Systematic Understanding of the Mechanism of Salvianolic Acid A via Computational Target Fishing
Abstract
:1. Introduction
2. Results
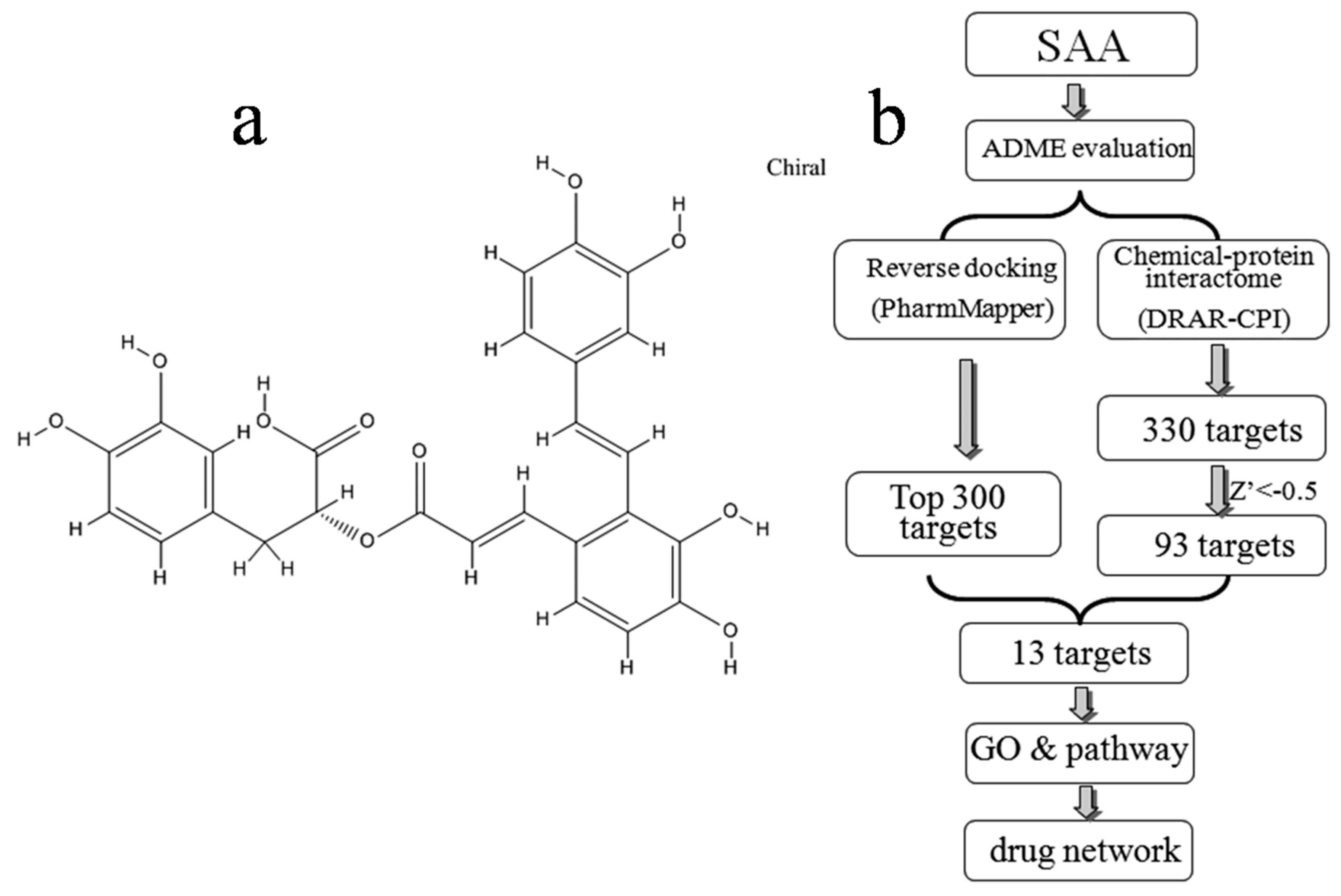
2.1. ADME-Related Properties of SAA
2.2. Identification of Potential Targets
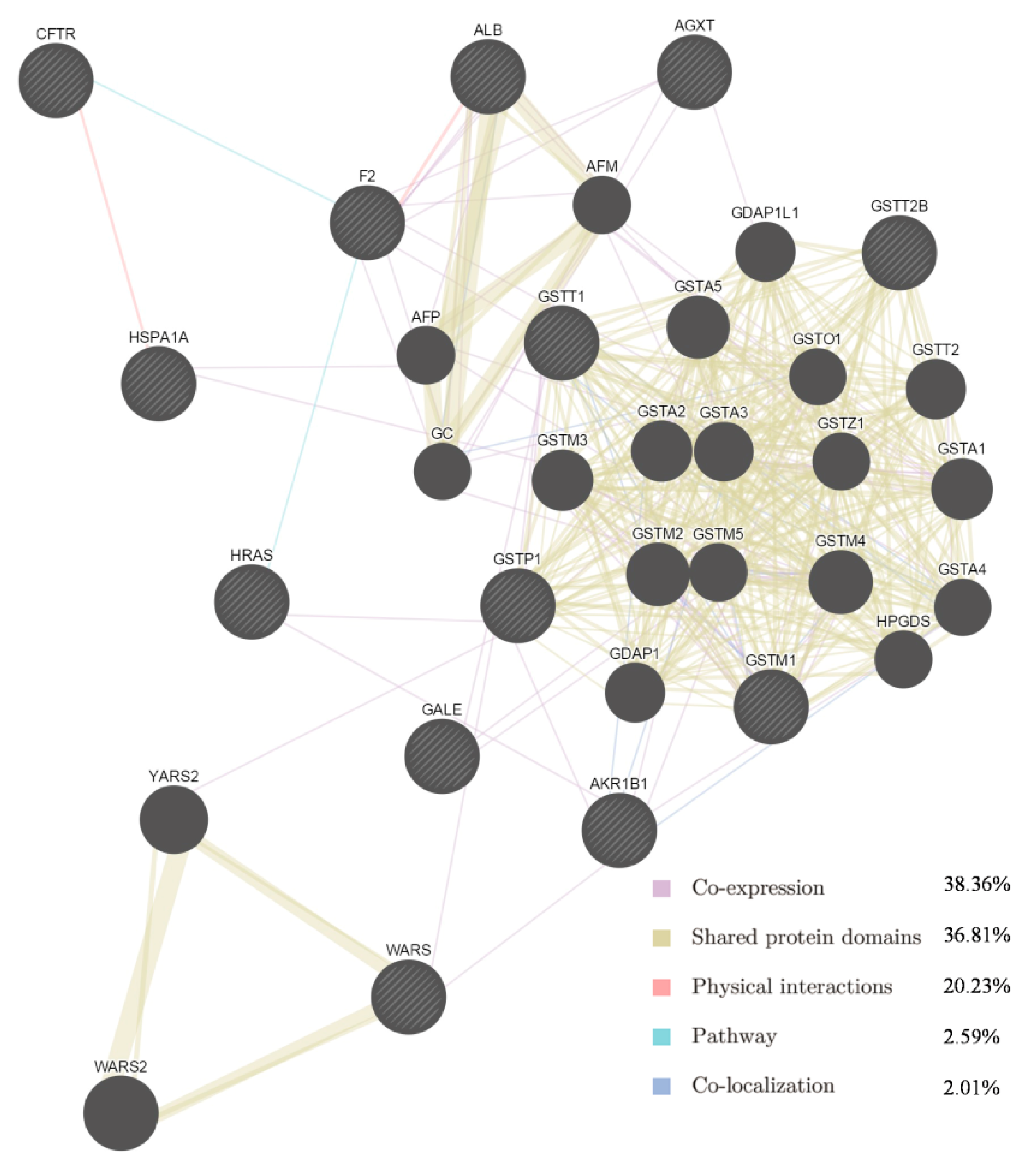
2.3. Analysis by GeneMANIA
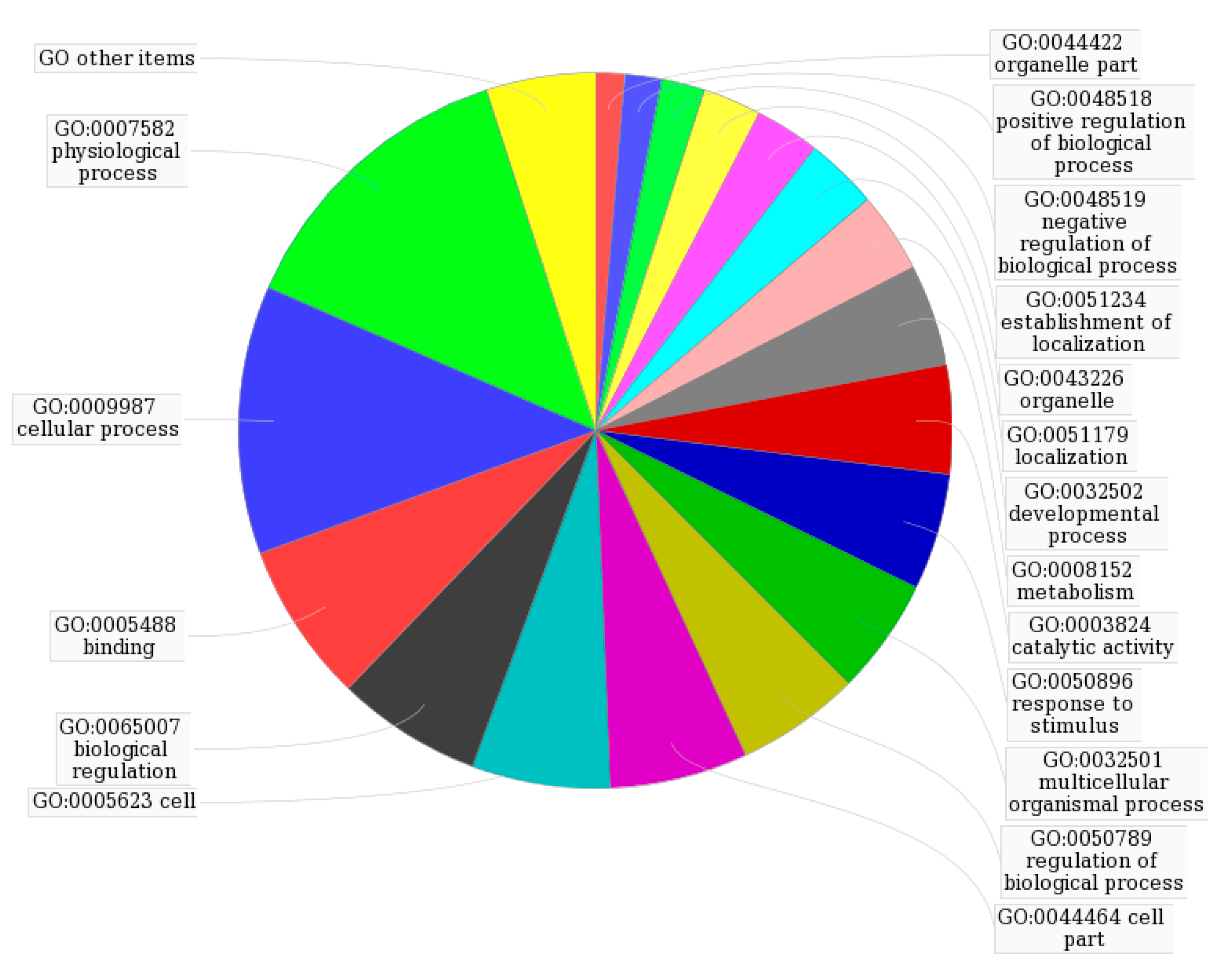
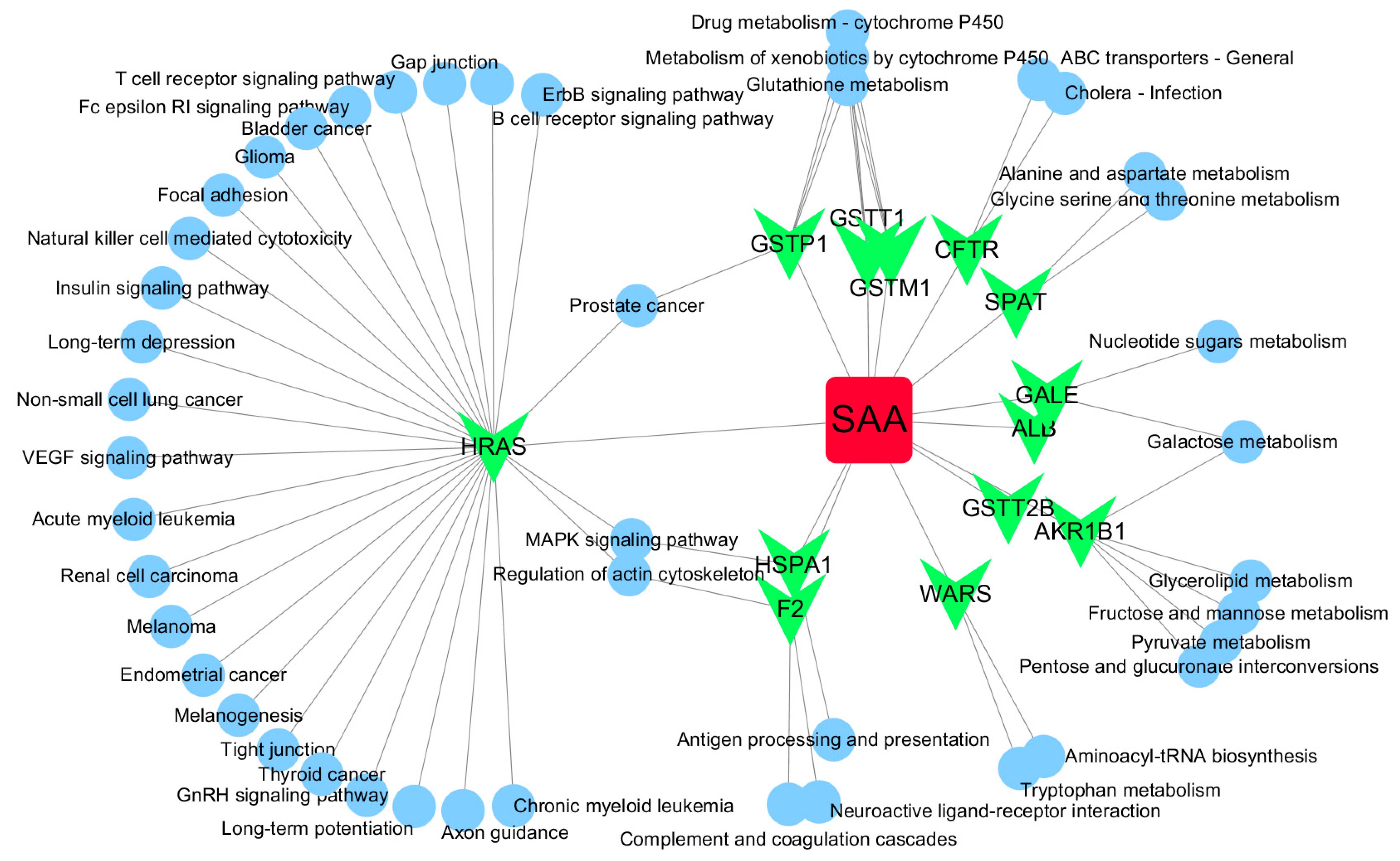
2.4. GO and Pathway Analysis, and Network Construction
3. Discussion
4. Materials and Methods
4.1. Evaluation of Drug-Likeness Using the TCMSP Server
4.2. Computational Target Fishing by PharmMapper and DRAR-CPI
4.3. Analysis by GeneMANIA
4.4. GO and Pathway Analysis, and Network Construction
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schenone, M.; Dancik, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Hong, C.Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Zhao, Y.; Yang, X.; Fang, L.; Wang, S.; Shi, L.; Yu, X.; Yang, H.; Sun, J.; et al. Research progress of salvianolic acid A. Zhongguo Zhong Yao Za Zhi 2011, 36, 2603–2609. [Google Scholar] [PubMed]
- Liu, G.T.; Zhang, T.M.; Wang, B.E.; Wang, Y.W. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem. Pharmacol. 1992, 43, 147–152. [Google Scholar] [CrossRef]
- Fan, H.Y.; Yang, M.Y.; Qi, D.; Zhang, Z.K.; Zhu, L.; Shang-Guan, X.X.; Liu, K.; Xu, H.; Che, X. Salvianolic acid A as a multifunctional agent ameliorates doxorubicin-induced nephropathy in rats. Sci. Rep. 2015, 5, 12273. [Google Scholar] [CrossRef] [PubMed]
- Hurle, M.R.; Yang, L.; Xie, Q.; Rajpal, D.K.; Sanseau, P.; Agarwal, P. Computational drug repositioning: From data to therapeutics. Clin. Pharmacol. Ther. 2013, 93, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, H.; Reagan, K.; Xu, X.; Mendrick, D.L.; Slikker, W., Jr.; Tong, W. In silico drug repositioning: What we need to know. Drug Discov. Today 2013, 18, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.Q. Computational target fishing: What should chemogenomics researchers expect for the future of in silico drug design and discovery? Future Med. Chem. 2014, 6, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Ling, Q.Z.; Chen, S.J. Identification of a potential target of capsaicin by computational target fishing. Evid. Based Complement. Altern. Med. 2015, 2015, 983951. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J. A potential target of tanshinone iia for acute promyelocytic leukemia revealed by inverse docking and drug repurposing. Asian Pac. J. Cancer Prev. 2014, 15, 4301–4305. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Guo, Z.; Tao, W.; Yang, Y.; Xu, X.; et al. Tcmsp: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Gifford, E. Admet in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Leeson, P.D. Molecular inflation, attrition and the rule of five. Adv. Drug Deliv. Rev. 2016, 101, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Zheng, C.; Huang, C.; Chen, X.; Guo, Z.; Fu, Y.; Liu, J.; Wang, Y. Systematic understanding the mechanisms of vitiligo pathogenesis and its treatment by qubaibabuqi formula. J. Ethnopharmacol. 2016, 190, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, Q.; Guo, Z.; Fu, Y.; Chen, X.; Shar, P.A.; Shahen, M.; Zhu, J.; Xue, J.; Bai, Y.; et al. Systems pharmacology dissection of the integrated treatment for cardiovascular and gastrointestinal disorders by traditional Chinese medicine. Sci. Rep. 2016, 6, 32400. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, L.; Lee, Y.M.; Kalesh, K.A.; Ong, Y.S.; Lim, J.; Jee, J.E.; Sun, H.; Lee, S.S.; Hua, Z.C.; et al. Target identification of natural and traditional medicines with quantitative chemical proteomics approaches. Pharmacol. Ther. 2016, 162, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cereto-Massague, A.; Ojeda, M.J.; Valls, C.; Mulero, M.; Pujadas, G.; Garcia-Vallve, S. Tools for in silico target fishing. Methods 2015, 71, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tammali, R.; Srivastava, S.K.; Ramana, K.V. Targeting aldose reductase for the treatment of cancer. Curr. Cancer Drug Targets 2011, 11, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V. Aldose reductase: New insights for an old enzyme. Biomol. Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Du, G.H.; Qiu, Y.; Tian, Y.E.; Zhang, J.T. Prevention of galactose-induced cataractogenesis in rats by salvianolic acid A. Yao Xue Xue Bao 1995, 30, 561–566. [Google Scholar] [PubMed]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. Ras isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, Y.; Yan, C.H.; Li, L.N.; Chen, X.G. Inhibition of tumor growth by s-3-1, a synthetic intermediate of salvianolic acid A. J. Asian Nat. Prod. Res. 2002, 4, 271–280. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, C.C.; Townsend, D.M.; Tew, K.D. Glutathione s-transferase polymorphisms: Cancer incidence and therapy. Oncogene 2006, 25, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.P.; Wei, S.Q.; Zhang, L.Q.; Gao, X.C.; Yu, N.N.; Bi, S.; Cui, H. Preventive effect of danshensu on selenite-induced cataractogenesis in cultured rat lens. Clin. Experiment. Ophthalmol. 2013, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.N.; Yan, K.; Cui, Y.L.; Fan, G.W.; Wang, Y.F. Protective effect of salvia miltiorrhiza and carthamus tinctorius extract against lipopolysaccharide-induced liver injury. World J. Gastroenterol. 2015, 21, 9079–9092. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Han, J.M.; Kim, D.W.; Yi, M.H.; Son, S.W.; Kim, Y.A.; Choi, M.K.; Son, C.G. Ethanol extract of astragali radix and salviae miltiorrhizae radix, myelophil, exerts anti-amnesic effect in a mouse model of scopolamine-induced memory deficits. J. Ethnopharmacol. 2014, 153, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, W.; Lei, Q.; Tao, X.; You, H.; Xie, P. Salvianolate increases heat shock protein expression in a cerebral ischemia-reperfusion injury model. Neural. Regen. Res. 2013, 8, 2327–2335. [Google Scholar] [PubMed]
- Li, Q.; Li, X.; Li, C.; Chen, L.; Song, J.; Tang, Y.; Xu, X. A network-based multi-target computational estimation scheme for anticoagulant activities of compounds. PLoS ONE 2011, 6, e14774. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y. A biomedical investigation of the hepatoprotective effect of radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, S.; Zhang, W.; Zheng, X.; Hu, S.; Pang, C.; Lu, J.; Xing, J.; Dong, Y. Salvianolic acid A reverses paclitaxel resistance in human breast cancer mcf-7 cells via targeting the expression of transgelin 2 and attenuating pi3 k/akt pathway. Phytomedicine 2014, 21, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, S.; Yang, Q.; Cai, J.; Zhang, W.; You, H.; Xing, J.; Dong, Y. Salvianolic acid A reverses the paclitaxel resistance and inhibits the migration and invasion abilities of human breast cancer cells by inactivating transgelin 2. Cancer Biol. Ther. 2015, 16, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xu, J.X. Salvianic acid A protects human neuroblastoma sh-sy5y cells against MPP+-induced cytotoxicity. Neurosci. Res. 2005, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Chen, J.; Yuan, X.; Jiang, Z.; Chen, W. Salvianolic acid A positively regulates pten protein level and inhibits growth of a549 lung cancer cells. Biomed. Rep. 2013, 1, 213–217. [Google Scholar] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. Pharmmapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, J.; Shi, L.; Mikailov, M.; Zhu, H.; Wang, K.; He, L.; Yang, L. Drar-cpi: A server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011, 39, W492–W498. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The genemania prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Name | MW | AlogP | Hdon | Hacc | OB (%) | Caco-2 | BBB | DL | FSAF | TPSA | RBN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SAA | 494.48 | 4.14 | 7 | 10 | 2.96 | −0.56 | −1.62 | 0.7 | 0.4 | 184.98 | 9 |
| Rank | PDB ID | Name | Target Gene |
|---|---|---|---|
| 1 | 1T40 | Aldose reductase | AKR1B1 |
| 2 | 2C3Q | Glutathione S-transferase theta-1 | GSTT1 |
| 3 | 2BX8 | Serum albumin | ALB |
| 4 | 1LJR | Glutathione S-transferase theta-2 | GSTT2B |
| 5 | 1XMI | Cystic fibrosis transmembrane conductance regulator | CFTR |
| 6 | 1EK5 | UDP-glucose 4-epimerase | GALE |
| 7 | 2E8A | Heat shock 70 kDa protein 1 | HSPA1 |
| 8 | 1H0C | Serine-pyruvate aminotransferase | SPAT |
| 9 | 11GS | Glutathione S-transferase P | GSTP1 |
| 10 | 5P21 | GTPase HRas | HRAS |
| 11 | 1A3B | Prothrombin | F2 |
| 12 | 1XWK | Glutathione S-transferase Mu 1 | GSTM1 |
| 13 | 1R6T | Tryptophanyl-tRNA synthetase, cytoplasmic | WARS |
| GO Term | Count | Percentage |
|---|---|---|
| GO:0007582 Physiological process | 41 | 0.1348684211 |
| GO:0009987 Cellular process | 37 | 0.1217105263 |
| GO:0005488 Binding | 22 | 0.0723684211 |
| GO:0065007 Biological regulation | 20 | 0.0657894737 |
| GO:0005623 Cell | 19 | 0.0625 |
| GO:0044464 Cell part | 19 | 0.0625 |
| GO:0050789 Regulation of biological process | 17 | 0.0559210526 |
| GO:0032501 Multicellular organismal process | 16 | 0.0526315789 |
| GO:0050896 Response to stimulus | 16 | 0.0526315789 |
| GO Other items | 15 | 0.0493421053 |
| GO:0003824 Catalytic activity | 15 | 0.0493421053 |
| GO:0008152 Metabolism | 14 | 0.0460526316 |
| GO:0032502 Developmental process | 11 | 0.0361842105 |
| GO:0051179 Localisation | 10 | 0.0361842105 |
| GO:0043226 Organelle | 9 | 0.0296052632 |
| GO:0051234 Establishment of localisation | 8 | 0.0263157895 |
| GO:0048519 Negative regulation of biological process | 6 | 0.0197368421 |
| GO:0048518 Positive regulation of biological process | 5 | 0.0164473684 |
| GO:0044422 Organelle part | 4 | 0.0131578947 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-J.; Cui, M.-C. Systematic Understanding of the Mechanism of Salvianolic Acid A via Computational Target Fishing. Molecules 2017, 22, 644. https://doi.org/10.3390/molecules22040644
Chen S-J, Cui M-C. Systematic Understanding of the Mechanism of Salvianolic Acid A via Computational Target Fishing. Molecules. 2017; 22(4):644. https://doi.org/10.3390/molecules22040644
Chicago/Turabian StyleChen, Shao-Jun, and Ming-Chao Cui. 2017. "Systematic Understanding of the Mechanism of Salvianolic Acid A via Computational Target Fishing" Molecules 22, no. 4: 644. https://doi.org/10.3390/molecules22040644





