Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity
Abstract
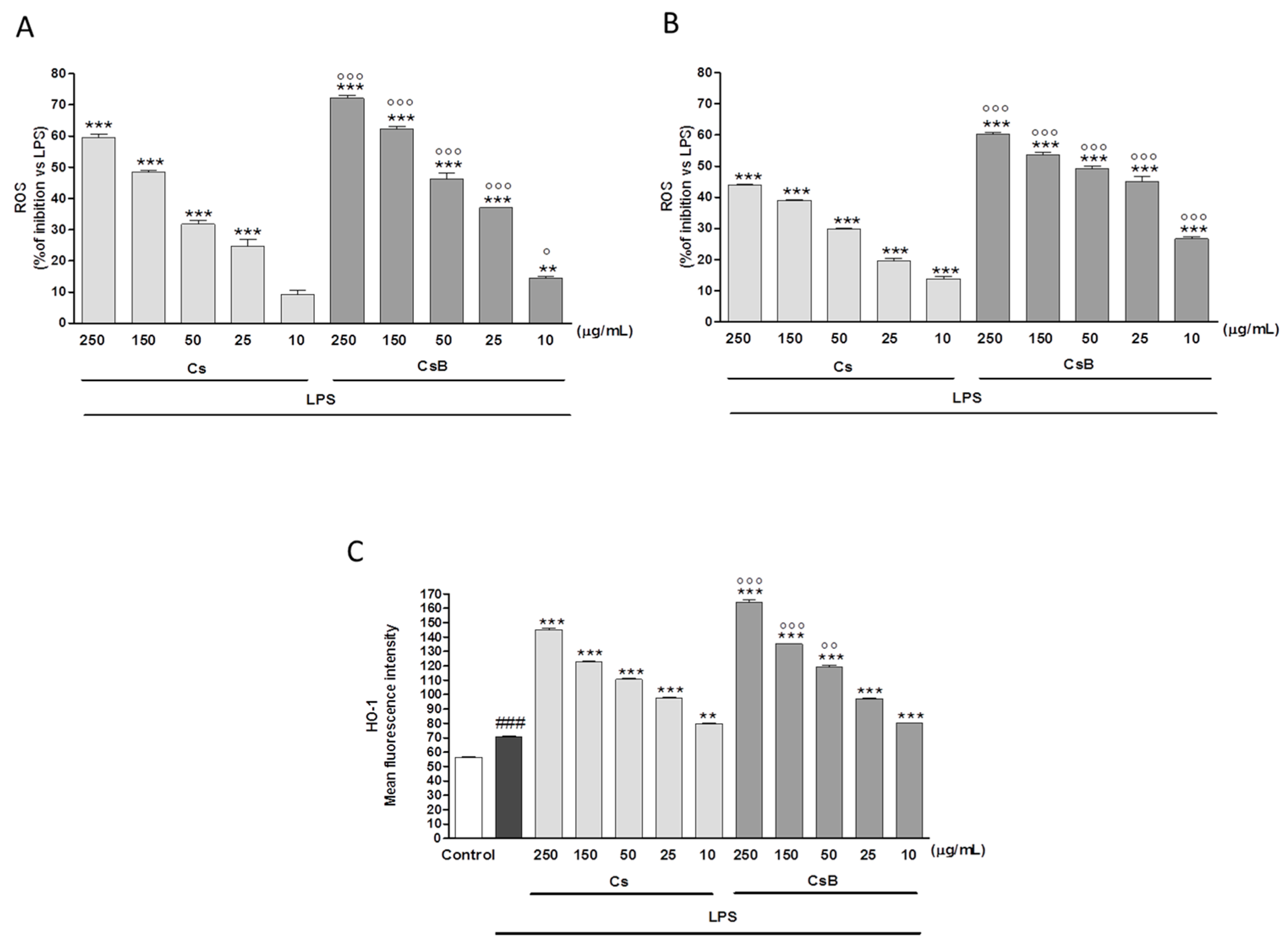
:1. Introduction
2. Results
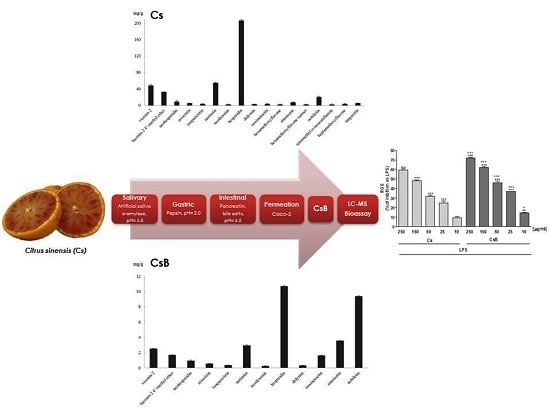
2.1. Qualitative and Quantitative Polyphenolic Profile of Citrus sinensis
2.2. Gastro-Intestinal Digestion and Bioavailability Assessment of Citrus sinensis Juice Flavonoids
2.3. Citrus sinensis and Bioavailable Fractions Did Not Affect Cell Viability
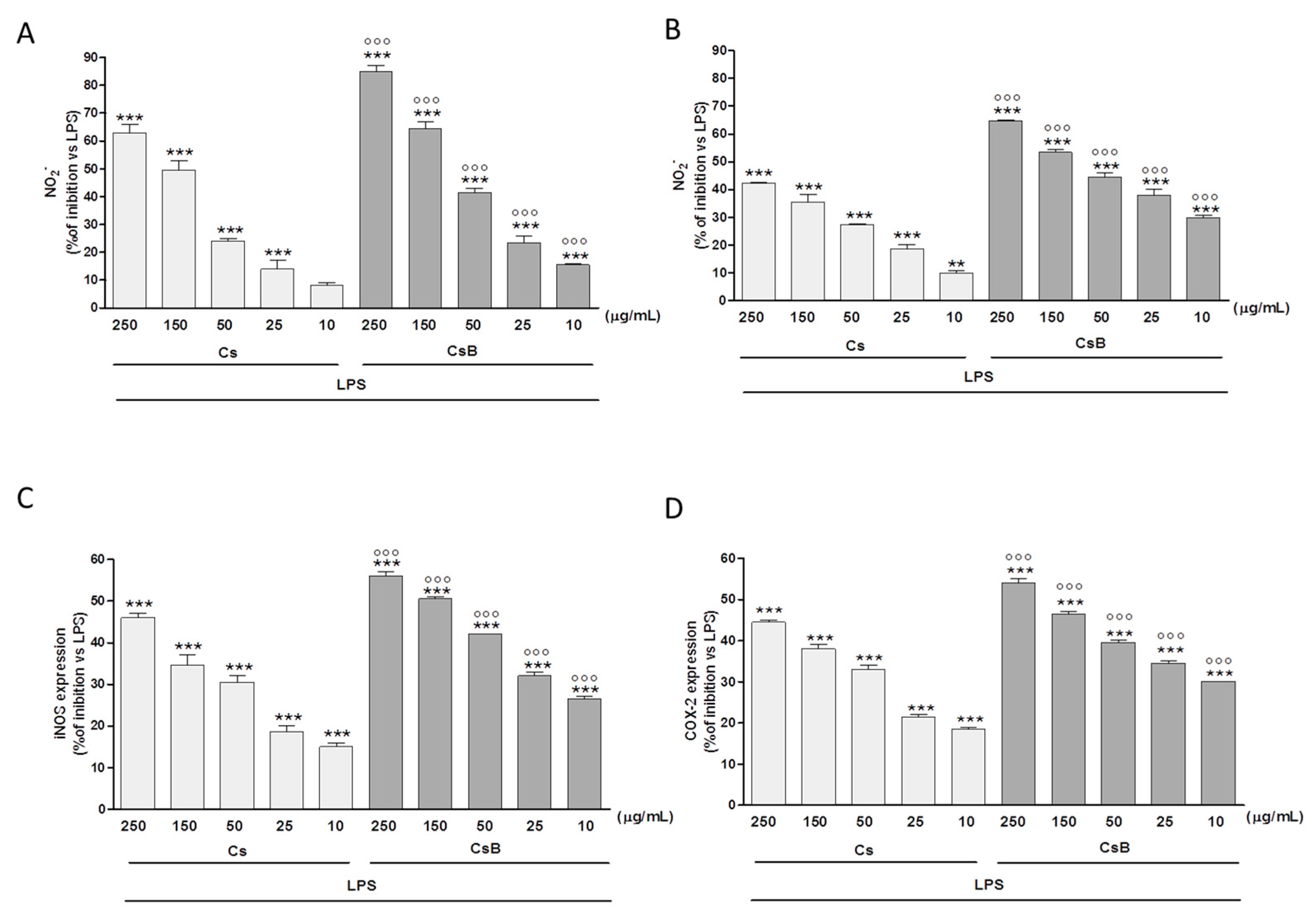
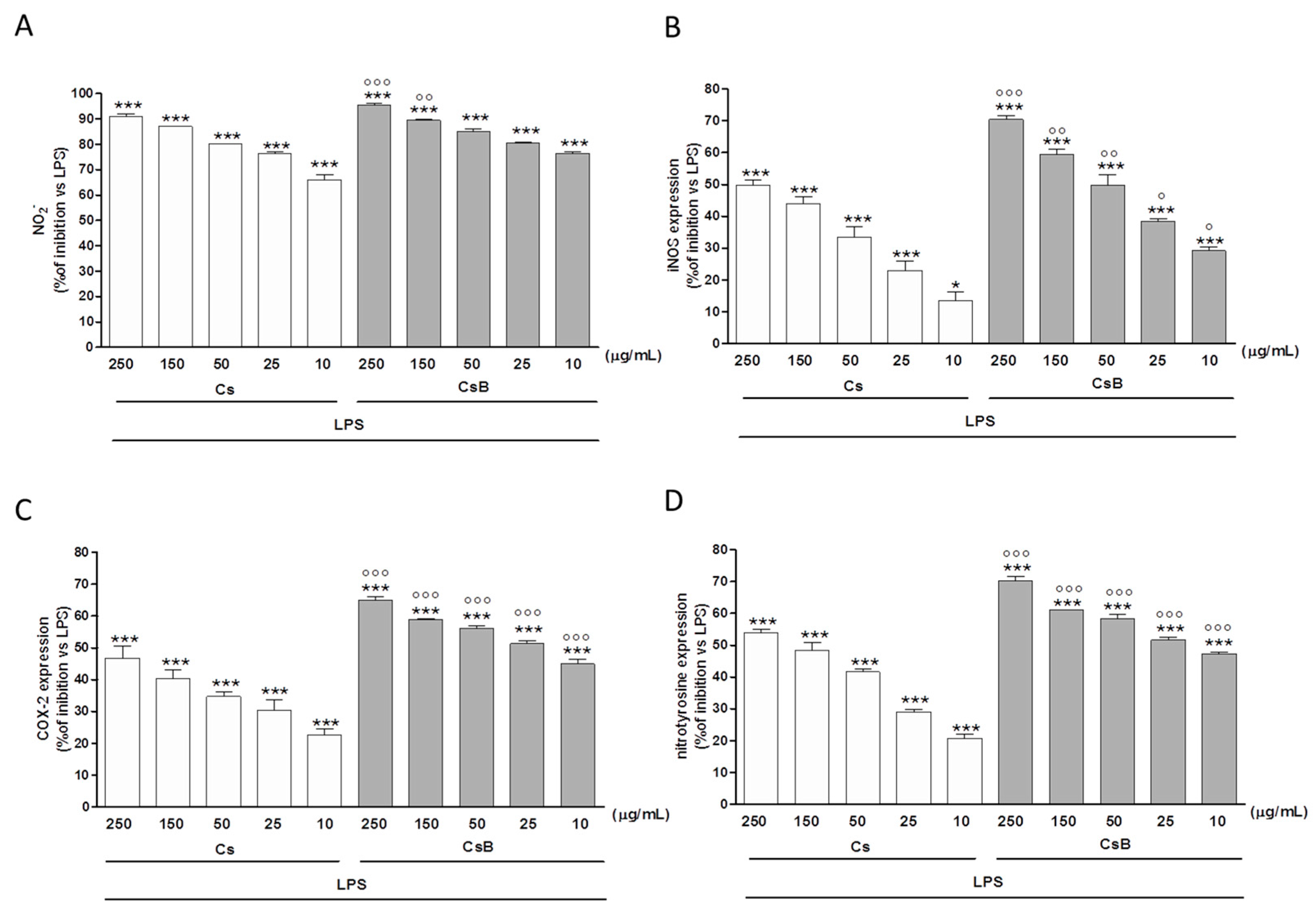
2.4. Effect of Citrus Extracts on LPS-Induced NO, iNOS and COX-2
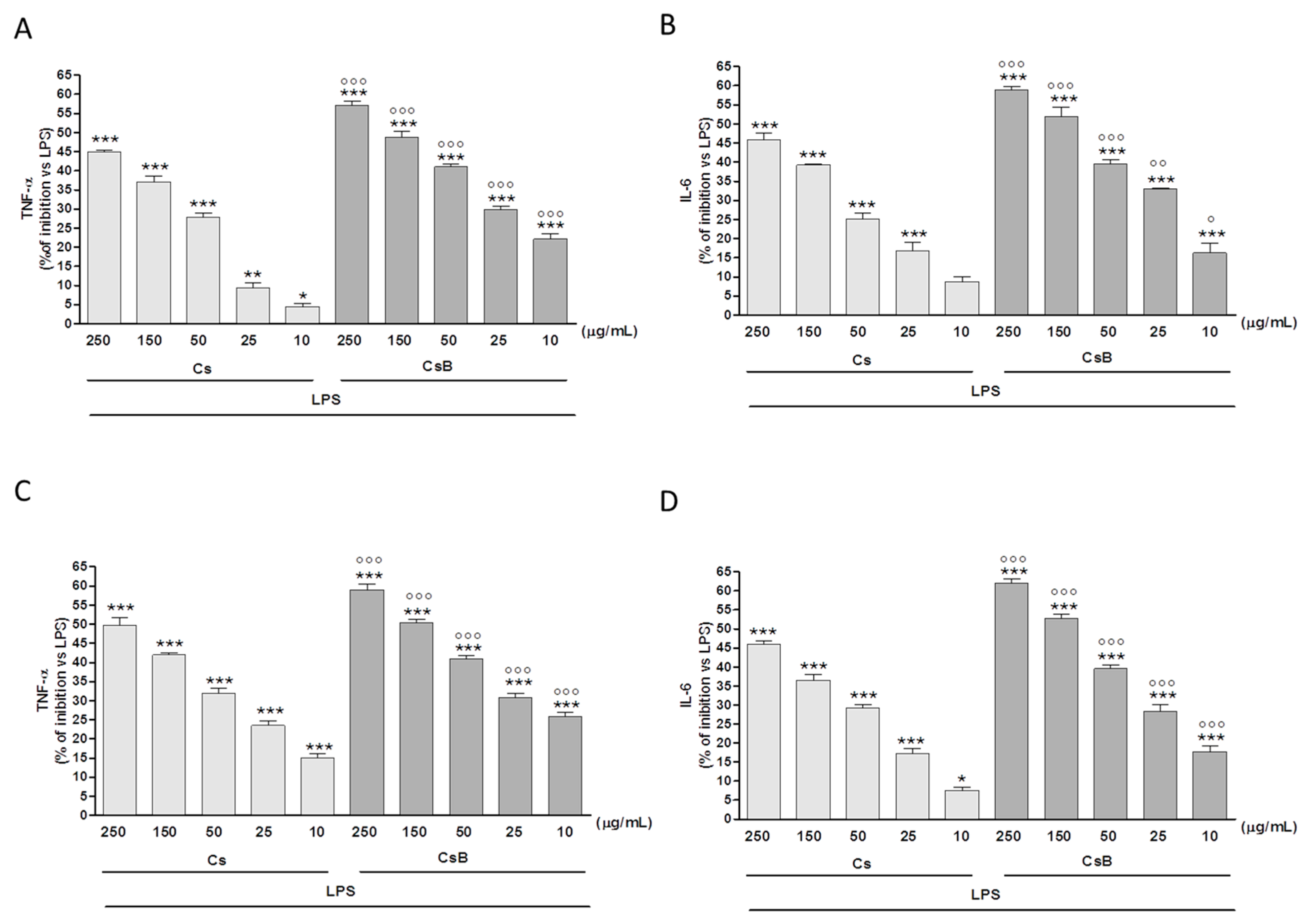
2.5. Effect of Citrus Extracts on LPS-Induced TNF-α and IL-6 Production
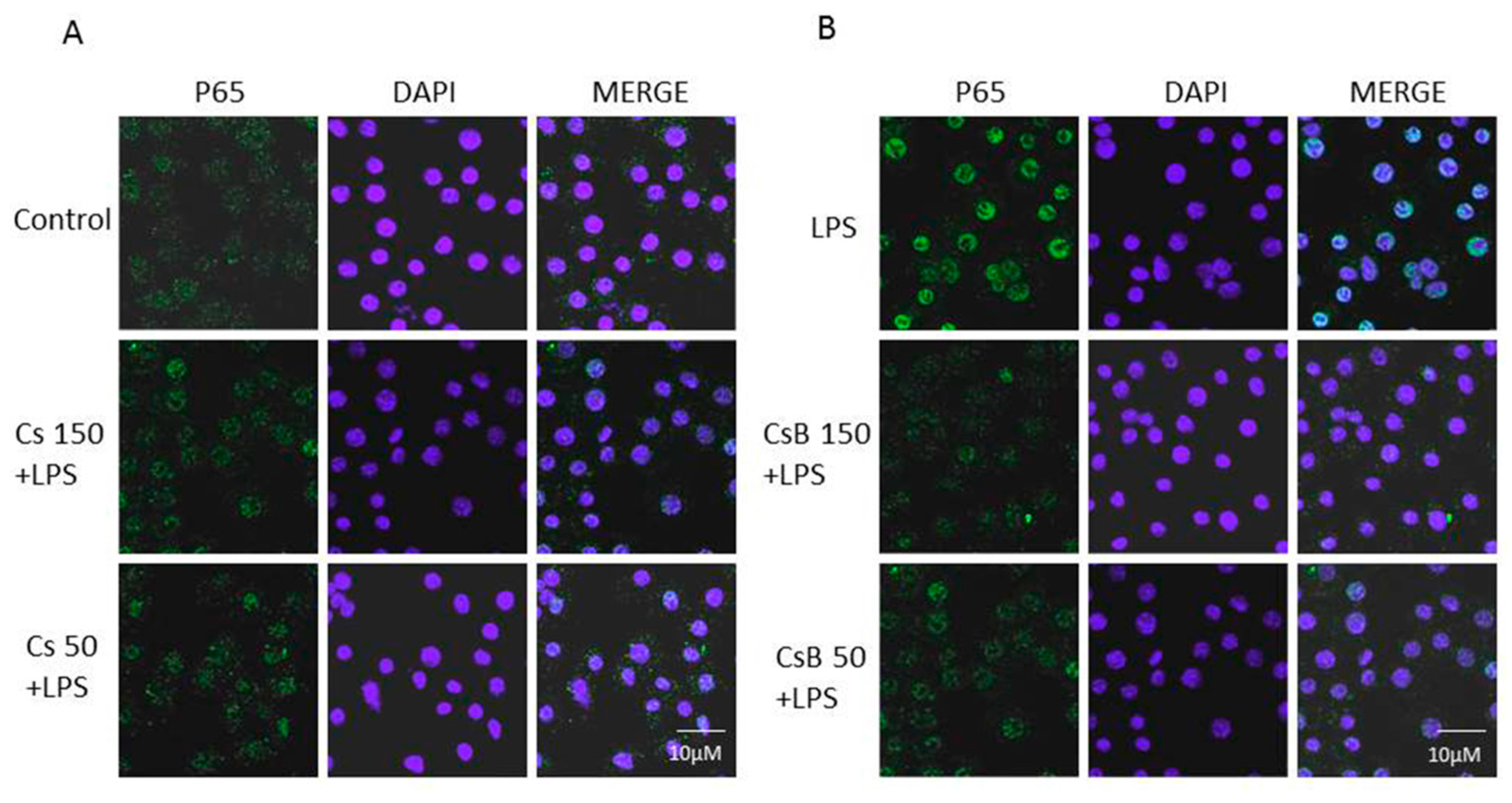
2.6. Effect of Citrus Extracts on p65 NF-kB Nuclear Translocation
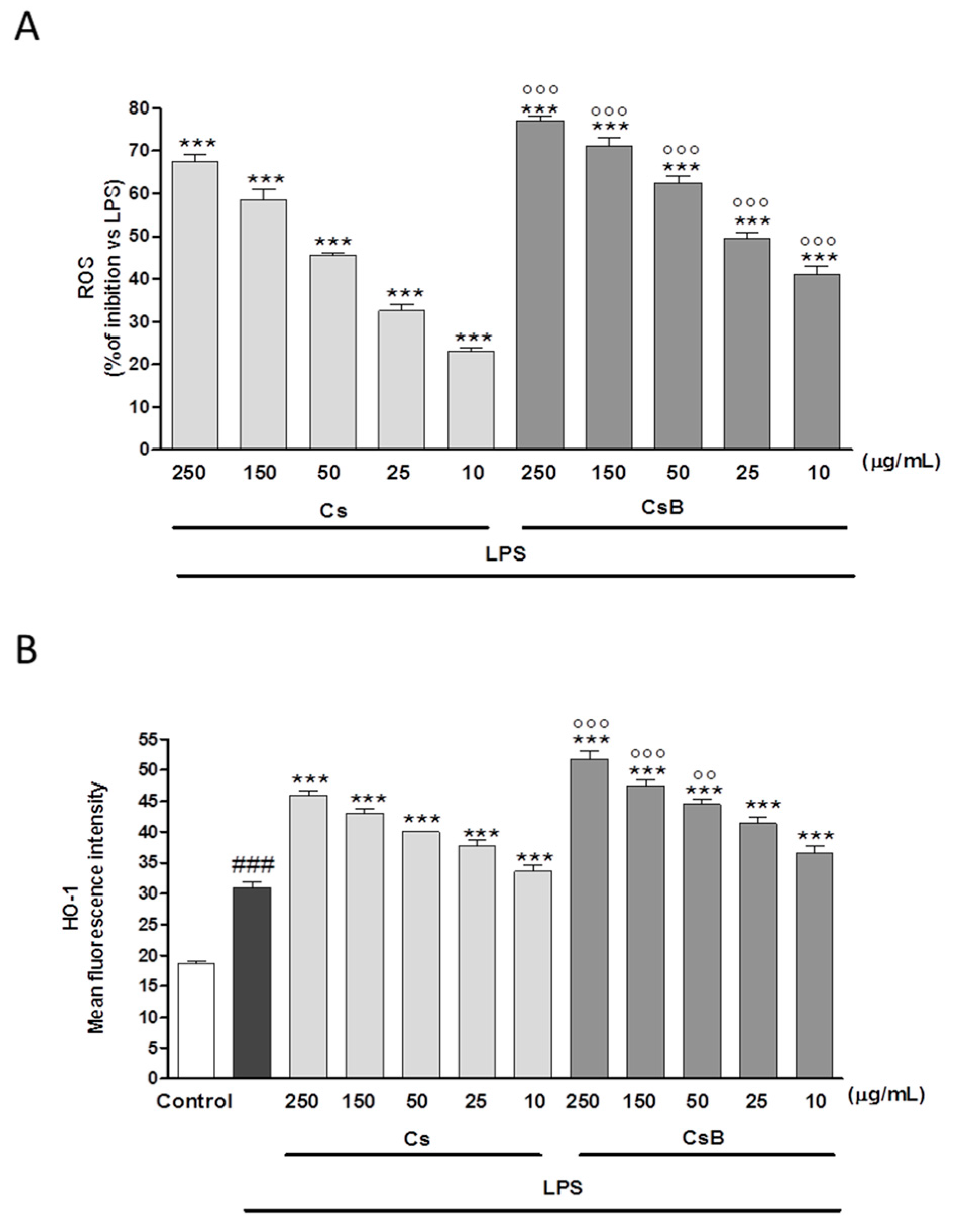
2.7. Effect of Citrus Extracts on LPS-Induced ROS and Induced HO-1 Expression
2.8. CsB and Cs Suppress LPS-Mediated Inflammatory Response in Mouse Primary Macrophages
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Sample Preparation
4.3. Instrumentation
4.4. UHPLC PDA Conditions
4.5. UHPLC-ESI-IT-TOF Conditions
4.6. In Vitro Gastrointestinal Digestion
4.7. Cell Culture
4.7.1. Caco-2 Cell Line
4.7.2. J774A.1 Macrophage Cell Line
4.7.3. Primary Murine Peritoneal Macrophages
4.8. Experimental Procedures
4.9. Antiproliferative Activity
4.10. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.11. Immunofluorescence Analysis with Confocal Microscopy in Macrophages J774A.1
4.12. Nitrite Determination and Cytofluorimetry Evaluation of iNOS, COX-2 and HO-1 Expression and Nitrotyrosine Formation in J774A.1
4.13. TNF-α and IL-6 Determination in Macrophages J774A.1
4.14. NO Determination and iNOS, COX-2, Nitrotyrosine, ROS and HO-1 Detection in Mouse Peritoneal Macrophages
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hauser, A.B.; Stinghen, A.E.; Kato, S.; Bucharles, S.; Aita, C.; Yuzawa, Y.; Pecoits-Filho, R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit. Dial. Int. 2008, 28, 183–187. [Google Scholar]
- Pike, J.; Chandra, R.K. Effect of vitamin and trace element supplementation on immune indices in healthy elderly. Int. J. Vitaminol. Nutr. Res. 1995, 65, 117–121. [Google Scholar]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.G.; Jones, K.H.; Jiang, Z.; Lantry, L.E.; Southwell-Keely, P.; Kohar, I.; Thornton, D.E. Cytotoxicity of Tocopherols and Their Quinones in Drug-Sensitive and Multidrug-Resistant LeUKemia Cells. Lipids 1998, 33, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Mazumder, U.K.; Pal, D.; Mishra, M.L.; Maity, S. Flavonoids of Enhydra Fluctuans exhibits analgesic and anti-inflammatory activity in different animal models. Pak. J. Pharm. Sci. 2011, 24, 369–375. [Google Scholar] [PubMed]
- Braca, A.; Dal Piaz, F.; Marzocco, S.; Autore, G.; Vassallo, A.; De Tommasi, N. Triterpene derivatives as inhibitors of protein involved in the inflammatory process: Molecules interfering with phospholipase A2, cycloxygenase, and lipoxygenase. Curr. Drug Targets 2011, 12, 302–321. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bioaccessibility and antioxidant activity of grape. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K.; Guthrie, N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcia, O.; Castillo, J.; Sabater, F.; Del Rio, J.A. Characterization of a S-adenosyl-l-methionine: Eriodictyol 4′-O-methyltransferase from Citrus aurantium. Developmental changes in the levels of 4′-O-methoxyflavonoids and S-adenosyl-l-methionine: Eriodictyol 4′-O-methyltransferase activity. Plant Physiol. Biochem. 1995, 33, 263–271. [Google Scholar]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Larrosa, M.; Escudero, E.; Zafrilla, M.P.; Cerdá, B.; Boza, J.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J. Agric. Food Chem. 2010, 58, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1148–G1154. [Google Scholar] [PubMed]
- Park, K.I.; Park, H.S.; Kim, M.K.; Hong, G.E.; Nagappan, A.; Lee, H.J.; Yumnam, S.; Lee, W.S.; Won, C.K.; Shin, S.C.; et al. Flavonoids identified from Korean Citrus aurantium L. inhibit non-small cell lung cancer growth in vivo and in vitro. J. Funct. Foods 2014, 7, 287–297. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Oulianova, N.; Cheng, D.; Huebert, N.; Chen, Y. Human oral drugs absorption is correlated to their in vitro uptake by brush border membrane vesicles. Int. J. Pharm. 2007, 336, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, M.H.; Wang, Z.; Lambros, T.; Ho, C.T. Biological Activity, Metabolism and Separation of Polymethoxyflavonoids from Citrus Peels. Tree For. Sci. Biotechnol. 2008, 2, 36–51. [Google Scholar]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Brown, D.M.; Donaldson, K.; Borm, P.J.; Schins, R.P.; Dehnhardt, M.; Gilmour, P.; Jimenez, L.A.; Stone, V. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L344–L353. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Galleggiante, V.; De Santis, S.; Cavalcanti, E.; Scarano, A.; De Benedictis, M.; Serino, G.; Caruso, M.L.; Mastronardi, M.; Pinto, A.; Campiglia, P.; et al. Dendritic Cells Modulate Iron Homeostasis and Inflammatory Abilities Following Quercetin Exposure. Curr. Pharm. Des. 2017, in press. [Google Scholar]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- McAnlis, G.T.; McEneny, J.; Pearce, J.; Young, I.S. The effect of various dietary flavonoids on the susceptibility of low density lipoproteins to oxidation in vitro using both metallic and non-metallic oxidising agents. Biochem. Soc. Trans. 1997, 25, 142S. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Chen, K.Y.; Ho, C.T.; Li, S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci. Hum. Wellness 2014, 3, 26–35. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the structures of flavonoids and their NF-κB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011, 21, 6036–6041. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Campiglia, P.; Stiuso, P.; Ritieni, A.; Novellino, E. Nutraceutical potential of polyphenolic fractions from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 140, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Popolo, A.; Bianco, G.; Sorrentino, R.; Pinto, A.; Autore, G.; Marzocco, S. The Uremic Toxin Indoxyl Sulphate Enhances Macrophage Response to LPS. PLoS ONE 2013, 8, e76778. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemia, M.; KchoUK, M.E.; Senatore, F.; Autore, G.; Marzocco, S.; De Feo, V.; Bruno, M. Antiproliferative activity of hexane extract from Tunisian Cistus libanotis, Cistus monspeliensis and Cistus villosus. Chem. Cent. J. 2013, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Del Regno, M.; Adesso, S.; Popolo, A.; Quaroni, A.; Autore, G.; Severino, L.; Marzocco, S. Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells. Toxicol. Appl. Pharmacol. 2015, 285, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Pepe, G.; Sommella, E.; Manfra, M.; Scopa, A.; Sofo, A.; Tenore, G.C.; Russo, M.; Di Gaudio, F.; Autore, G.; et al. Anti-inflammatory and antioxidant activity of polyphenolic extracts from Lactuca sativa (var. Maravilla de Verano) under different farming methods. J. Sci. Food Agric. 2016, 96, 4194–4206. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC prfiling and effects on LPS-stimulated J774A.1 macrophages of flavonoids from bergamot (Citrus bergamia) juice, an underestimated waste product with high anti-inflammatory potential. J. Funct. Foods 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds present in Citrus sinensis are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S.; et al. Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity. Molecules 2017, 22, 623. https://doi.org/10.3390/molecules22040623
Pepe G, Pagano F, Adesso S, Sommella E, Ostacolo C, Manfra M, Chieppa M, Sala M, Russo M, Marzocco S, et al. Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity. Molecules. 2017; 22(4):623. https://doi.org/10.3390/molecules22040623
Chicago/Turabian StylePepe, Giacomo, Francesco Pagano, Simona Adesso, Eduardo Sommella, Carmine Ostacolo, Michele Manfra, Marcello Chieppa, Marina Sala, Mariateresa Russo, Stefania Marzocco, and et al. 2017. "Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity" Molecules 22, no. 4: 623. https://doi.org/10.3390/molecules22040623