Synthesis and Self-Assembly of Shape Amphiphiles Based on POSS-Dendron Conjugates
Abstract
:1. Introduction
2. Results
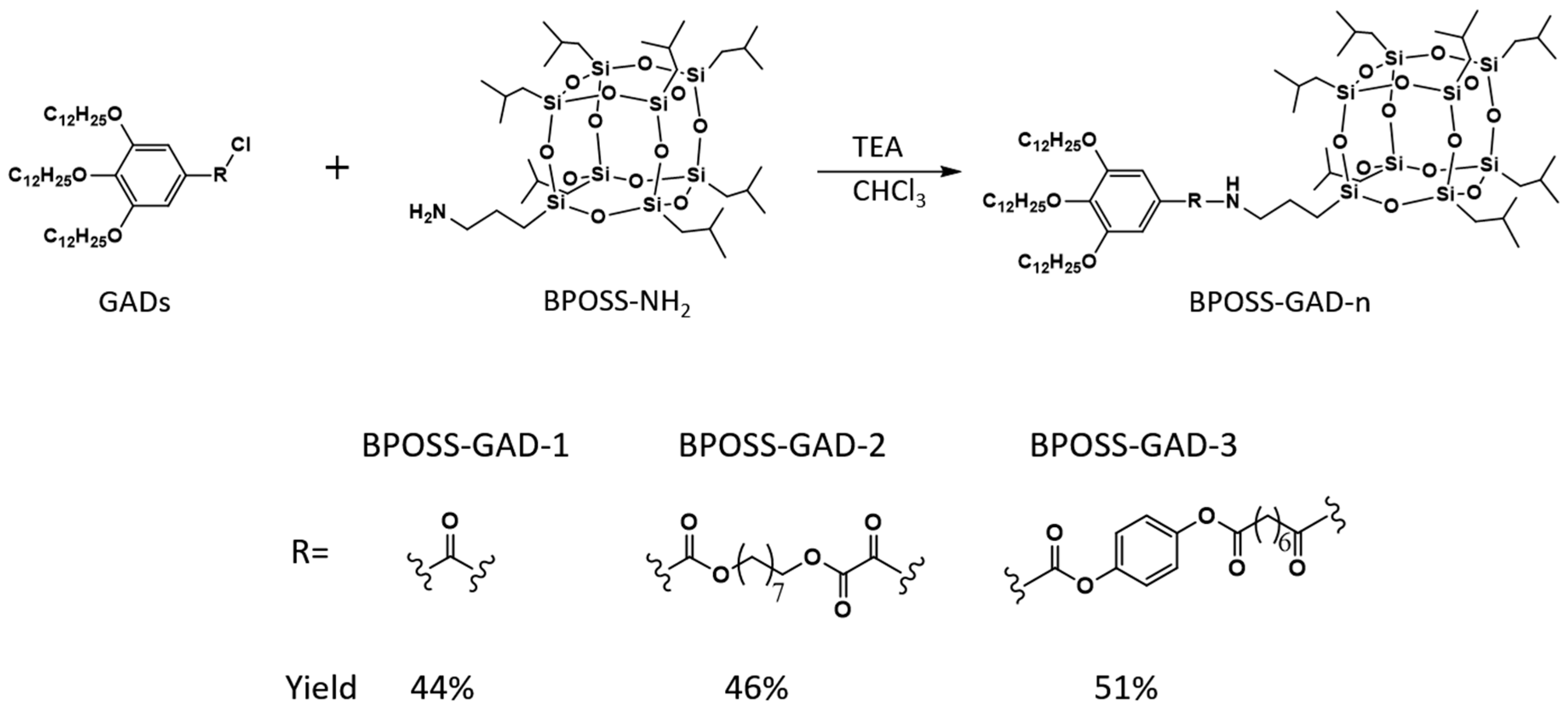
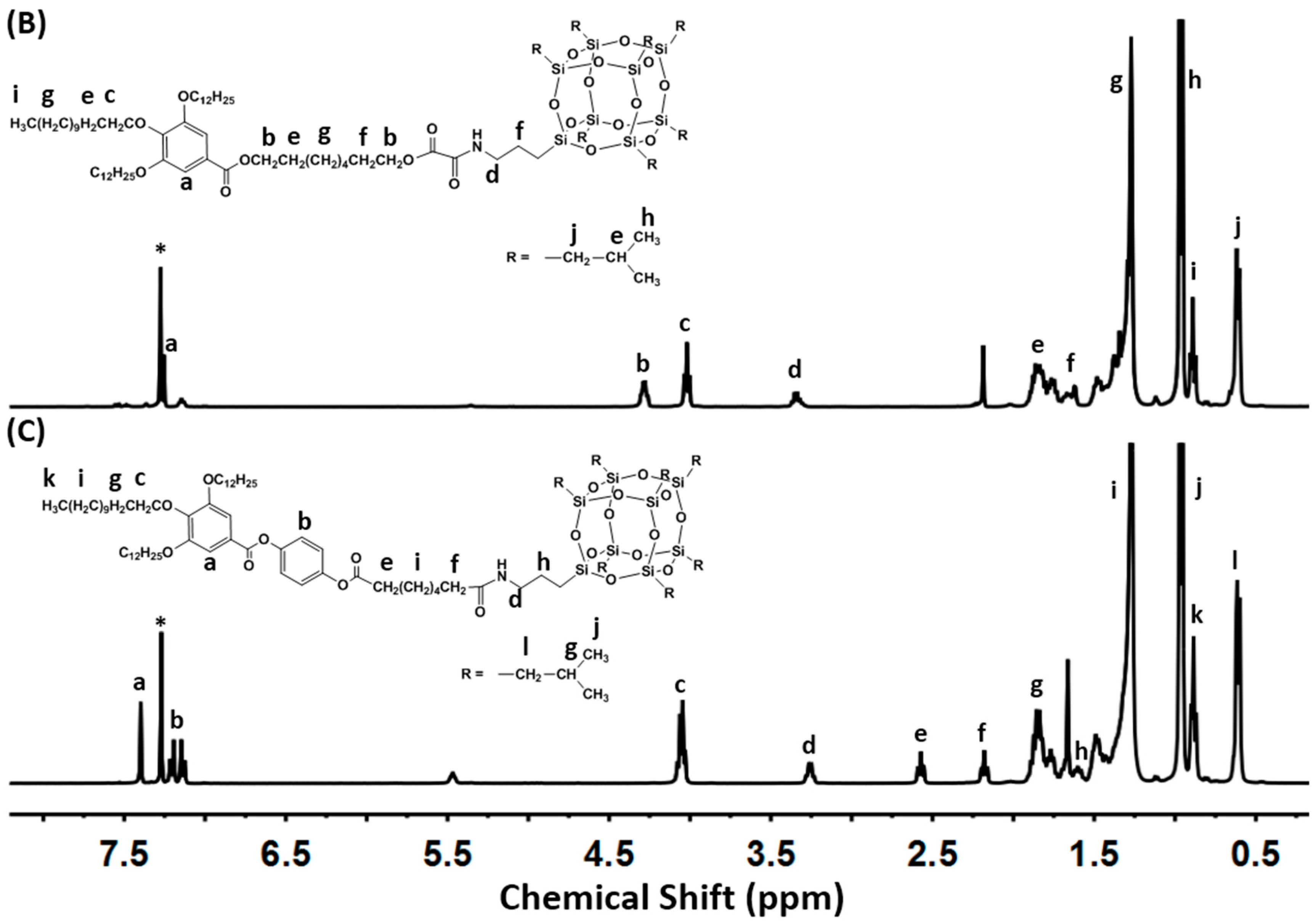
2.1. Molecular Synthesis and Characterizations
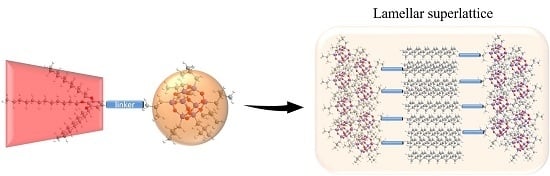
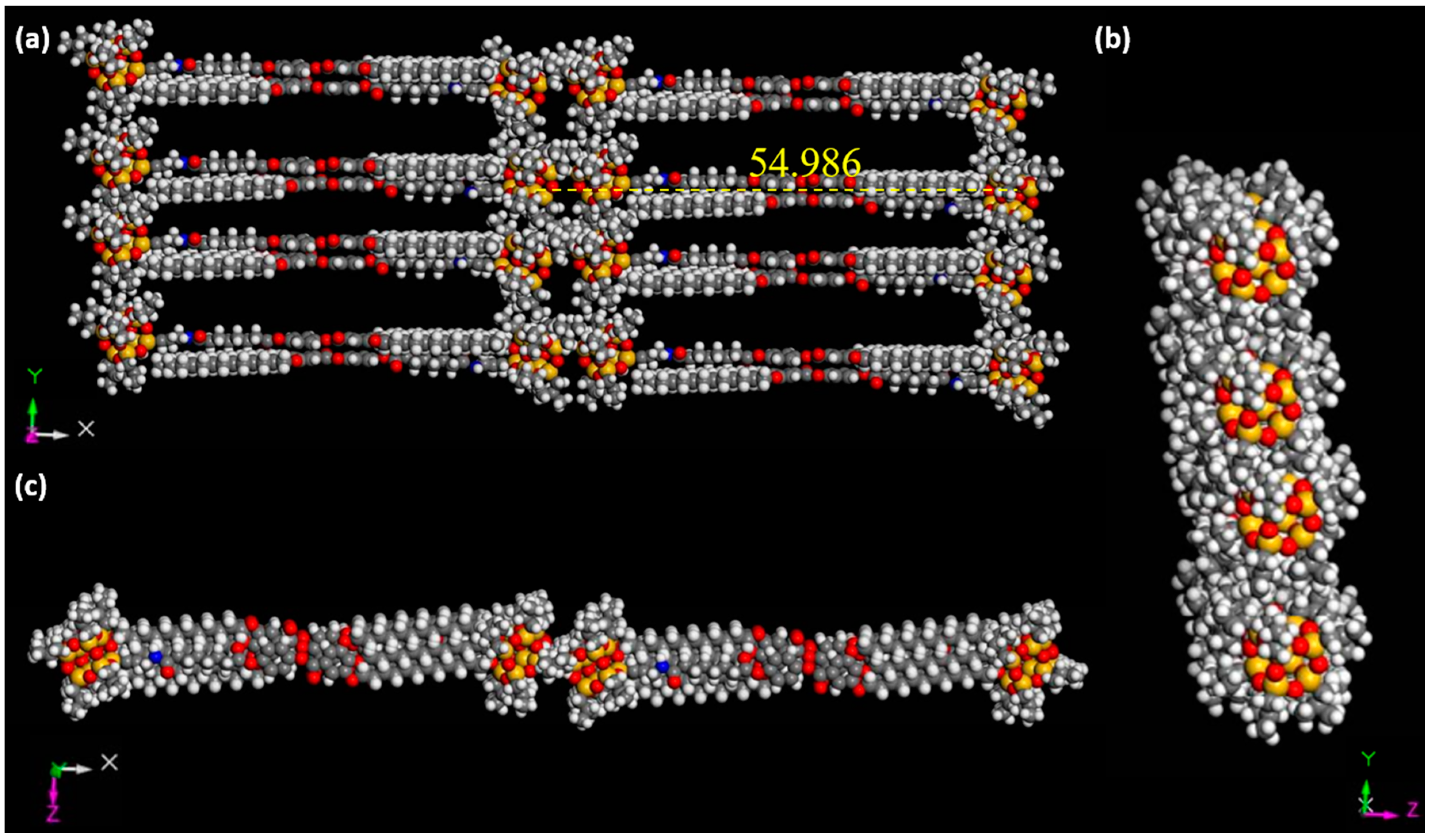
2.2. Structures in the Condensed State
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. General Synthetic Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsu, C.H.; Dong, X.H.; Lin, Z.; Ni, B.; Lu, P.; Jiang, Z.; Tian, D.; Shi, A.C.; Thomas, E.L.; Cheng, S.Z. Tunable Affinity and Molecular Architecture Lead to Diverse Self-Assembled Supramolecular Structures in Thin Films. ACS Nano 2016, 10, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Date, R.W.; Bruce, D.W. Shape Amphiphiles: Mixing Rods and Disks in Liquid Crystals. J. Am. Chem. Soc. 2003, 125, 9012–9013. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, S.C.; Horsch, M.A.; Iacovella, C.R.; Zhang, Z.; Chan, E.R.; Zhang, X. Self-assembly of anisotropic tethered nanoparticle shape amphiphiles. Curr. Opin. Colloid Interface Sci. 2005, 10, 287–295. [Google Scholar] [CrossRef]
- Feldmann, C.; Jüstel, T.; Ronda, C.R.; Schmidt, P.J. Inorganic luminescent materials: 100 years of research and application. Adv. Funct. Mater. 2003, 13, 511–516. [Google Scholar] [CrossRef]
- Zhong, Y.; Trinh, M.T.; Chen, R.; Wang, W.; Khlyabich, P.P.; Kumar, B.; Xu, Q.; Nam, C.-Y.; Sfeir, M.Y.; Black, C.; et al. Efficient Organic Solar Cells with Helical Perylene Diimide Electron Acceptors. J. Am. Chem. Soc. 2014, 136, 15215–15221. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.; Strijkers, G.J.; van Tilborg, G.A.; Cormode, D.P.; Fayad, Z.A.; Nicolay, K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 2009, 42, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, P.F.; Engel, M.; Glotzer, S.C. Predictive self-assembly of polyhedra into complex structures. Science 2012, 337, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Horsch, M.A.; Lamm, M.H.; Glotzer, S.C. Tethered Nano Building Blocks: Toward a Conceptual Framework for Nanoparticle Self-Assembly. Nano Lett. 2003, 3, 1341–1346. [Google Scholar] [CrossRef]
- Hudson, S.D.; Jung, H.-T.; Percec, V.; Cho, W.-D.; Johansson, G.; Ungar, G.; Balagurusamy, V.S.K. Direct Visualization of Individual Cylindrical and Spherical Supramolecular Dendrimers. Science 1997, 278, 449–452. [Google Scholar] [CrossRef]
- Zeng, X.; Ungar, G.; Liu, Y.; Percec, V.; Dulcey, A.E.; Hobbs, J.K. Supramolecular dendritic liquid quasicrystals. Nature 2004, 428, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Ungar, G.; Liu, Y.; Zeng, X.; Percec, V.; Cho, W.D. Giant supramolecular liquid crystal lattice. Science 2003, 299, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular Nanoparticles Are Unique Elements for Macromolecular Science: From “Nanoatoms” to Giant Molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Tu, Y.; Wang, C.-L.; Van Horn, R.M.; Tsai, C.-C.; Graham, M.J.; Sun, B.; Lotz, B.; Zhang, W.-B.; Cheng, S.Z.D. Hierarchical structure and polymorphism of a sphere-cubic shape amphiphile based on a polyhedral oligomeric silsesquioxane-[60]fullerene conjugate. J. Mater. Chem. 2011, 21, 14240–14247. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, W.B.; Yu, X.; Yue, K.; Sun, H.J.; Hsu, C.H.; Hsu, C.S.; Joseph, J.; Modarelli, D.A.; Cheng, S.Z. Facile synthesis and photophysical properties of sphere-square shape amphiphiles based on porphyrin-[60] fullerene conjugates. Chem. Asian J. 2013, 8, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, W.B.; Van Horn, R.M.; Tu, Y.; Gong, X.; Cheng, S.Z.; Sun, Y.; Tong, M.; Seo, J.; Hsu, B.B.; et al. A porphyrin–fullerene dyad with a supramolecular “double-cable” structure as a novel electron acceptor for bulk heterojunction polymer solar cells. Adv. Mater. 2011, 23, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-W.; Huang, C.-F.; Wu, K.-Y.; Wu, S.-L.; Chang, S.-T.; Cheng, Y.-J.; Wang, C.-L. Flat-on ambipolar triphenylamine/C60 nano-stacks formed from the self-organization of a pyramid-sphere-shaped amphiphile. Chem. Sci. 2016, 7, 2768–2774. [Google Scholar] [CrossRef]
- Kaiser, T.E.; Wang, H.; Stepanenko, V.; Würthner, F. Supramolecular Construction of Fluorescent J-Aggregates Based on Hydrogen-Bonded Perylene Dyes. Angew. Chem. Int. Ed. 2007, 46, 5541–5544. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Sun, B.; Tsai, C.-C.; Tu, Y.; Leng, S.; Li, K.; Kang, Z.; Horn, R.M.V.; Li, X.; Zhu, M.; et al. Synthesis, Self-assembly, and Crystal Structure of a Shape-Persistent Polyhedral-Oligosilsesquioxane-Nanoparticle-Tethered Perylene Diimide. J. Phys. Chem. B 2010, 114, 4802–4810. [Google Scholar] [CrossRef]
- Yu, X.; Yue, K.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Liu, C.; Xin, Y.; Wang, H.F.; Shi, A.C.; Newkome, G.R.; et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Natl. Acad. Sci. USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hsu, C.H.; Wang, J.; Mei, S.; Dong, X.; Li, Y.; Li, M.; Liu, H.; Zhang, W.; Aida, T.; et al. Self-assembly. Selective assemblies of giant tetrahedra via precisely controlled positional interactions. Science 2015, 348, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Collet, J.P.; Xu, G.; Zhu, L. Supramolecular Self-Assembly in a Disk-Cube Dyad Molecule Based on Triphenylene and Polyhedral Oligomeric Silsesquioxane (POSS). Chem. Mater. 2006, 18, 3503–3512. [Google Scholar] [CrossRef]
- Liu, H.; Hsu, C.H.; Lin, Z.; Shan, W.; Wang, J.; Jiang, J.; Huang, M.; Lotz, B.; Yu, X.; Zhang, W.B.; et al. Two-dimensional nanocrystals of molecular Janus particles. J. Am. Chem. Soc. 2014, 136, 10691–10699. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, J.; Shan, W.; Guo, D.; Wang, J.; Hsu, C.H.; Huang, M.; Zhang, W.; Lotz, B.; Zhang, W.B.; et al. Manipulation of Self-Assembled Nanostructure Dimensions in Molecular Janus Particles. ACS Nano 2016, 10, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Custers, J.P.A.; Hersmis, M.C.; Meuldijk, J.; Vekemans, J.A.J.M.; Hulshof, L.A. 3,4,5-Tri-Dodecyloxybenzoic Acid: Combining Reaction Engineering and Chemistry in the Development of an Attractive Tool to Assist Scaling Up Solid–Liquid Reactions. Org. Process Res. Dev. 2002, 6, 645–651. [Google Scholar] [CrossRef]
- Percec, V.; Aqad, E.; Peterca, M.; Rudick, J.G.; Lemon, L.; Ronda, J.C.; De, B.B.; Heiney, P.A.; Meijer, E.W. Steric communication of chiral information observed in dendronized polyacetylenes. J. Am. Chem. Soc. 2006, 128, 16365–16372. [Google Scholar] [CrossRef] [PubMed]
- Asahi Kasei Chemicals Corporation. Process for Production of Powder of Cage Silsesquioxane Compound. Patent 12/444,285, 20080410, 1 April 2010. [Google Scholar]
- Li, Y.; Zhang, W.B.; Hsieh, I.F.; Zhang, G.; Cao, Y.; Li, X.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z. Breaking symmetry toward nonspherical Janus particles based on polyhedral oligomeric silsesquioxanes: Molecular design, “click” synthesis, and hierarchical structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds BPOSS-GAD-1, BPOSS-GAD-2, BPOSS-GAD-3 are available from the authors. |

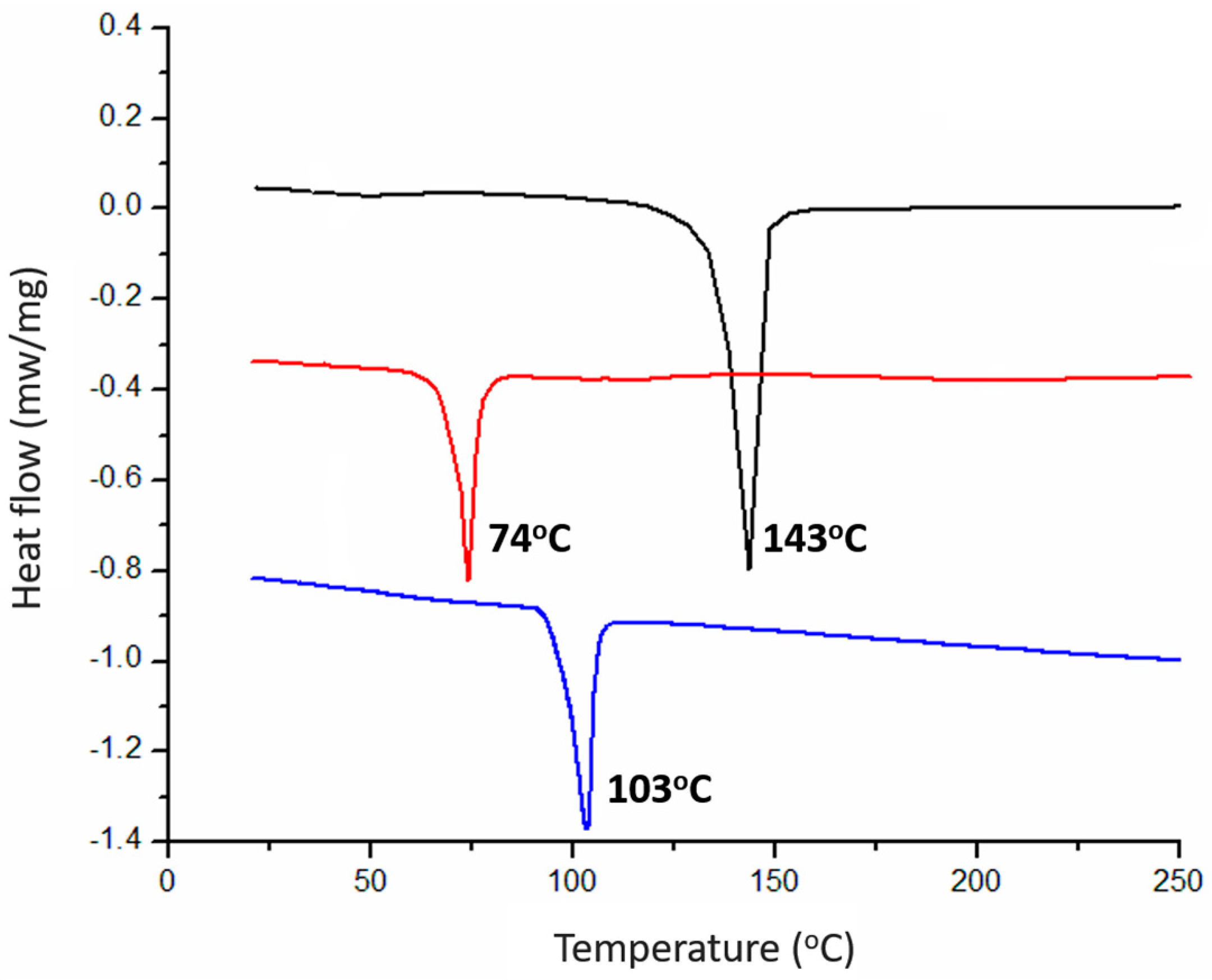
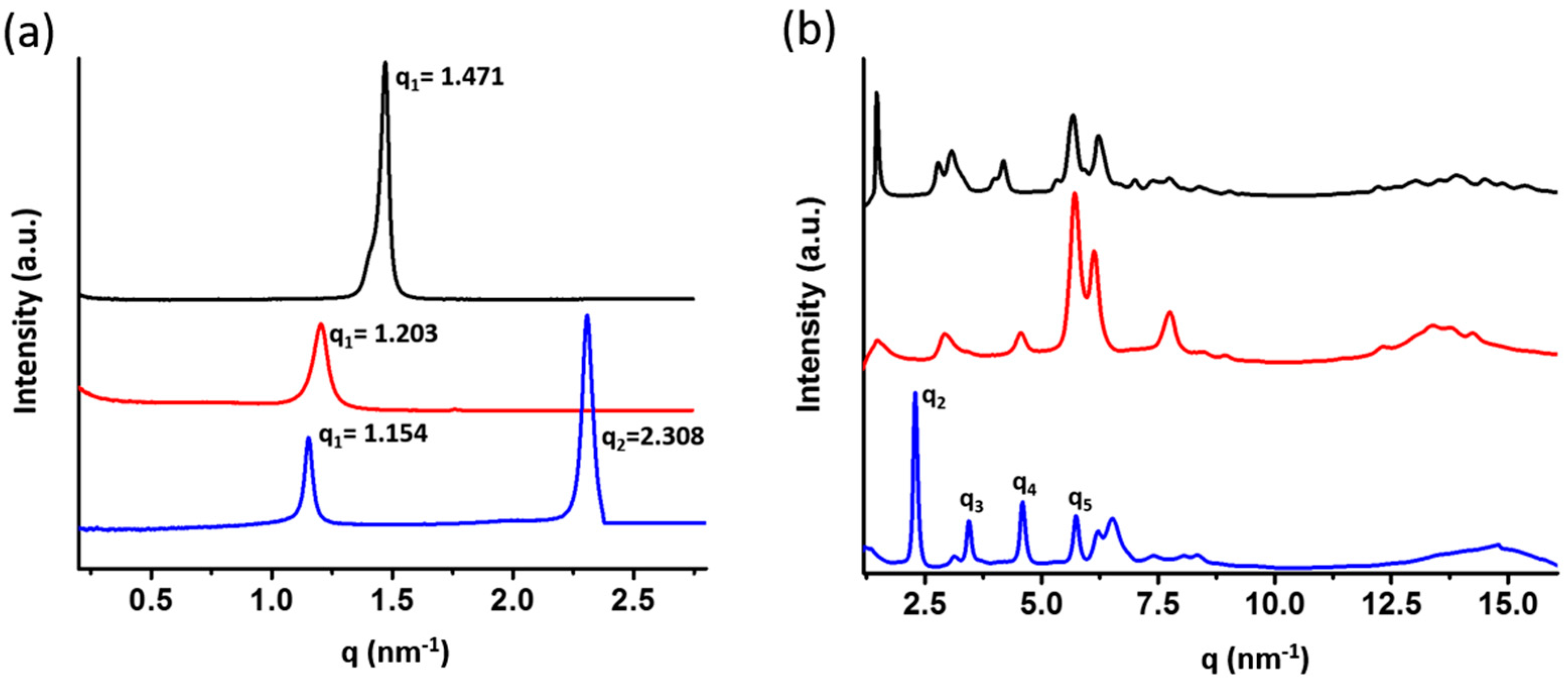
| Sample | q (nm−1) a | d-Spacing (Å) b | Tm (°C) | Morphology |
|---|---|---|---|---|
| BPOSS-GAD-1 | 1.471 | 42.70 | 143 | crystal |
| BPOSS-GAD-2 | 1.203 | 52.20 | 74 | crystal |
| BPOSS-GAD-3 | 1.154 | 54.42 | 103 | crystal with LAM superlattice |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Ding, M.; Xu, Y.; Zhao, F.; Dai, H.; Miao, X.-R.; Yang, S.; Li, H. Synthesis and Self-Assembly of Shape Amphiphiles Based on POSS-Dendron Conjugates. Molecules 2017, 22, 622. https://doi.org/10.3390/molecules22040622
Shao Y, Ding M, Xu Y, Zhao F, Dai H, Miao X-R, Yang S, Li H. Synthesis and Self-Assembly of Shape Amphiphiles Based on POSS-Dendron Conjugates. Molecules. 2017; 22(4):622. https://doi.org/10.3390/molecules22040622
Chicago/Turabian StyleShao, Yu, Minyuan Ding, Yujie Xu, Fangjia Zhao, Hui Dai, Xia-Ran Miao, Shuguang Yang, and Hui Li. 2017. "Synthesis and Self-Assembly of Shape Amphiphiles Based on POSS-Dendron Conjugates" Molecules 22, no. 4: 622. https://doi.org/10.3390/molecules22040622