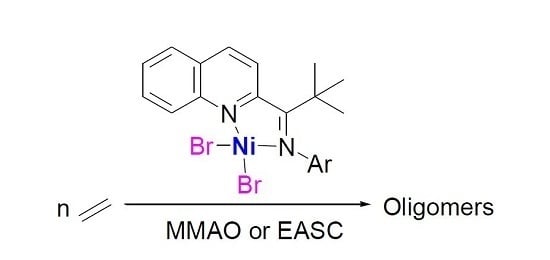
N-(2,2-Dimethyl-1-(quinolin-2-yl)propylidene) arylaminonickel Complexes and Their Ethylene Oligomerization
Abstract
:1. Introduction
2. Results
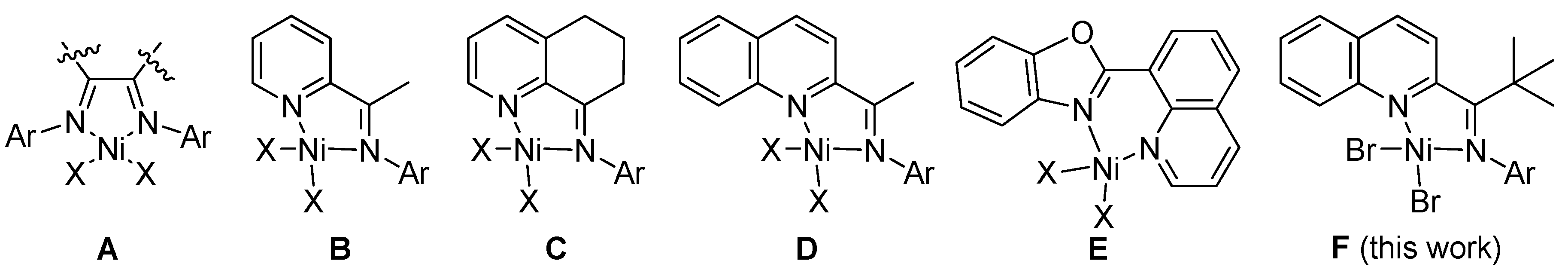
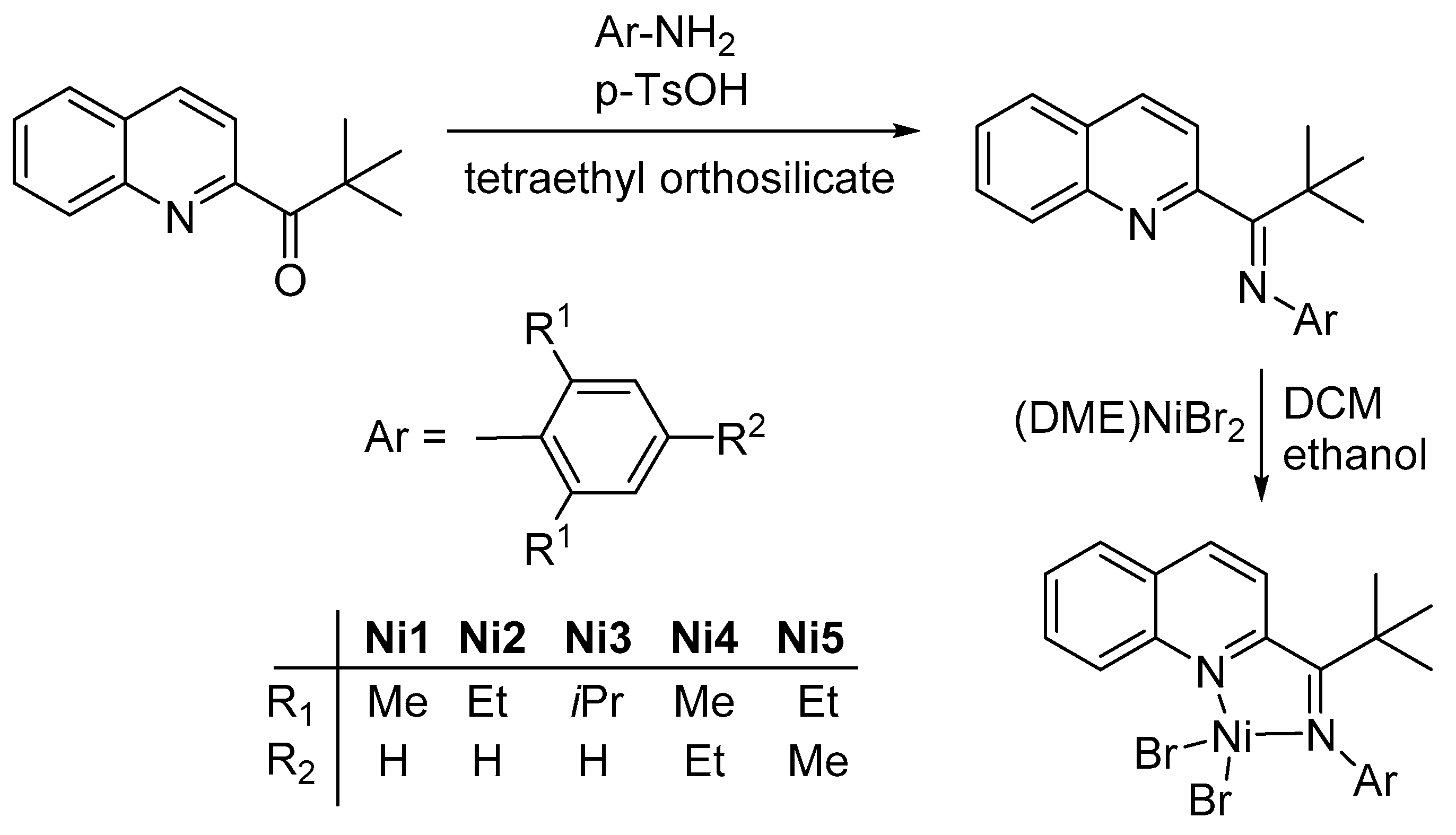
2.1. Synthesis and Characterization of Ligands and Nickel Complexes
2.2. Ethylene Oligomerization
2.2.1. Ethylene Oligomerization in Presence of EASC
2.2.2. Ethylene Oligomerization in Presence of MMAO
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization of Ligands
3.3. Synthesis and Characterization of Nickel Complexes
3.4. General Procedure for Ethylene Oligomerization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(I1)-and Ni(I1)-based batalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Keim, W.; Kowaldt, F.H.; Goddard, R.; Kruger, C. Novel coordination of (benzoy1methylene) triphenylphosphorane in a nickel oligomerization catalyst. Angew. Chem. Int. Ed. Engl. 1978, 17, 466–467. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo-and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.-H.; Redshaw, C. Recent progress on nickel-based systems for ethylene oligo-/polymerization catalysis. J. Organomet. Chem. 2014, 751, 717–741. [Google Scholar] [CrossRef]
- Schmid, M.; Eberhardt, R.; Klinga, M.; Leskela, M.; Rieger, B. New C2V-and chiral C2-symmetric olefin polymerization catalysts based on nickel(II) and palladium(II) diimine complexes bearing 2,6-diphenyl aniline moieties: Synthesis, structural characterization, and first insight into polymerization properties. Organometallics 2001, 20, 2321–2330. [Google Scholar] [CrossRef]
- Zou, H.; Zhu, F.; Wu, Q.; Ai, J.; Lin, S.A. Synthesis of long-chain-branched polyethylene by ethylene homopolymerization with a novel nickel(II) α-diimine catalyst. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1325–1330. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; Yu, J.; Yang, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.-H. Synthesis, characterization and ethylene polymerization behavior of nickel dihalide complexes bearing bulky unsymmetrical α-diimine ligands. Catal. Sci. Technol. 2012, 2, 415–422. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, W.; Liu, W.; Wang, L.; Redshaw, C.; Sun, W.-H. Unsymmetrical α-diiminonickel bromide complexes: Synthesis, characterization and investigation of their catalytic behavior toward ethylene. Catal. Sci. Technol. 2013, 3, 2737–2745. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-;diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, S.; Huang, C.; Solan, G.A.; Hao, X.; Sun, W.-H. Balancing high thermal stability with high activity in diaryliminoacenaphthene-Nickel(II) catalysts for ethylene polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Yuan, Y.; Du, S.; Sun, Y.; Solan, G.A.; Guo, C.; Sun, W.-H. Raising the N-aryl fluoride content in unsymmetrical diaryliminoacenaphthylenes as a route to highly active Nickel(II) catalysts in ethylene polymerization. Dalton Trans. 2016, 45, 18313–18323. [Google Scholar] [CrossRef] [PubMed]
- Laine, T.V.; Piironen, U.; Lappalainen, K.; Klinga, M.; Aitola, E.; Leskelä, M. Pyridinylimine-based nickel(II) and palladium(II) complexes: Preparation, structural characterization and use as alkene polymerization catalysts. J. Organomet. Chem. 2000, 606, 112–124. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, D.; Zhang, T.; Sun, W.-H.; Chen, J.; Ren, Q.; Liu, D.; Zheng, G.; Chen, W. Bridged bis-pyridinylimino dinickel(II) complexes: Syntheses, characterization, ethylene oligomerization and polymerization. J. Organomet. Chem. 2005, 690, 1739–1749. [Google Scholar] [CrossRef]
- Yue, E.; Zhang, L.; Xing, Q.; Cao, X.P.; Hao, X.; Redshaw, C.; Sun, W.-H. 2-(1-(2-Benzhydrylnaphthylimino)ethyl)pyridylnickel halides: Synthesis, characterization, and ethylene polymerization behavior. Dalton Trans. 2014, 43, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zeng, Y.; Huang, W.; Hao, X.; Sun, W.-H. N-(5,6,7-Trihydroquinolin-8-ylidene)arylaminonickel dichlorides as highly active single-site pro-catalysts in ethylene polymerization. Dalton Trans. 2011, 40, 8436–8443. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Zeng, Y.; Zhang, L.; Ni, C.; Hao, X.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization behaviour of N-(2-Substituted-5,6,7-trihydroquinolin-8-ylidene)arylaminonickel dichlorides. New J. Chem. 2011, 35, 178–183. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Sun, W.-H.; Redshaw, C. Synthesis, characterization and ethylene polymerization behavior of 8-(nitroarylamino)-5,6,7-trihydroquinolylnickel dichlorides: Influence of the nitro group and impurities on catalytic activity. ACS Catal. 2011, 1, 1213–1220. [Google Scholar] [CrossRef]
- Sun, Z.; Yue, E.; Qu, M.; Oleynik, I.V.; Oleynik, I.I.; Li, K.; Liang, T.; Zhang, W.; Sun, W.-H. 8-(2-Cycloalkylphenylimino)-5,6,7-trihydroquinolylnickel halides: Polymerizing ethylene to highly branched and lower molecular weight polyethylenes. Inorg. Chem. Front. 2015, 2, 223–227. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Liang, T.; Zhao, Z.; Hu, X.; Sun, W.-H. Rigid geometry 8-arylimino-7,7-dimethyl-5,6-dihydroquinolyl nickel bromides: Single-site active species towards ethylene polymerization. New J. Chem. 2016, 40, 9329–9336. [Google Scholar] [CrossRef]
- Song, S.; Zhao, W.; Wang, L.; Redshaw, C.; Wang, F.; Sun, W.-H. 2-(1-Aryliminoethylidene)quinolylnickel(II) dibromides: Synthesis, characterization and ethylene dimerization capability. J. Organomet. Chem. 2011, 696, 3772–3778. [Google Scholar] [CrossRef]
- Hao, P.; Song, S.; Xiao, T.; Li, Y.; Redshaw, C.; Sun, W.-H. Highly active 8-benzoxazolyl-or 8-benzothiazolyl-2-alkylquinolinylnickel(II) complexes for ethylene dimerization and vinyl polymerization of norbornene. Polyhedron 2013, 52, 1138–1144. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhang, S.; Jie, S.; Zhang, W.; Li, Y.; Ma, H.; Chen, J.; Wedeking, K.; Fröhlich, R. Synthesis, characterization and ethylene oligomerization studies of nickel complexes bearing 2-imino-1,10-phenanthrolines. J. Organomet. Chem. 2006, 691, 4196–4203. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Wang, X.; Mahmood, Q.; Hao, X.; Hu, X.; Guo, C.Y.; Solan, G.A.; Sun, W.-H. Highly branched unsaturated polyethylenes achievable using strained imino-cyclopenta[b]pyridyl-nickel precatalysts. Polym. Chem. 2017, 8, 995–1005. [Google Scholar] [CrossRef]
- Huang, F.; Sun, Z.; Du, S.; Yue, E.; Ba, J.; Hu, X.; Liang, T.; Galland, G.B.; Sun, W.-H. Ring-tension adjusted ethylene polymerization by aryliminocycloheptapyridylnickel complexes. Dalton Trans. 2015, 44, 14281–14292. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, F.; Qu, M.; Yue, E.; Oleynik, I.V.; Oleynik, I.I.; Zeng, Y.; Liang, T.; Li, K.; Zhang, W.; et al. Targeting polyethylene waxes: 9-(2-Cycloalkylphenylimino)-5,6,7,8-tetrahydrocyclo heptapyridylnickel halides and their ethylene polymerization. RSC Adv. 2015, 5, 77913–77921. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhang, W.; Gao, T.; Tang, X.; Chen, L.; Li, Y.; Jin, X. Synthesis and characterization of N-(2-pyridyl)benzamide-based nickel complexes and their activity for ethylene oligomerization. J. Organomet. Chem. 2004, 689, 917–929. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, D.; Jie, S.; Sun, W.-H.; Chen, J. Nickel(II) complexes bearing phosphinooxazoline ligands: Synthesis, structures and their ethylene oligomerization behaviors. J. Organomet. Chem. 2005, 690, 3918–3928. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, W.-H.; Li, T.; Yang, X. Influence of electronic effect on catalytic activity of bis(imino)pyridyl Fe(II) and bis(imino)pyrimidyl Fe(II) complexes. J. Mol. Catal. A Chem. 2004, 218, 119–124. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Li, Y.; Pan, L.; Li, Y.; Hu, N. Fe(II) and Co(II) pyridinebisimine complexes bearing different substituents on ortho-and para-position of imines: Synthesis, characterization and behavior of ethylene polymerization. J. Organomet. Chem. 2005, 690, 1233–1239. [Google Scholar] [CrossRef]
- Song, S.; Xiao, T.; Liang, T.; Wang, F.; Redshaw, C.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization behaviour of 8-(1-aryliminoethylidene)quinaldinylnickel dihalides. Catal. Sci. Technol. 2011, 1, 69–75. [Google Scholar] [CrossRef]
Sample Availability: Samples of the organic compounds and nickel complexes are available from the authors. |
| Entry | Co-Cat | Al/Ni | Activity b | Oligomer Distribution c (%) | ||
|---|---|---|---|---|---|---|
| C4/ΣC | C6/ΣC | C≥8/ΣC | ||||
| 1 | Et2AlCl | 400 | 1.07 | 55.0 | 42.1 | 2.9 |
| 2 | EASC | 400 | 1.33 | 28.9 | 35.5 | 35.6 |
| 3 | MAO | 1500 | 0.38 | 73.4 | 9.1 | 17.5 |
| 4 | MMAO | 1500 | 1.25 | 9.1 | 87.1 | 3.8 |
| 5 | Et3Al | 400 | 0.05 | 100 | ||
| Entry | Cat. | Al/Ni | t (min) | T (°C) | Activity b | Oligomer Distribution c (%) | ||
|---|---|---|---|---|---|---|---|---|
| C4/ΣC | C6/ΣC | C≥8/ΣC | ||||||
| 1 | Ni1 | 200 | 30 | 30 | 0.64 | 31.1 | 45.8 | 23.1 |
| 2 | Ni1 | 300 | 30 | 30 | 0.81 | 33.2 | 49.0 | 17.8 |
| 3 | Ni1 | 400 | 30 | 30 | 1.33 | 28.9 | 35.5 | 35.6 |
| 4 | Ni1 | 500 | 30 | 30 | 2.17 | 21.4 | 24.7 | 53.9 |
| 5 | Ni1 | 600 | 30 | 30 | 1.81 | 10.7 | 24.6 | 64.7 |
| 6 | Ni1 | 700 | 30 | 30 | 1.52 | 9.91 | 26.4 | 63.7 |
| 7 | Ni1 | 800 | 30 | 30 | 1.11 | 8.65 | 19.4 | 72.0 |
| 8 | Ni1 | 500 | 30 | 20 | 1.38 | 29.5 | 22.6 | 47.9 |
| 9 | Ni1 | 500 | 30 | 40 | 3.34 | 15.7 | 22.9 | 61.4 |
| 10 | Ni1 | 500 | 30 | 50 | 2.78 | 10.2 | 18.8 | 71.0 |
| 11 | Ni1 | 500 | 30 | 60 | 2.49 | 8.20 | 15.1 | 76.7 |
| 12 | Ni1 | 500 | 15 | 40 | 5.99 | 16.8 | 18.1 | 65.1 |
| 13 | Ni1 | 500 | 45 | 40 | 3.15 | 14.7 | 25.6 | 59.7 |
| 14 | Ni1 | 500 | 60 | 40 | 3.01 | 16.0 | 26.3 | 57.7 |
| 15 | Ni2 | 500 | 30 | 40 | 4.06 | 15.1 | 16.3 | 68.6 |
| 16 | Ni3 | 500 | 30 | 40 | trace | |||
| 17 | Ni4 | 500 | 30 | 40 | 3.03 | 16.5 | 20.1 | 63.4 |
| 18 | Ni5 | 500 | 30 | 40 | 3.90 | 16.8 | 18.8 | 64.4 |
| 19 d | Ni1 | 500 | 30 | 40 | 3.91 | 18.7 | 26.0 | 55.3 |
| Entry | Cat. | Al/Ni | t (min) | T (°C) | Activity b | Oligomer Distribution c (%) | ||
|---|---|---|---|---|---|---|---|---|
| C4/ΣC | C6/ΣC | C≥8/ΣC | ||||||
| 1 | Ni1 | 1000 | 30 | 30 | 1.10 | 9.1 | 80.8 | 10.1 |
| 2 | Ni1 | 2000 | 30 | 30 | 1.45 | 8.9 | 78.1 | 13.0 |
| 3 | Ni1 | 2500 | 30 | 30 | 1.71 | 7.6 | 75.7 | 16.7 |
| 4 | Ni1 | 3000 | 30 | 30 | 1.63 | 6.7 | 71.4 | 21.9 |
| 5 | Ni1 | 3500 | 30 | 30 | 1.54 | 9.0 | 73.1 | 17.9 |
| 6 | Ni1 | 2500 | 30 | 20 | 1.52 | 9.7 | 81.3 | 9.0 |
| 7 | Ni1 | 2500 | 30 | 40 | 1.75 | 7.8 | 88.4 | 3.8 |
| 8 | Ni1 | 2500 | 30 | 50 | 1.67 | 6.8 | 88.9 | 4.3 |
| 9 | Ni1 | 2500 | 30 | 60 | 1.49 | 5.2 | 90.0 | 4.8 |
| 10 | Ni1 | 2500 | 15 | 40 | 3.18 | 8.8 | 88.1 | 3.1 |
| 11 | Ni1 | 2500 | 45 | 40 | 0.89 | 6.7 | 88.9 | 4.4 |
| 12 | Ni1 | 2500 | 60 | 40 | 0.68 | 6.0 | 90.5 | 3.5 |
| 13 | Ni2 | 2500 | 30 | 40 | 1.81 | 7.5 | 86.9 | 5.6 |
| 14 | Ni4 | 2500 | 30 | 40 | 1.50 | 5.9 | 89.7 | 4.4 |
| 15 | Ni5 | 2500 | 30 | 40 | 1.65 | 8.1 | 83.4 | 8.5 |
| 16 d | Ni1 | 2500 | 30 | 40 | 1.91 | 8.7 | 89.0 | 2.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, H.; Zhao, T.; Wang, Y.; Ban, Q.; Sun, W.-H. N-(2,2-Dimethyl-1-(quinolin-2-yl)propylidene) arylaminonickel Complexes and Their Ethylene Oligomerization. Molecules 2017, 22, 630. https://doi.org/10.3390/molecules22040630
Suo H, Zhao T, Wang Y, Ban Q, Sun W-H. N-(2,2-Dimethyl-1-(quinolin-2-yl)propylidene) arylaminonickel Complexes and Their Ethylene Oligomerization. Molecules. 2017; 22(4):630. https://doi.org/10.3390/molecules22040630
Chicago/Turabian StyleSuo, Hongyi, Tong Zhao, Yiqing Wang, Qing Ban, and Wen-Hua Sun. 2017. "N-(2,2-Dimethyl-1-(quinolin-2-yl)propylidene) arylaminonickel Complexes and Their Ethylene Oligomerization" Molecules 22, no. 4: 630. https://doi.org/10.3390/molecules22040630