An Expedient Total Synthesis of Triciribine
Abstract
:1. Introduction
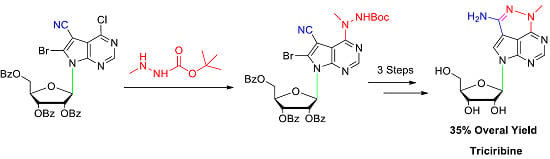
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 6-Bromo-4-chloro-5-cyano-7-[2,3,5-tri-O-benzoyl-β-d-ribofuranosyl]pyrrolo[2,3-d]pyrimidine (5)
3.2. Synthesis of 6-Bromo-5-cyano-4-(1-methylhydrazino)-7-[2,3,5-tri-O-benzoyl-β-d-ribofuranosyl]pyrrolo[2,3-d]pyrimidine (6)
3.3. Synthesis of 6-Bromo-5-cyano-4-(2-(tert-butoxycarbonyl)-1-methylhydrazinyl)-7-[2,3,5-tri-O-benzoyl-β-d-ribofuranosyl]pyrrolo[2,3-d]pyrimidine (9)
3.4. Synthesis of 6-Amino-7-bromo-4-methyl-8-(2,3,5-tri-O-benzoyl-β-d-ribofuranosyl)pyrrolo[4,3,2-de]pyrimido [4,5-c]pyridazine (10)
3.5. Synthesis of 6-Amino-4-methyl-8-(2,3,5-tri-O-benzoyl-β-d-ribofuranosyl)pyrrolo[4,3,2-de]pyrimido[4,5-c]pyridazine (11)
3.6. Synthesis of 6-Amino-4-methyl-8-(β-d-ribofuranosyl)pyrrolo[4,3,2-de]pyrimido[4,5-c]pyridazine (Triciribine, TCN, 1)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- De Clercq, E. The next ten stories on antiviral drug discovery (part E): Advents, advances, and adventures. Med. Res. Rev. 2010, 31, 118–160. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiretroviral drugs. Curr. Opin. Pharmacol. 2010, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Schram, K.H.; Townsend, L.B. The synthesis of 6-amino-4-methyl-8-(β-d-ribofuranosyl)(4-H,8-H)pyrrolo[4,3,2-de]pyrimido[4,5-c]pyridazine, a new tricyclic nucleoside. Tetrahedron Lett. 1971, 49, 4757–4760. [Google Scholar] [CrossRef]
- Wotring, L.L.; Passiatore, J.E.; Roti Roti, J.L.; Townsend, L.B. Effects of the tricyclic nucleoside 6-amino-4-methyl-8-(β-d-ribofuranosyl)pyrrolo[4,3,2-de]pyrimido[4,5-c]pyridazine on the viability and cell cycle distribution of L1210 cells in vitro. Cancer Res. 1985, 45, 6355–6361. [Google Scholar] [PubMed]
- Mittelman, A.; Casper, E.S.; Young, C.W. Phase I study of tricyclic nucleoside phosphate. Cancer Treat. 1983, 67, 159–162. [Google Scholar]
- Feun, L.G.; Savaraj, N.; Bodey, G.P.; Krakoff, I. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984, 44, 3608–3612. [Google Scholar] [PubMed]
- O’Connell, M.J.; Rubin, J.; Moertel, C.G. Phase II clinical of tricyclic nucleoside phosphate for advanced colorectal cancer. Cancer Treat. 1987, 71, 333–334. [Google Scholar]
- Feun, L.G.; Blessing, J.A.; Barrett, R.J.; Hanjani, P.A. A Phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix: A gynecologic oncology group study. Am. J. Clin. Oncol. 1993, 16, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dan, H.C.; Sun, M.; Herlyn, M.; Sebti, S.M.; Cheng, J.Q. Akt/Protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004, 64, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Yamauchi, T.; Husain, K.; Sebti, S.; Malafa, M. Triciribine phosphate monohydrate, an AKT inhibitor, enhances gemcitabine activity in pancreatic cancer cells. Anticancer Res. 2015, 35, 4599–4604. [Google Scholar] [PubMed]
- Christopher, R.G.; Domenico, C.; Robert, M.W. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Investig. New Drugs 2011, 29, 1381–1389. [Google Scholar]
- Porcari, A.R.; Townsend, L.B. An improved total synthesis of triciribine: A tricyclic nucleoside with antineoplastic and antiviral properties. Nucleosides Nucleotides Nucleic Acids 2004, 23, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Kim, J.S.; Hilfinger, J. Expedient total synthesis of triciribine and its prodrugs. Synth. Commun. 2012, 42, 358–374. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Dou, Y.H.; Xiao, Q. First total synthesis of a naturally occurring iodinated 5′-deoxyxylofuranosyl marine nucleoside. Mar. Drugs 2012, 10, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, R.C.; Xiao, Q.; Ju, Y. An efficient approach for the synthesis of 1′-O-α-methyl pyrrolo[2,3-d]pyrimidine isonucleosides. Synthesis 2011, 8, 1213–1218. [Google Scholar]
- Dou, Y.H.; Ding, H.X.; Xiao, Q. A total synthesis of mycalisine A. Chin. Chem. Lett. 2013, 24, 379–382. [Google Scholar] [CrossRef]
- Schram, K.H.; Townsend, L.B. Pyrrolopyrimidine nucleosides. Part XI. Influence of amino-groups at C-4 and C-6 or an amino-group at C-6 on the reactivity of a 5-cyano-group in pyrrolo[2,3-d] pyrimidine nucleosides. J. Chem. Soc. Perkin Trans. 1 1975, 1253–1257. [Google Scholar] [CrossRef]
- Chung, F.L.; Schram, K.H.; Panzica, R.P.; Earl, R.A.; Wotring, L.L.; Townsend, L.B. Synthesis of certain [6:5:6] linear tricyclic nucleosides as potential antitumor agents. J. Med. Chem. 1980, 23, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Amblard, F.; Zhang, H.W.; McBrayer, T.R.; Detorio, M.A.; Whitaker, T.; Coats, S.J.; Schinazi, R.F. Synthesis and evaluation of Janus type nucleosides as potential HCV NS5B polymerase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3385–3388. [Google Scholar] [CrossRef] [PubMed]
- Moussodia, R.O.; Acherar, S.; Bordessa, A.; Vanderesse, R.; Jamart-Gregoire, B. An expedient and short synthesis of chiral α-hydrazinoesters: Synthesis and conformational analysis of 1:1 [α/α-Nα-hydrazino] mers. Tetrahedron 2012, 68, 4682–4692. [Google Scholar] [CrossRef]
- Kavoosi, S.; Rayala, R.; Walsh, B.; Barrios, M.; Gonzalez, W.G.; Miksovska, J.; Mathivathanan, L.; Raptis, R.G.; Wnuk, S.F. Synthesis of 8-(1, 2, 3-triazol-1-yl)-7-deazapurine nucleosides by azide–alkyne click reactions and direct C-H bond functionalization. Tetrahedron Lett. 2016, 57, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Sabat, N.; Postova Slavetinska, L.; Klepetarova, B.; Hocek, M. C–H Phosphonation of pyrrolopyrimidines: synthesis of substituted 7-and 9-deazapurine-8-phosphonate derivatives. J. Org. Chem. 2016, 81, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Seela, F.; Peng, X.; Budow, S. Advances in the synthesis of 7-deazapurine-pyrrolo[2, 3-d]pyrimidine 2′-deoxyribonucleosides including d-and l-enantiomers, fluoro derivatives and 2',3'-dideoxyribonucleosides. Curr. Org. Chem. 2007, 11, 427–462. [Google Scholar] [CrossRef]
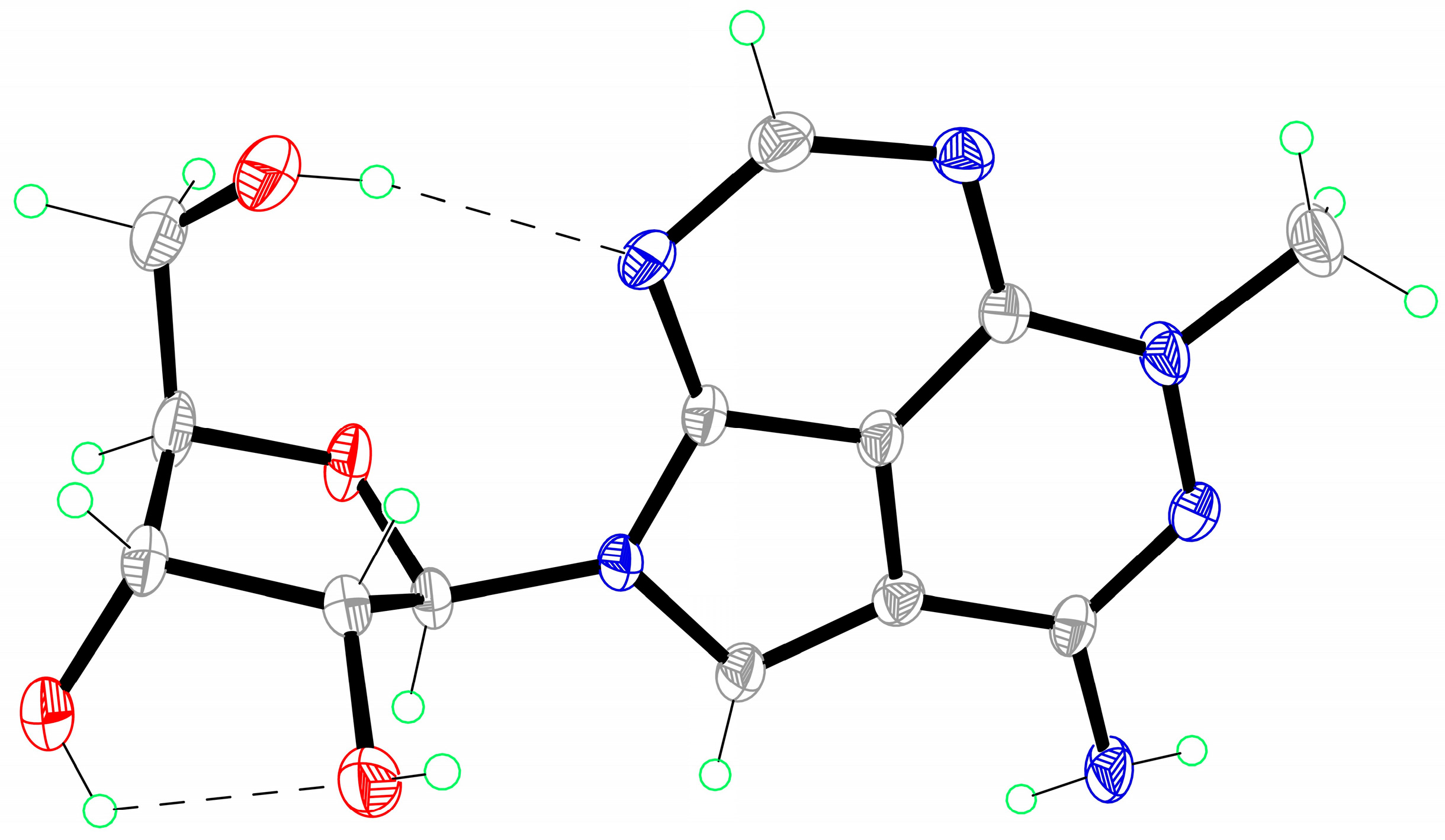
- Crystallographic data for triciribine 1: C13H16N6O4, M = 374.37, crystal dimensions 0.26 × 0.28 × 0.30 mm, orthorhombic, space group, C2221 (No. 20). CCDC 1520825 contains the supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Date Centre via www.ccdc.cam.ac.uk/date_request/cif.
Sample Availability: Samples of all the reported compounds are available from the Prof. Dr. Qiang Xiao. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Ruan, Z.; Ding, H.; Zhou, Y.; Xiao, Q. An Expedient Total Synthesis of Triciribine. Molecules 2017, 22, 643. https://doi.org/10.3390/molecules22040643
Hu C, Ruan Z, Ding H, Zhou Y, Xiao Q. An Expedient Total Synthesis of Triciribine. Molecules. 2017; 22(4):643. https://doi.org/10.3390/molecules22040643
Chicago/Turabian StyleHu, Chen, Zhizhong Ruan, Haixin Ding, Yirong Zhou, and Qiang Xiao. 2017. "An Expedient Total Synthesis of Triciribine" Molecules 22, no. 4: 643. https://doi.org/10.3390/molecules22040643