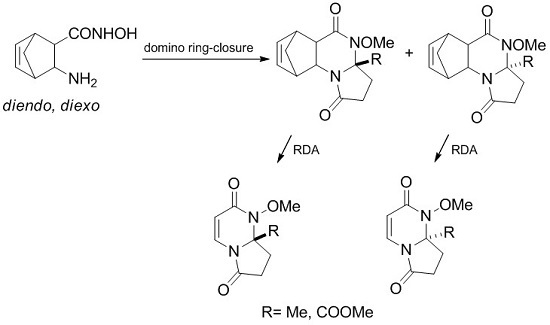
Synthesis of Pyrrolo[1,2-a]pyrimidine Enantiomers via Domino Ring-Closure followed by Retro Diels-Alder Protocol
Abstract
:1. Introduction
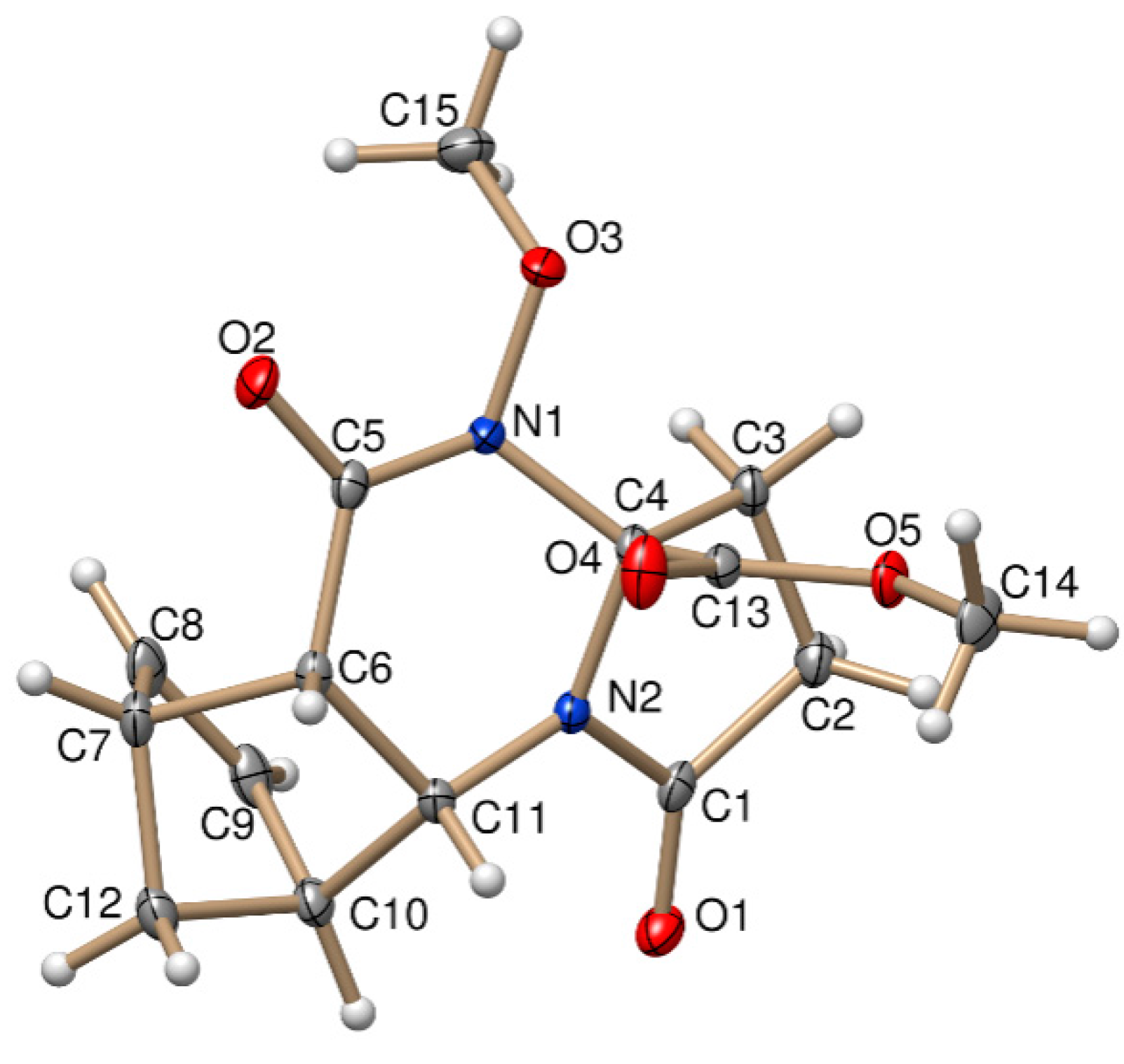
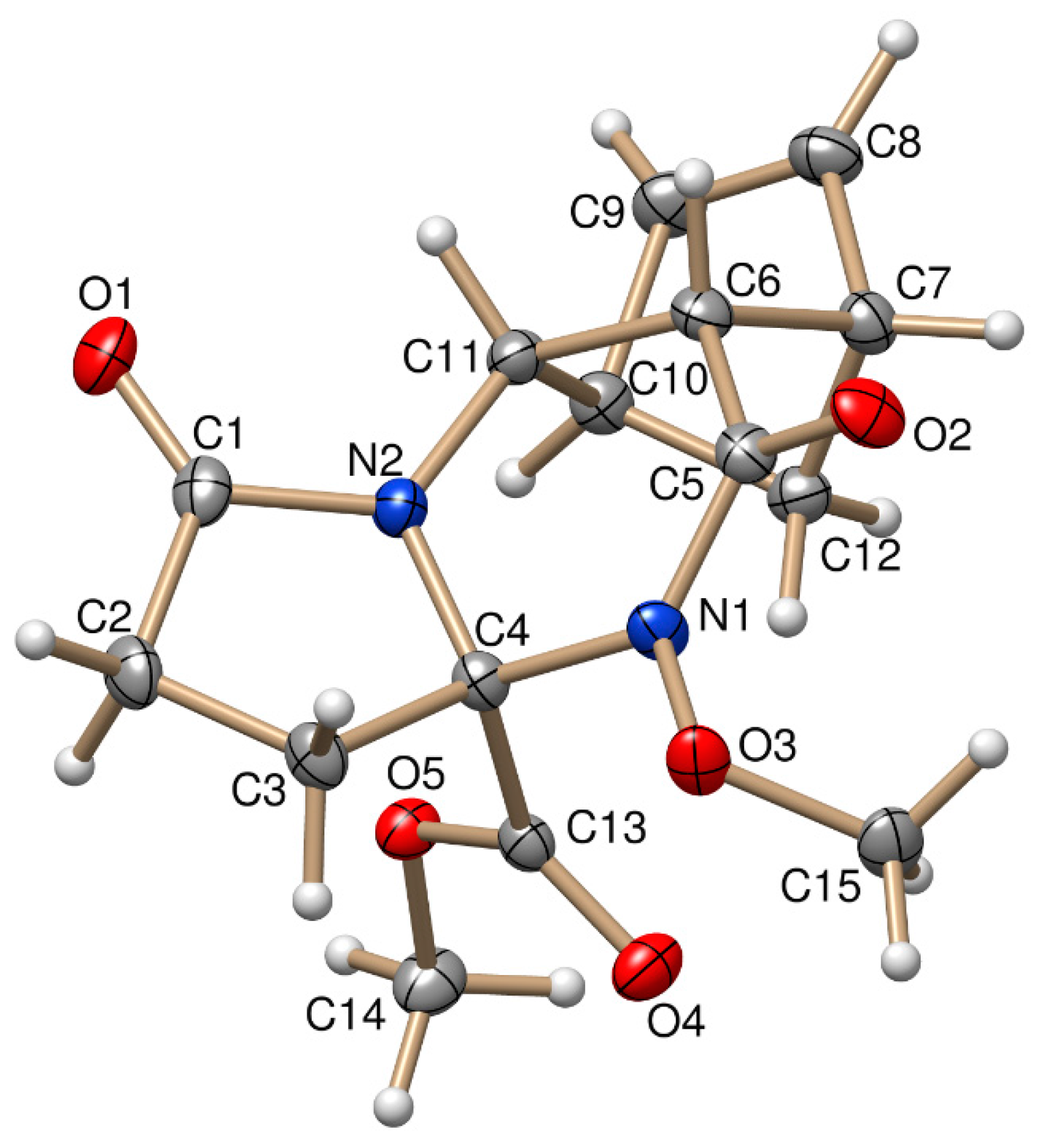
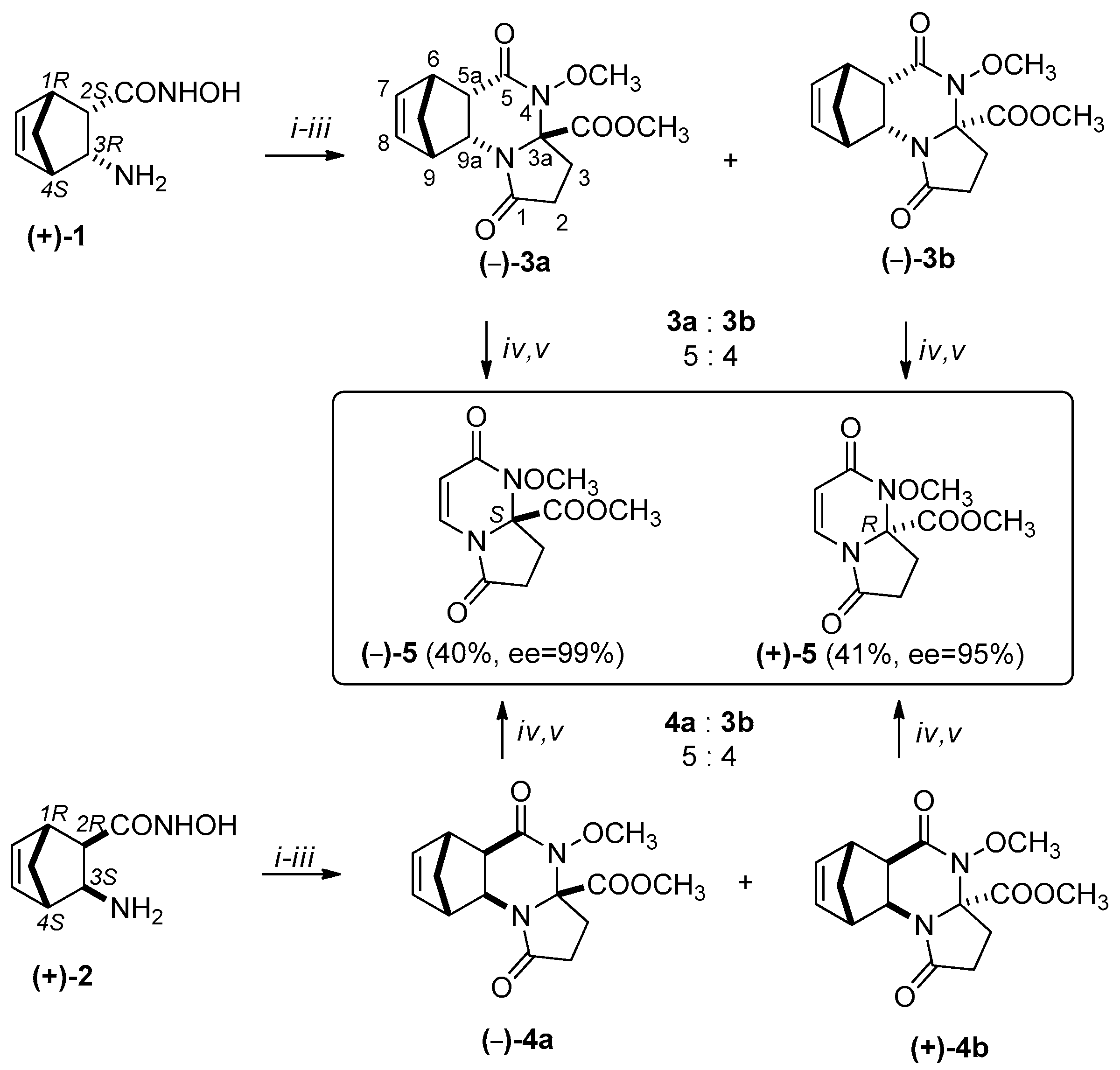
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of New Compounds
3.2.1. Synthesis of Methanopyrrolo[1,2-a]quinazoline Derivatives (–)-3a, (–)-3b, (–)-4a, and (+)-4b
3.2.2. Synthesis of Pyrrolo[1,2-a]pyrimidines (+)-5 and (–)-5
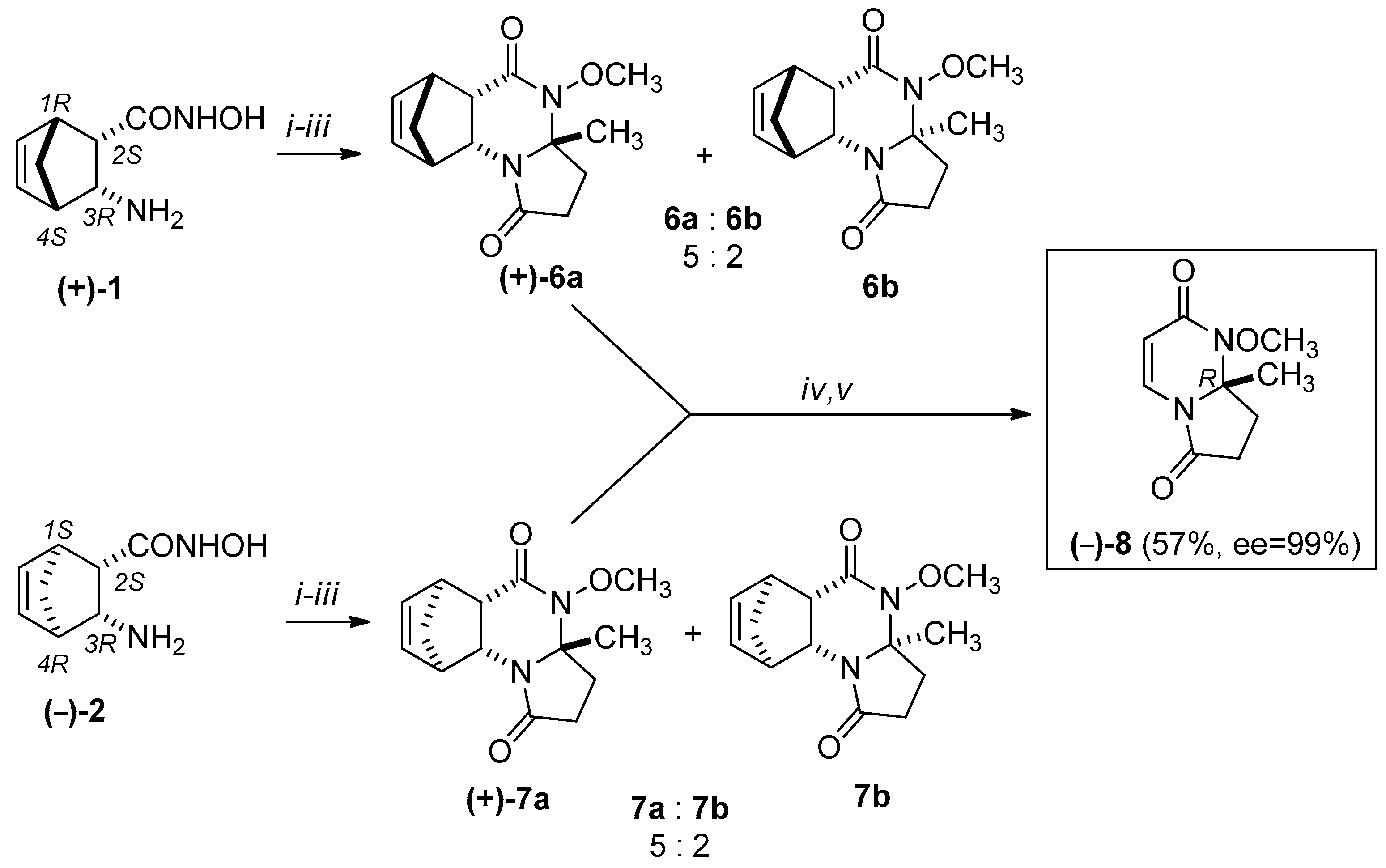
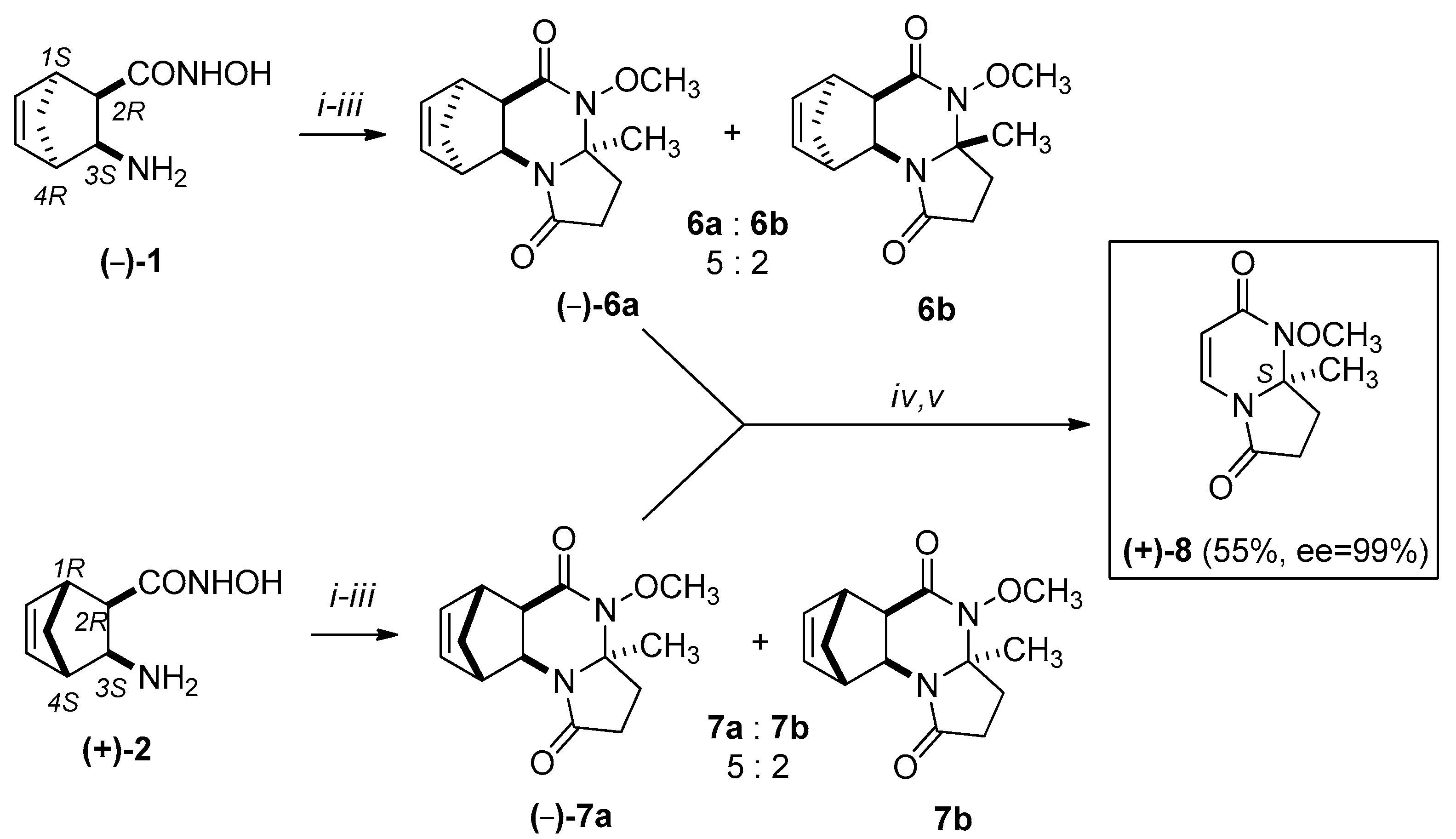
3.2.3. Synthesis of Methanopyrrolo[1,2-a]quinazoline Derivatives (+)-6a, (–)-6a, (+)-7a, and (–)-7a
3.2.4. Synthesis of Pyrrolo[1,2-a]pyrimidines (+)-8 and (–)-8
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lossen, H. Über die Oxalohydroxamsäure. Liebigs Ann. 1869, 150, 314–322. [Google Scholar] [CrossRef]
- Marmion, C.J.; Parker, J.P.; Nolan, K.B. Hydroxamic acids: An important class of metalloenzyme inhibitors. In Comprehensive Inorganic Chemistry II; Reedijk, J., Poeppelmeier, K., Eds.; Royal College of Surgeons: Dublin, Ireland, 2013; Volume 3, pp. 683–708. [Google Scholar]
- Bertrand, S.; Hélesbeux, J.-J.; Larcher, G.; Duval, O. Hydroxamate, a key pharmacophore exhibiting a wide range of biological activities. Mini-Rev. Med. Chem. 2013, 13, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Samuni, U.; Goldstein, S. The mechanism underlying nitroxyl and nitric oxide formation from hydroxamic acids. Biochim. Biophys. Acta 2012, 1820, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.M.; Almaraza, A.E.; Barib, S.E.; Olabeb, J.A.; Amorebietaa, V.T. The HNO donor ability of hydroxamic acids upon oxidation with cyanoferrates (III). J. Coord. Chem. 2015, 68, 3236–3246. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S. Hydroxamic acids, recent breakthroughs in stereoselective synthesis and biological evaluations. Curr. Org. Synth. 2016, 13, 659–668. [Google Scholar] [CrossRef]
- Mishra, R.C.; Tripathi, R.; Katiyar, D.; Tewari, N.; Singh, D.; Tripathia, R.P. Synthesis of glycosylated β-Amino hydroxamates as new class of antimalarials. Bioorg. Med. Chem. 2003, 11, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
- Zarember, K.A.; Cruz, A.R.; Huang, C.; Gallin, J.I. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigates. Antimicrob. Agents Chemother. 2009, 53, 2654–2656. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K. Ciclopirox: An overview. Int. J. Dermatol. 2001, 40, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Grassadonia, A.; Cioffi, P.; Simiele, F.; Iezzi, L.; Zilli, M.; Natoli, C. Role of hydroxamate-based histone deacetylase inhibitors (Hb-HDACIs) in the treatment of solid malignancies. Cancers 2013, 5, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, C.-N.; Hajji, N.; Oliver, E.; Cotroneo, E.; Wharton, J.; Wang, D.; Li, M.; McKinsey, T.A.; Stenmark, K.R. Histone deacetylation inhibition in pulmonary hypertension: Therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 2012, 126, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsuo, K.; Nakanishi, A.; Hatano, T.; Izeki, H.; Ishida, Y.; Mori, W. Syntheses and anti-inflammatory and analgesic activities of hydroxamic acids and acid hydrazides. Chem. Pharm. Bull. 1983, 31, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E. Target practice: HDAC inhibitors for schizophrenia. Nat. Neurosci. 2012, 15, 1180–1181. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Granchi, C. Bioactive heterocycles containing endocyclic N-hydroxy groups. Eur. J. Med. Chem. 2015, 97, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, W.; Rotzinger, B. Isoindolo[2,1-a]quinazoline Derivatives for Stabilization of Organic Materials. US 9321902 B2, 26 April 2016. [Google Scholar]
- Alagha, A.; Parthasarathi, L.; Gaynor, D.; Müller-Bunz, H.; Starikova, Z.A.; Farkas, E.; O’Brien, E.C.; Gil, M.-J.; Nolan, K.B. Metal complexes of cyclic hydroxamates. Synthesis and crystal structures of 3-hydroxy-2-methyl-3H-quinazolin-4-one (ChaH) and of its Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes. Inorg. Chim. Acta 2011, 368, 58–66. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Synthesis and application of N-hydroxylamine derivatives as potential replacements for HOBt. Eur. J. Org. Chem. 2009, 10, 1499–1501. [Google Scholar] [CrossRef]
- Tardibono, L.P.; Miller, M.J. Synthesis and anticancer activity of new hydroxamic acid containing 1,4-benzodiazepines. Org. Lett. 2009, 11, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Shemchuk, L.A.; Chernykh, V.P.; Krys’kiv, O.S. Reaction of anthranilic acid amides with cyclic anhydrides. Russ. J. Org. Chem. 2006, 42, 382–387. [Google Scholar] [CrossRef]
- Shpernat, Y.; Mizhiritskii, M. 3-Hydroxy-4-oxo-1,2,3-triazines and Derivatives thereof for Amide and Ester Bond Formation. WO 2005007634 A1, 27 January 2005. [Google Scholar]
- Tanaka, K.; Matsuo, K.; Nakanishi, A.; Kataoka, Y.; Takase, K.; Otsuki, S. Syntheses of cyclic hydroxamic acid derivatives, and their chelating abilities and biological activities. Chem. Pharm. Bull. 1988, 36, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Raymond, K.N. Efficient route to highly water-soluble aromatic cyclic hydroxamic acid ligands. Eur. J. Org. Chem. 2008, 16, 2697–2700. [Google Scholar] [CrossRef]
- Coutts, R.T; Pound, N.J. Preparation of an aromatic hydroxylamine and some cyclic hydroxamic acids, and their reaction with hydrochloric acid. Can. J. Chem. 1970, 48, 1859–1864. [Google Scholar] [CrossRef]
- Bauer, L.; Nambury, C.N.V. The Synthesis of trans-2,4-Dioxo-3-hydroxydecahydroquinazoline. J. Org. Chem. 1961, 26, 1106–1109. [Google Scholar] [CrossRef]
- Giglio, B.C.; Alexanian, E.J. Alkene hydrofunctionalization using hydroxamic acids: A radical-mediated approach to alkene hydration. Org. Lett. 2014, 16, 4304–4307. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Defoin, A.; Salomon, E.; Tarnus, C.; Wetterholm, A.; Haeggström, J.Z. Synthesis and structure activity relationships of novel non-peptidic metallo-aminopeptidase inhibitors. Bioorg. Med. Chem. 2006, 14, 7241–7257. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, J.; Kunisch, F.; Matzke, M.; Militzer, H.-C.; Schmidt, A.; Schönfeld, W. Novel antifungal β-amino acids: Synthesis and activity against Candida albicans. Bioorg. Med. Chem. Lett. 2003, 13, 433–436. [Google Scholar] [CrossRef]
- Wang, Y.; Papamichelakis, M.; Chew, W.; Sellstedt, J.; Noureldin, R.; Tadayon, S.; Daigneault, S. Development of a suitable process for the preparation of a TNF-α converting enzyme inhibitor, WAY-281418. Org. Proc. Res. Dev. 2008, 12, 1253–1260. [Google Scholar] [CrossRef]
- Sengupta, P.; Puri, C.S.; Chokshi, H.A.; Sheth, C.K.; Midha, A.S.; Chitturi, T.R.; Thennati, R.; Murumkar, P.R.; Yadav, M.R. Synthesis, preliminary biological evaluation and molecular modeling of some new heterocyclic inhibitors of TACE. Eur. J. Org. Med. Chem. 2011, 46, 5549–5555. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.-W.; Chen, L.; Lu, Z.; Xue, C.-B.; Liu, R.-Q.; Covington, M.B.; Qian, M.; Wasserman, Z.R.; Vaddi, K.; Christ, D.D.; et al. Discovery of β-benzamido hydroxamic acids as potent, selective, and orally bioavailable TACE inhibitors. Biorg. Med. Chem. Lett. 2008, 18, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fekete, B.; Palkó, M.; Mándity, I.; Haukka, M.; Fülöp, F. A domino ring-closure followed by retro-Diels-Alder reaction for the preparation of pyrimido[2,1-a]isoindole enantiomers. Eur. J. Org. Chem. 2016, 21, 3519–3527. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Popa, M.M. Pyrrolo[1,2-a]quinazolines. Synthesis and biological properties. ARKIVOC 2014, 2014, 428–452. [Google Scholar]
- Reddy, S.B.V.; Reddy, B.P.; Reddy, V.G.P.; Siriwardena, A. An efficient lactamisation/N-acyliminium Pictet—Spengler domino strategy for the diasteroselective synthesis of polyhydroxylated quinoxalinone, β-carboline and quinazolinone derivatives. Org. Biomol. Chem. 2016, 14, 4276–4282. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.S.; Keivanloo, A.; Nasr-Isfahani, H.; Bamoniri, A. Synthesis of novel 1,5-disubstituted pyrrolo[1,2-a]quinazolines and their evaluation for anti-bacterial and anti-oxidant activities. RSC Adv. 2016, 6, 92663–92669. [Google Scholar] [CrossRef]
- Acosta, P.; Ortiz, A.; Insuasty, B.; Abonia, R.; Quiroga, J. Synthesis and study of the electronic properties of pyrazolo[1,5-c]pyrrolo[1,2-a]quinazoline and pyrazolo[1,5-c]pyrido[1,2-a]quinazoline derivatives. Monatsh Chem. 2017, 148, 237–244. [Google Scholar] [CrossRef]
- Jin, R.-Z.; Zhang, W.-T.; Zhou, Y.-J.; Wang, X.-S. Iodine-catalyzed synthesis of 5H-phthalazino[1,2-b]quinazoline and isoindolo[2,1-a]quinazoline derivatives via a chemoselective reaction of 2-aminobenzohydrazide and 2-formylbenzoic acid in ionic liquids. Tetrahedron Lett. 2016, 57, 2515–2519. [Google Scholar] [CrossRef]
- Zhang, W.-T.; Qiang, W.-W.; Yao, C.-S.; Wang, X.-S. Iodine-catalyzed synthesis of fused tetracyclic pyridazino[6,1-b]pyrrolo [1,2-a]quinazolin-9(1H)-one derivatives via a tandem reaction. Tetrahedron 2016, 72, 2178–2185. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Lu, L.; Zhou, Y.-J.; Wang, X.-S. Iodine-catalyzed synthesis of pyrrolo[1,2-a]quinazoline-3a-carboxylic acid derivatives in ionic liquids. Res. Chem. Intermed. 2013, 39, 3327–3335. [Google Scholar] [CrossRef]
- Bradar, B.; Reich, E. Biochemical and biological properties of 5-bromotubercidin: Differential effects on cellular DNA-directed and viral RNA-directed RNA synthesis. Bioorg. Med. Chem. 2008, 16, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Glunz, P.W.; Douty, B.P.; Decicco, C.P. Design and synthesis of bicyclic pyrimidinone-based HCV NS3 protease inhibitors. Bioorg. Med. Chem. 2003, 13, 785–788. [Google Scholar] [CrossRef]
- Ivanov, M.A.; Aleksandrova, L.A. Bicyclic furano-, pyrrolo-, and thiopheno[2,3-d] derivatives of pyrimidine nucleosides: Synthesis and antiviral properties. Russ. J. Bioorg. Chem. 2013, 39, 22–39. [Google Scholar] [CrossRef]
- Kumar, V.P.; Cisneros, J.A.; Frey, K.M.; Castellanos-Gonzalez, A.; Wang, Y.; Gangjee, A.; White, A.C., Jr.; Jorgensen, W.L.; Anderson, K.S. Structural studies provide clues for analog design of specific inhibitors of Cryptosporidium hominis thymidylate synthase—Dihydrofolate reductase. Bioorg. Med. Chem. Lett. 2014, 24, 4158–4161. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; El-Domany, R.A.; El-Hameed, R.H.A. Synthesis of certain pyrrole derivatives as antimicro-bial agents. Acta Pharm. 2009, 59, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Awazu, Y.; Mizutani, A.; Nagase, Y.; Iwata, H.; Oguro, Y.; Miki, H.; Imamura, S.; Hori, A. A novel pyrrolo[3, 2-d]pyrimidine derivative, as a vascular endothelial growth factor receptor and platelet-derived growth factor receptor tyrosine kinase inhibitor, shows potent antitumor activity by suppression of tumor angiogenesis. Cancer Sci. 2012, 103, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.D.; Wilson, J.W.; Deanda, F.; Patnaik, S.; Redman, A.M.; Yang, B.; Shewchuk, L.; Sabbatini, P.; Leesnitzer, M.A.; Groy, A.; et al. Discovery of 4,6-bis-anilino-1H-pyrrolo[2,3-d]pyrimidines: Potent inhibitors of the IGF-1R receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2009, 19, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Cherian, C.S.; Desmoulin, K.; Wang, L.; Polin, L.; White, K.; Kushner, J.; Stout, M.; Hou, Z.; Gangjee, A.; Matherly, L.H. Therapeutic targeting malignant mesothelioma with a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate via its selective uptake by the proton-coupled folate transporter. Cancer Chemother. Pharmacol. 2013, 71, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Coumar, M.S.; Wu, J.-S.; Leou, J.-S.; Tan, U.-K.; Chang, C.-Y.; Chang, T.-Y.; Lin, W.-H.; Hsu, J.T.-A.; Chao, Y.-S.; Wua, S.-Y.; et al. Aurora kinase A inhibitors: Identification, SAR exploration and molecular modeling of 6,7-dihydro-4H-pyrazolo-[1,5-a]pyrrolo[3,4-d]pyrimidine-5,8-dione scaffold. Bioorg. Med. Chem. Lett. 2008, 18, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Cuny, G.D.; Yu, P.B.; Laha, J.K.; Xing, X.; Liu, J.-F.; Lai, C.S.; Deng, D.Y.; Sachidanandan, C.; Bloch, K.D.; Peterson, R.T. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Ella, D.A.A E.; Ghorab, M.M.; Noaman, E.; Heibab, H.I.; Khalil, A.I. Molecular modeling study and synthesis of novel pyrrolo[2,3-d]pyrimidines and pyrrolotriazolopyrimidines of expected antitumor and radioprotective activities. Bioorg. Med. Chem. 2008, 16, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Furet, P.; Gerspacher, M.; Pissot-Soldermann, C. Design of two new chemotypes for inhibiting the Janus kinase 2 by scaffold morphing. Bioorg. Med. Chem. Lett. 2010, 20, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Kurup, S.; Ihnat, M.A.; Thorpe, J.E.; Shenoy, S.S. Synthesis and biological activity of N4-phenylsubstituted-6-(2,4-dichloro phenylmethyl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamines as vascular endothelial growth factor receptor-2 inhibitors and antiangiogenic and antitumor agents. Bioorg. Med. Chem. 2010, 18, 3575–3587. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A; Pavana, R.K.; Li, W.; Hamel, E.; Westbrook, C.; Mooberry, S.L. Novel water-soluble substituted pyrrolo[3,2-d]pyrimidines: Design, synthesis, and biological evaluation as antitubulin antitumor agents. Pharm. Res. 2012, 29, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, S.J.; Han, J.; Nřrsett, K.G.; Rydså, L.; Kjřbli, E.; Bugge, S.; Bjřrkřy, G.; Sundby, E.; Hoff, B.H. Identification of new 4-N-substituted 6-aryl-7H-pyrrolo[2,3-d]pyrimidine-4-amines as highly potent EGFR-TK inhibitors with Src-family activity. Eur. J. Pharm. Sci. 2014, 59, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, Y.; Miwa, K.; Seto, M.; Banno, H.; Ohta, Y.; Tamura, T.; Yusa, T.; Miki, H.; Kamiguchi, H.; Ikeda, Y.; et al. Design and synthesis of pyrrolo[3,2-d]pyrimidine HER2/EGFR dual inhibitors: Improvement of the physicochemical and pharmacokinetic profiles for potent in vivo anti-tumor efficacy. Bioorg. Med. Chem. 2012, 20, 6171–6180. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.M.; Yamini, L.; Vijjulathae, M. 3D QSAR of pyrrolo pyrimidine and thieno pyrimidines as human thymidylate synthase inhibitors. E-J. Chem. 2012, 9, 1699–1710. [Google Scholar] [CrossRef]
- Lauria, A.; Patella, C.; Abbate, I.; Martorana, A.; Almerico, A.M. Lead optimization through VLAK protocol: New annelated pyrrolo-pyrimidine derivatives as antitumor agents. Eur. J. Med. Chem. 2012, 55, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Temburnikar, K.W.; Ross, C.R.; Wilson, G.M.; Balzarini, J.; Cawrse, B.M.; Seley-Radtke, K.L. Antiproliferative activities of halogenated pyrrolo[3,2-d]pyrimidines. Bioorg. Med. Chem. 2015, 23, 4354–4363. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Chen, W.-H.; Wu, S.-G.; Tian, Y.-X.; Zhang, J.-J. Pyrrolo[3,2-d]pyrimidine derivatives as type II kinase insert domain receptor (KDR) inhibitors: CoMFA and CoMSIA studies. Int. J. Mol. Sci. 2012, 13, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Hondo, T.; Nagata, H.; Ogiyama, T.; Maeda, J.; Hoshii, H.; Kontani, T.; Kuromitsu, S.; Ohga, K.; Orita, M.; et al. Novel 7H-pyrrolo[2,3-d]pyrimidine derivatives as potent and orally active STAT6 inhibitors. Bioorg. Med. Chem. 2009, 17, 6926–6936. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Siracusa, M.A.; Modica, M.N.; Salerno, L.; Pedretti, A.; Vistoli, G.; Cagnotto, A.; Mennini, T.; Romeo, G. Synthesis and molecular modeling of 1H-pyrrolopyrimidine-2,4-dione derivatives as ligands for the α1-adrenoceptors. Bioorg. Med. Chem. 2011, 19, 5260–5276. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Romagnoli, R.; Saponaro, G.; Tabrizi, M.A.; Baraldi, S.; Pedretti, P.; Fusi, C.; Nassini, R.; Materazzi, S.; Geppetti, P.; et al. 7-Substituted-pyrrolo[3,2-d]pyrimidine-2,4-dione derivatives as antagonists of the transient receptor potential ankyrin 1 (TRPA1) channel: A promising approach for treating pain and inflammation. Bioorg. Med. Chem. 2012, 20, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Abe, N. Syntheses and some reactions of 4H-cyclohepta[4,5]pyrrolo[1,2-α]pyrimidin-4-ones. Bull. Chem. Soc. Jpn. 1987, 60, 1053–1056. [Google Scholar] [CrossRef]
- Acosta, P.; Insuasty, B.; Abonia, R.; Quiroga, J. Annelation of pyrrolo[1,2-a]pyrimidine and pyrido[1,2-a]pyrimidine systems to a pyrazolopyridine framework by a cascade of two cyclization reactions. Tetrahedron Lett. 2015, 56, 2917–2921. [Google Scholar] [CrossRef]
- Amarnath, V.; Madhav, R. A survey of methods for the preparation of pyrrolopyrimidines. Synthesis 1974, 12, 837–859. [Google Scholar] [CrossRef]
- De Coen, L.M.; Heugebaert, T.S.A.; García, D.; Stevens, C.V. Synthetic entries to and biological activity of pyrrolopyrimidines. Chem. Rev. 2016, 116, 80–139. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.; Kornienko, A. Multicomponent synthesis of the medicinally important pyrrolo[2,3-d]pyrimidine scaffold. Chem. Heterocycl. Compd. 2014, 50, 139–144. [Google Scholar] [CrossRef]
- Gao, J.; Henry, R.F.; Pagano, T.G.; Duerst, R.W.; Souers, A.J. A cascade reaction sequence en route to 7-substituted 2-aminopyrrolo[1,2-a]pyrimidine-4,6-diones and the corresponding acrylic acid derivatives. Tetrahedron Lett. 2007, 48, 7395–7398. [Google Scholar] [CrossRef]
- Miklós, F.; Tóth, Z.; Hänninen, M.M.; Sillanpää, R.; Forró, E.; Fülöp, F. Retro-Diels-Alder protocol for the synthesis of pyrrolo[1,2-a]pyrimidine and pyrimido[2,1-a]isoindole enantiomers. Eur. J. Org. Chem. 2013, 22, 4887–4894. [Google Scholar] [CrossRef]
- Prieur, V.; Heindler, N.; Rubio-Martínez, J.; Guillaumet, G.; Pujol, M.D. One-pot synthesis of 4-aminated pyrrolo[2,3-d]pyrimidines from alkynylpyrimidines under metal-catalyst-free conditions. Tetrahedron 2015, 71, 1207–1214. [Google Scholar] [CrossRef]
- Vincze, Z.; Pilipecz, M.V.; Scheiber, P.; Varga, T.R.; Tóth, G.; Nemes, P. Simple route to multisubstituted tetrahydropyrimidines. Tetrahedron 2015, 71, 6135–6142. [Google Scholar] [CrossRef]
- Cheng, R.; Guo, T.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. One-pot synthesis of quinazolinones from anthranilamides and aldehydes via p-toluenesulfonic acid catalyzed cyclocondensation and phenyliodine diacetate mediated oxidative dehydrogenation. Synthesis 2013, 45, 2998–3006. [Google Scholar] [CrossRef]
- Gao, Y.; Rubin, P.; Xiaoyi, N.; Zepp, C. Therapeutic Heterocyclic Compounds for the Treatment of Asthma and Allergy and Use Thereof. WO 2001070737 A3, 31 January 2002. [Google Scholar]
- Lajkó, G.; Orosz, T.; Grecsó, N.; Fekete, B.; Palkó, M.; Fülöp, F.; Lindner, W.; Péter, A.; Ilisz, I. High-performance liquid chromatographic enantioseparation of cyclic β-aminohydroxamic acids on zwitterionic chiral stationary phases based on Cinchona alkaloids. Anal. Chim. Acta 2016, 921, 84–94. [Google Scholar]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, B.; Palkó, M.; Haukka, M.; Fülöp, F. Synthesis of Pyrrolo[1,2-a]pyrimidine Enantiomers via Domino Ring-Closure followed by Retro Diels-Alder Protocol. Molecules 2017, 22, 613. https://doi.org/10.3390/molecules22040613
Fekete B, Palkó M, Haukka M, Fülöp F. Synthesis of Pyrrolo[1,2-a]pyrimidine Enantiomers via Domino Ring-Closure followed by Retro Diels-Alder Protocol. Molecules. 2017; 22(4):613. https://doi.org/10.3390/molecules22040613
Chicago/Turabian StyleFekete, Beáta, Márta Palkó, Matti Haukka, and Ferenc Fülöp. 2017. "Synthesis of Pyrrolo[1,2-a]pyrimidine Enantiomers via Domino Ring-Closure followed by Retro Diels-Alder Protocol" Molecules 22, no. 4: 613. https://doi.org/10.3390/molecules22040613