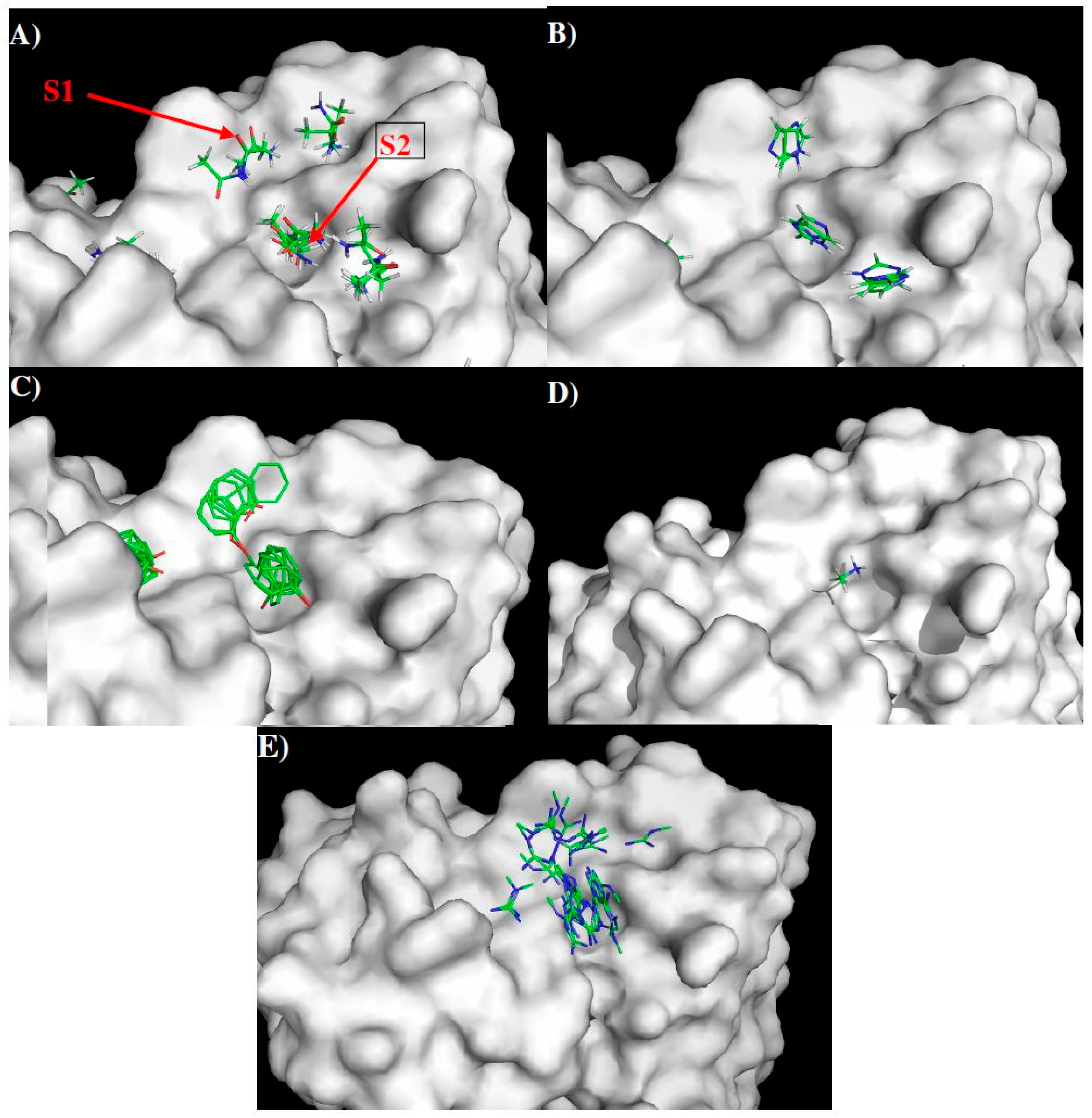


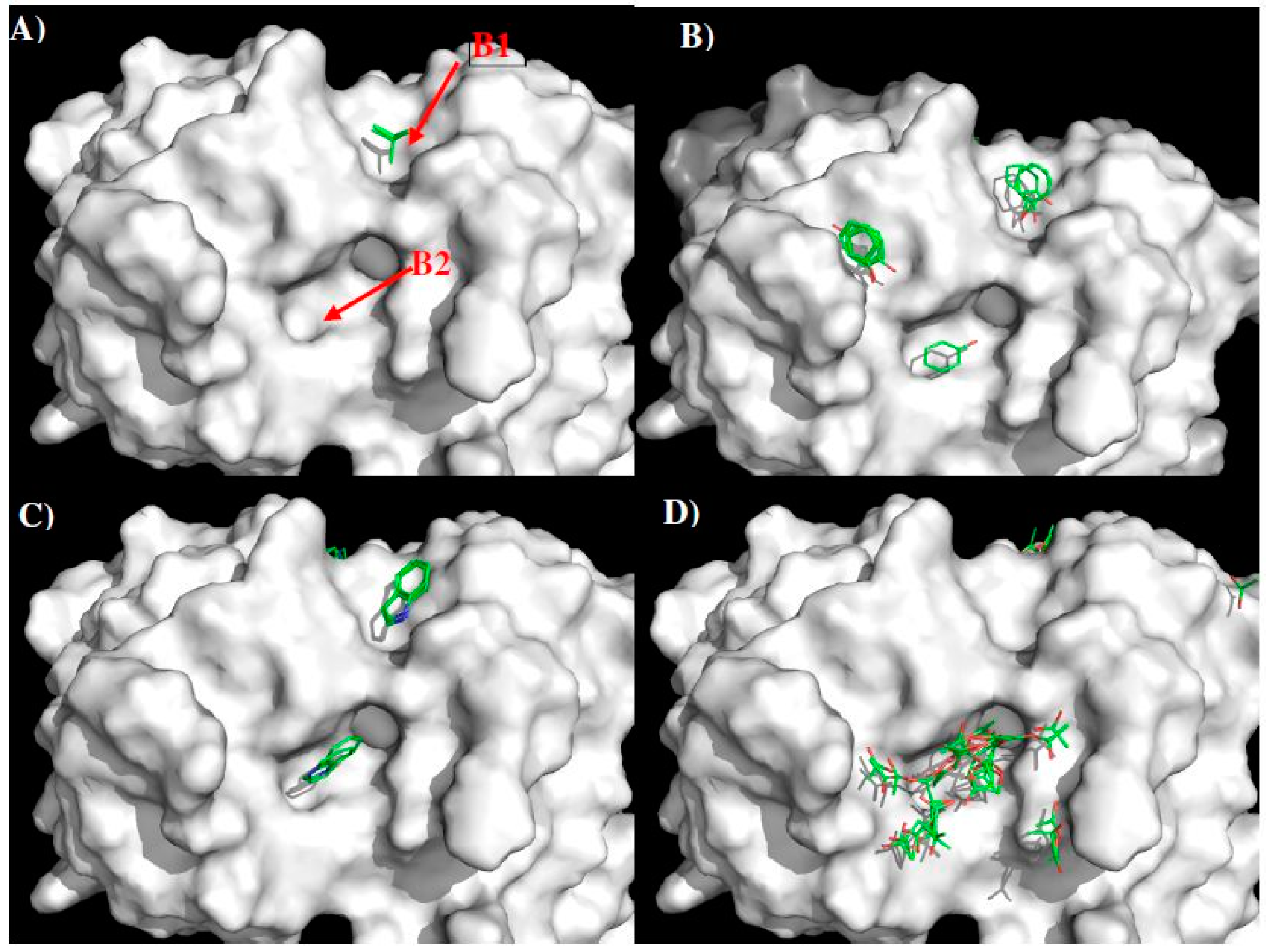
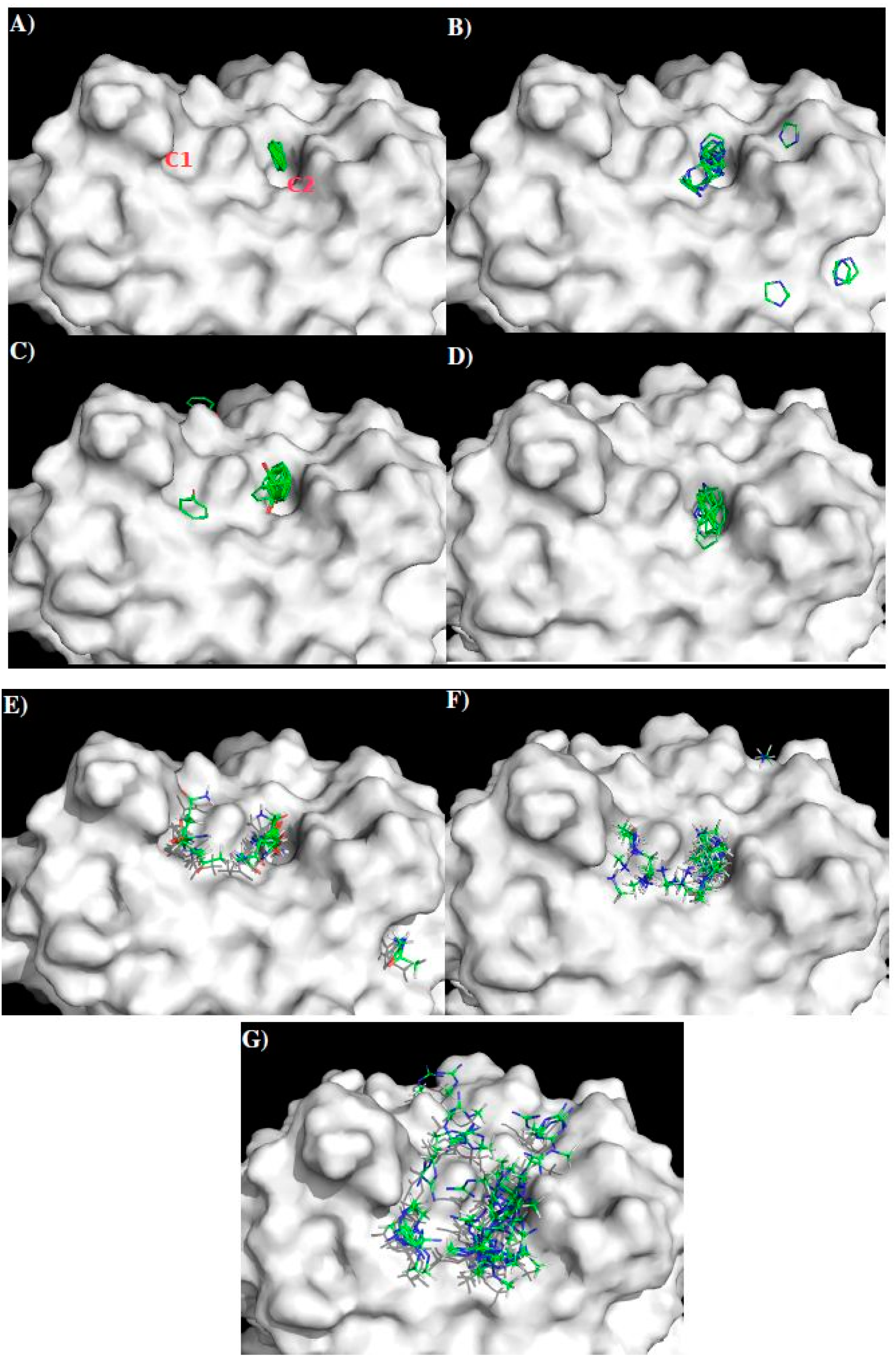
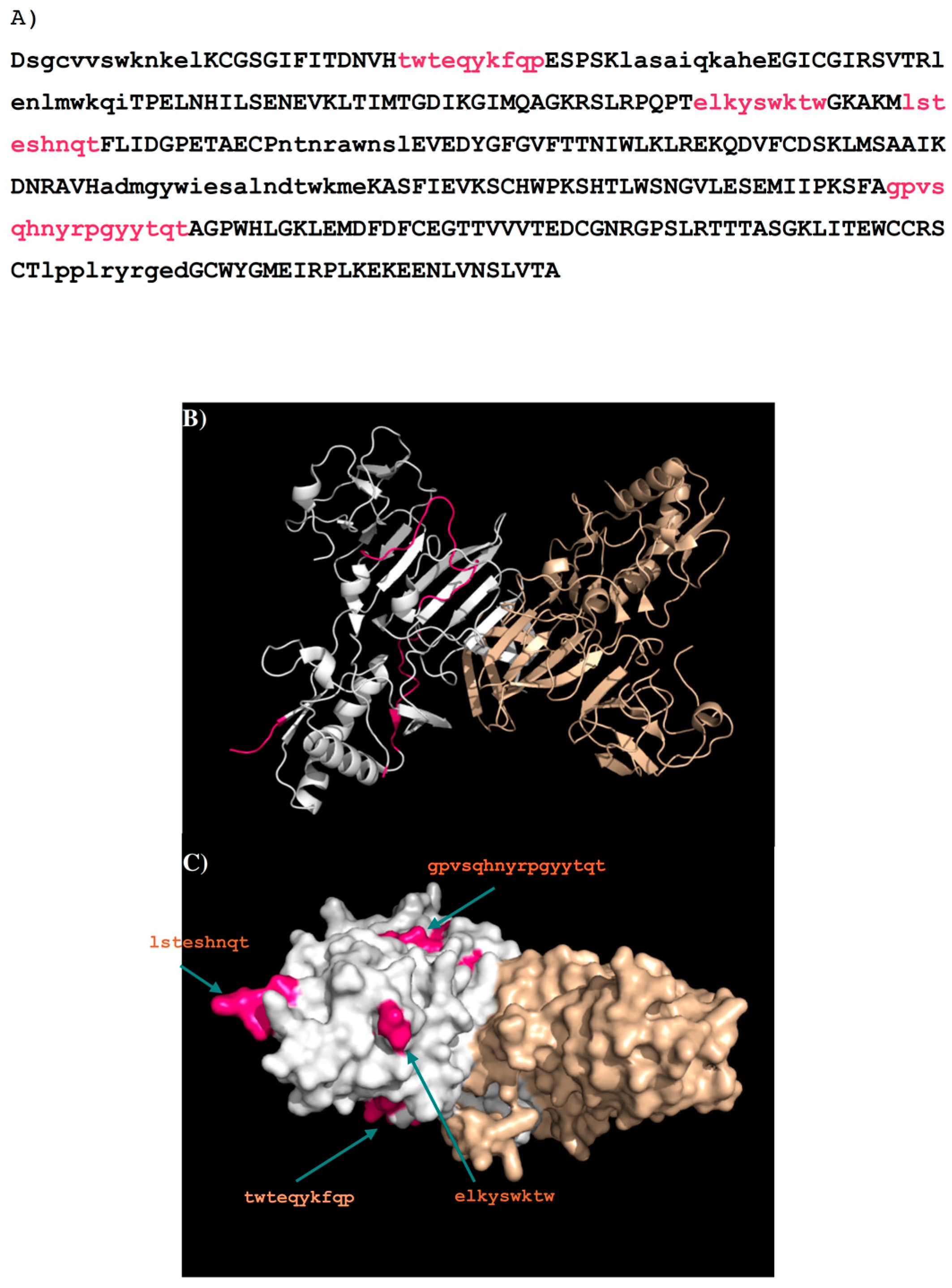
| Binding Surface | S1 | S2 |
|---|---|---|
| MCSS Minima Pattern | MGUA | MGUA |
| IMIA | MAMM | |
| PHEN | IMIA | |
| ACEM | PHEN | |
| ACEM | ||
| Sequence Pattern | R | R/K |
| H | H | |
| Y | Y | |
| Q/N | Q/N |
| Peptides | Regions | Sequence | Length (mer) | Secondary Structure | Surface Accessibility | Epitopes |
|---|---|---|---|---|---|---|
| 1H7.4-P1 | 5–13 | vvswknkel | 9 | Turn | Exposed a | |
| 1H7.4-P2 | 27–35 | twteqykfq | 9 | Loop | exposed | 1 |
| 1H7.4-P3 | 65–80 | nlmwkqitpelnhils | 16 | Helix | exposed | |
| 1H7.4-P4 | 97–116 | mqagkrslrpqptelkyswk | 20 | Loop | exposed | 2 |
| 1H7.4-P5 | 125–134 | steshnqtfl | 10 | Loop | exposed | 3 |
| 1H7.4-P6 | 143–151 | cpntnrawn | 9 | Loop | exposed | 4 |
| 1H7.4-P7 | 170–178 | klrekqdvf | 9 | Loop | exposed | 5 |
| 1H7.4-P8 | 187–195 | aikdnravh | 9 | 2β stands | buried | |
| 1H7.4-P9 | 249–264 | gpvsqhnyrpgyytqt | 16 | Loop | dimer interface b | |
| 1H7.4-P10 | 289–297 | edcgnrgps | 9 | Loop | exposed | 6 |
| 1H7.4-P11 | 318–327 | lpplryrged | 10 | β stand | buried |
| Binding Site | B1 | 11.50 Å | B2 |
|---|---|---|---|
| MCSS minima Pattern | IBUT | PHEN | |
| PHEN | INDO | ||
| INDO | ACET | ||
| Sequence Pattern | I/L/V | Gap of 2 amino acid | Y |
| Y | W | ||
| W | D/E |
| Peptides | Regions | Sequence | Length (mer) | Secondary Structure | Surface Accessibility | Epitopes |
|---|---|---|---|---|---|---|
| 1G5.3-P1 | 3–9 | gcvvswk | 7 | β stand | exposed a | |
| 1G5.3-P2 | 21–29 | itdnvhtwt | 9 | loop | buried | |
| 1G5.3-P3 | 67–82 | mwkqitpelnhilsen | 16 | helix | exposed | |
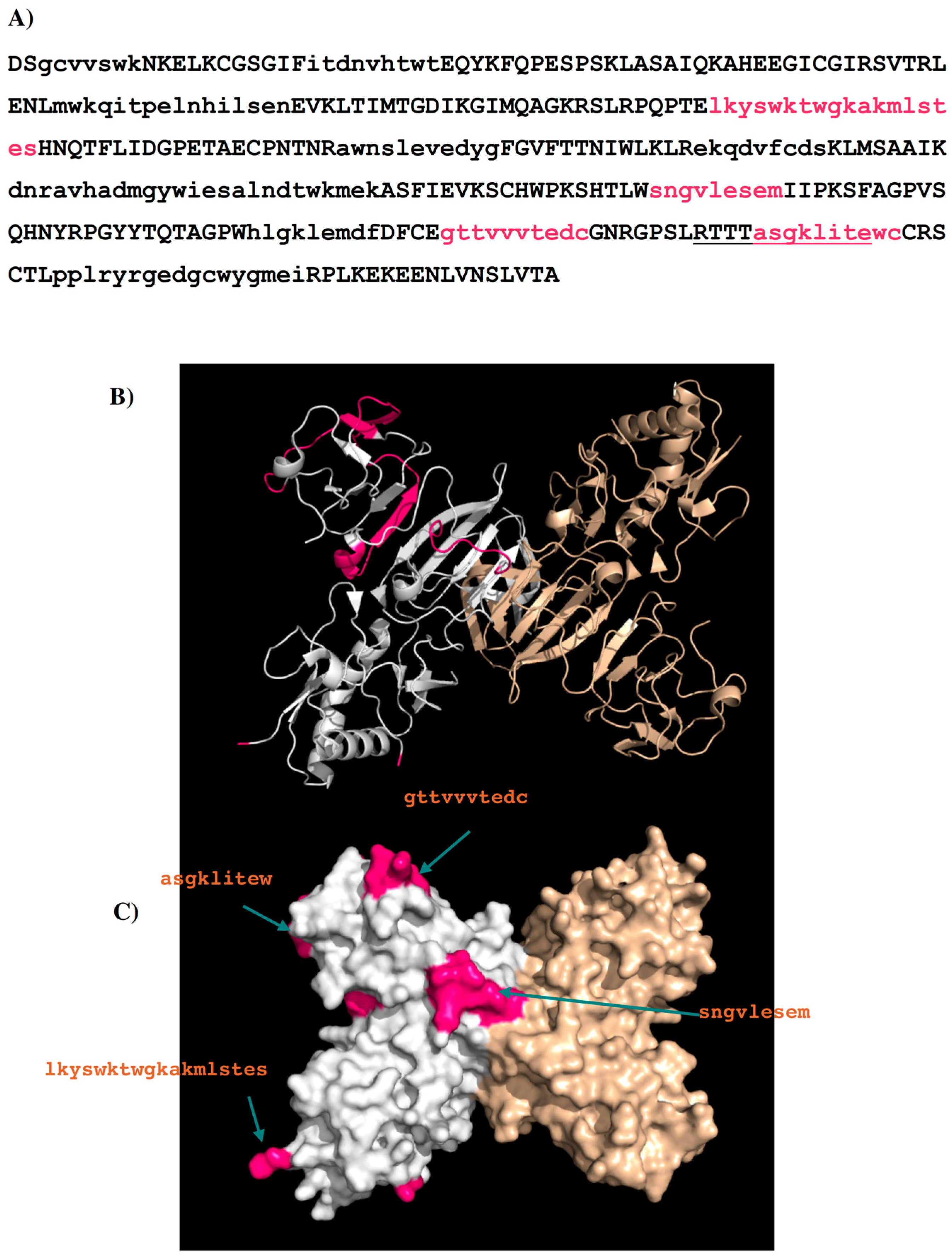
| 1G5.3-P4 | 111–128 | lkyswktwgkakmlstes | 18 | loop | exposed | I |
| 1G5.3-P5 | 149–159 | awnslevedyg | 11 | β stand | buried | |
| 1G5.3-P6 | 173–181 | ekqdvfcds | 9 | loop | buried b | |
| 1G5.3-P7 | 190–214 | dnravhadmgywiesalndtwkmek | 25 | 2β stands | buried | |
| 1G5.3-P8 | 233–241 | sngvlesem | 9 | loop | exposed | II |
| 1G5.3-P9 | 269–277 | hlgklemdf | 9 | β stand | buried | |
| 1G5.3-P10 | 282–291 | gttvvvtedc | 10 | loop | exposed | III |
| 1G5.3-P11 | 303–312 | asgklitewc | 10 | loop | exposed | IV |
| 1G5.3-P12 | 319–335 | pplryrgedgcwygmei | 17 | 2 β stands | buried |
| Binding Site | C1 | C2 |
|---|---|---|
| MCSS Minima Pattern | PHEN | BENZ |
| ACEM | IMIA | |
| MAMM | PHEN | |
| MGUA | INDO | |
| ACEM | ||
| MAMM | ||
| MGUA | ||
| Sequence Pattern | Y | F |
| Q/N | H | |
| R/K | Y | |
| W | ||
| Q/N | ||
| R/K |
| Peptides | Regions | Sequence | Length (mer) | Secondary Structure | Surface Accessibility | Epitopes |
|---|---|---|---|---|---|---|
| GUS2-P1 | 3–13 | gcvvswknkel | 11 | 2 β stands | exposed a | |
| GUS2-P2 | 27–36 | twteqykfqp | 10 | loop | exposed | A |
| GUS2-P3 | 42–51 | lasaiqkahe | 10 | helix | buried | |
| GUS2-P4 | 63–71 | lenlmwkqi | 9 | helix | exposed | |
| GUS2-P5 | 110–118 | elkyswktw | 9 | loop | exposed | B |
| GUS2-P6 | 124–132 | lsteshnqt | 9 | loop | exposed | C |
| GUS2-P7 | 145–153 | ntnrawnsl | 9 | loop | buried | |
| GUS2-P8 | 196–213 | admgywiesalndtwkme | 18 | 2 β stands | buried b | |
| GUS2-P9 | 249–264 | gpvsqhnyrpgyytqt | 16 | loop | exposed | D |
| GUS2-P10 | 318–327 | lpplryrged | 10 | β stand | buried |
| Functional Group | Abbreviation | Amino Acids | |
|---|---|---|---|
| Charged (−) | Acetate ion | ACET | ASP, GLU |
| Charged (+) | Methylguanidinium | MGUA | ARG |
| Charged (+) | Methylammonium | MAMM | LYS |
| Polar | Acetamide | ACEM | ASN, GLN |
| Polar | Methanol | MEOH | SER, THR |
| Hydrophobic | Methanethiol | MESH | CYS, MET |
| Aromatic Polar | Phenol | PHEN | TYR |
| Aromatic Polar | Indole | INDO | TRP |
| Aromatic Polar | Imidazole | IMIA | HIS |
| Aromatic Hydrophobic | Benzene | BENZ | PHE |
| Hydrophobic | Ibutane | IBUT | VAL, ILE, LEU |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.L.; Legge, F.S.; Lebani, K.; Mahler, S.M.; Young, P.R.; Watterson, D.; Treutlein, H.R.; Zeng, J. Computational Identification of Antibody Epitopes on the Dengue Virus NS1 Protein. Molecules 2017, 22, 607. https://doi.org/10.3390/molecules22040607
Jones ML, Legge FS, Lebani K, Mahler SM, Young PR, Watterson D, Treutlein HR, Zeng J. Computational Identification of Antibody Epitopes on the Dengue Virus NS1 Protein. Molecules. 2017; 22(4):607. https://doi.org/10.3390/molecules22040607
Chicago/Turabian StyleJones, Martina L., Fiona S. Legge, Kebaneilwe Lebani, Stephen M. Mahler, Paul R. Young, Daniel Watterson, Herbert R. Treutlein, and Jun Zeng. 2017. "Computational Identification of Antibody Epitopes on the Dengue Virus NS1 Protein" Molecules 22, no. 4: 607. https://doi.org/10.3390/molecules22040607






