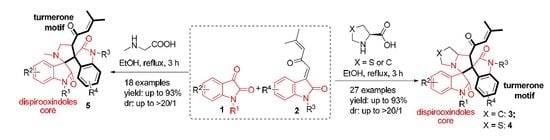
Molecular Hybridization-Guided One-Pot Multicomponent Synthesis of Turmerone Motif-Fused 3,3′-Pyrrolidinyl-dispirooxindoles via a 1,3-Dipolar Cycloaddition Reaction
Abstract
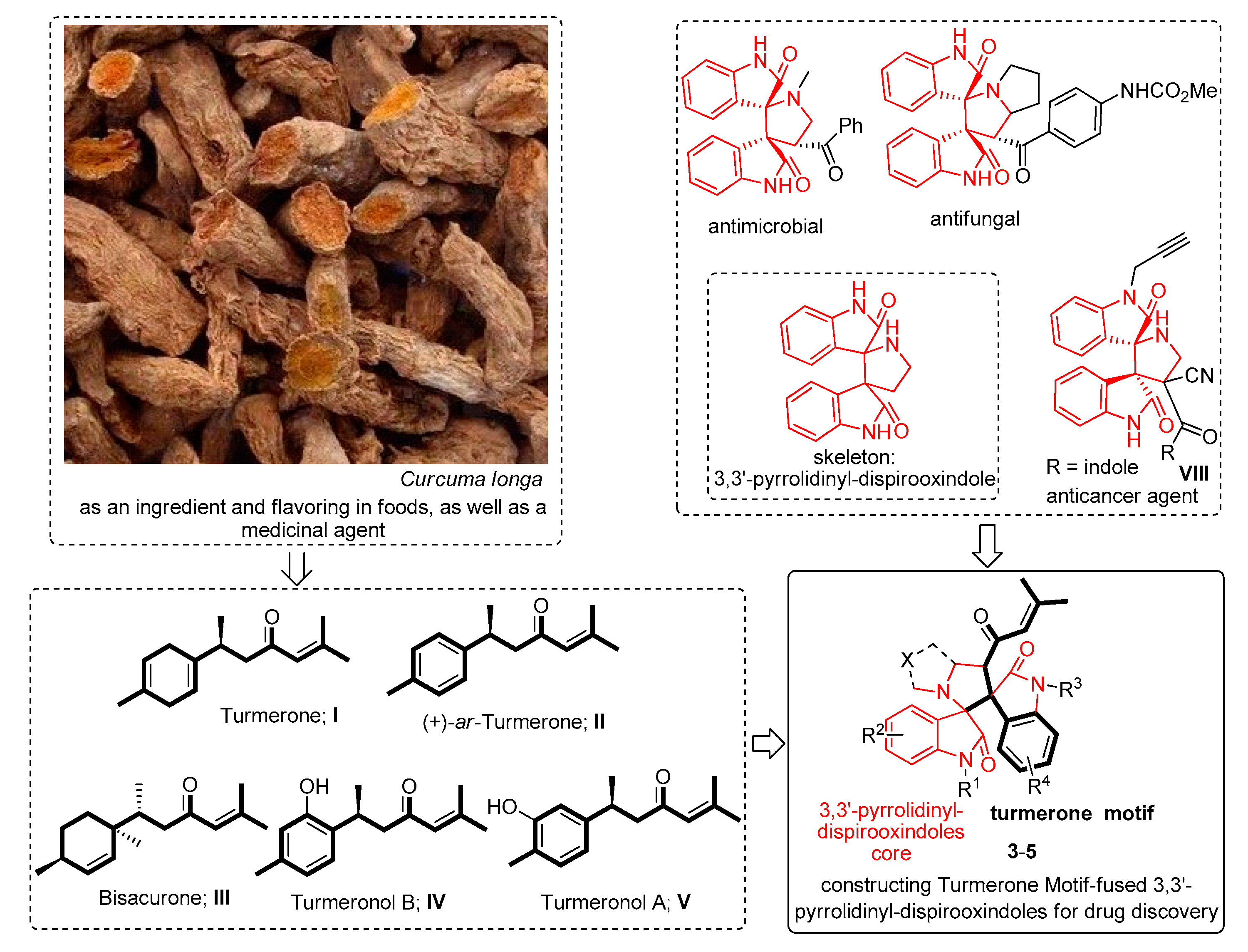
:1. Introduction
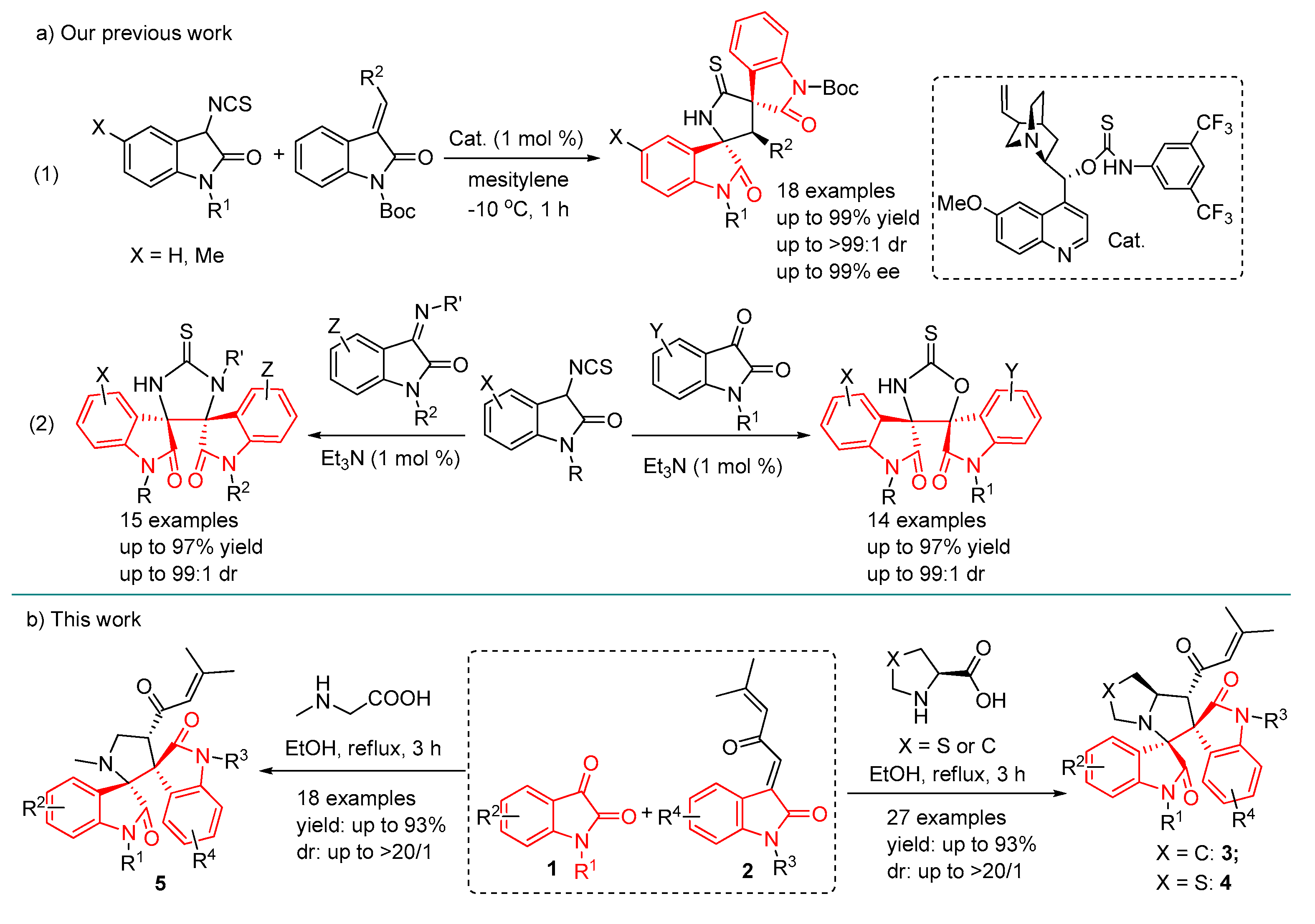
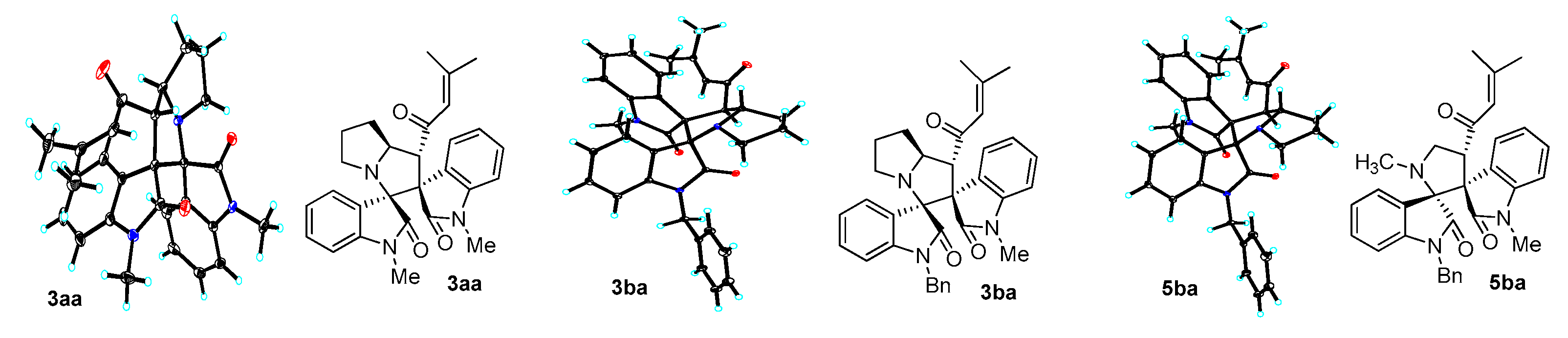
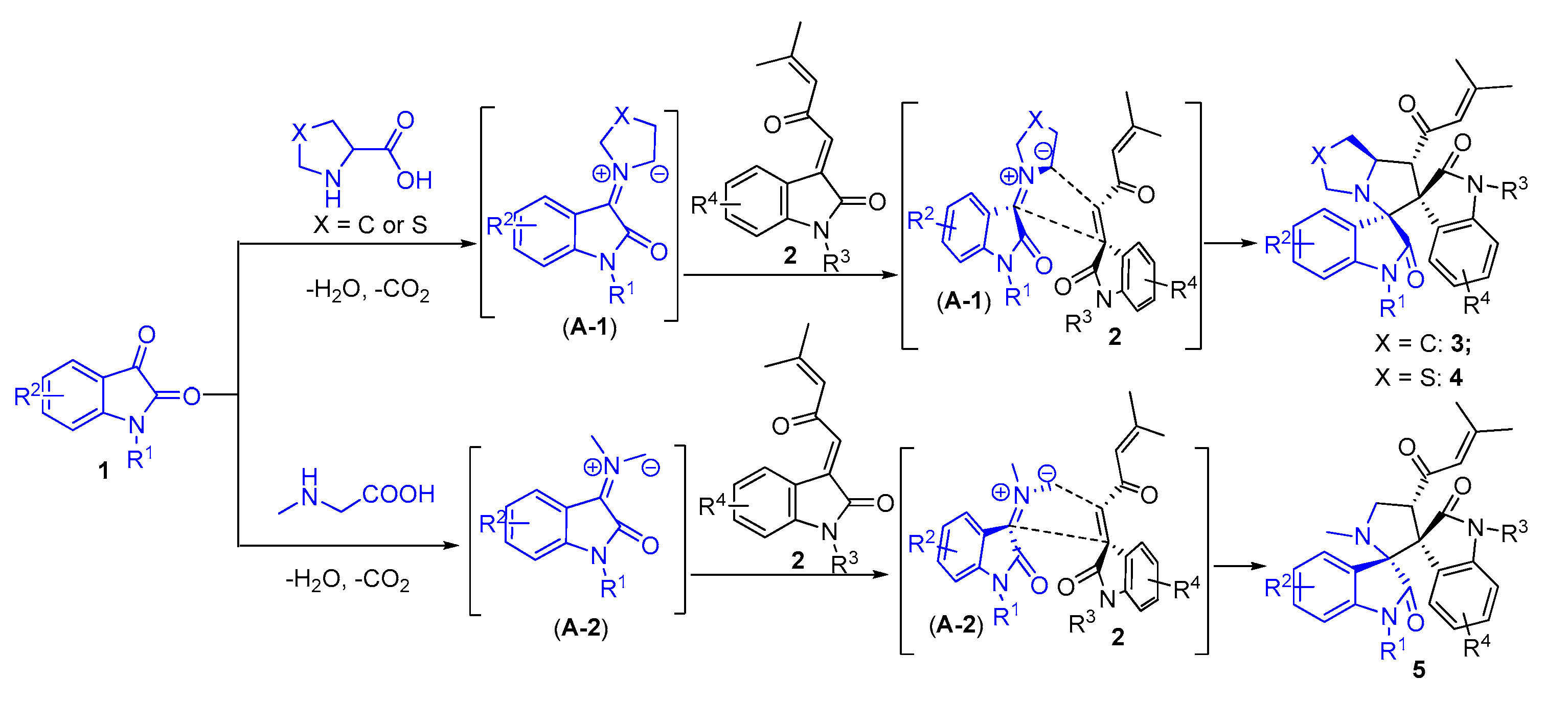
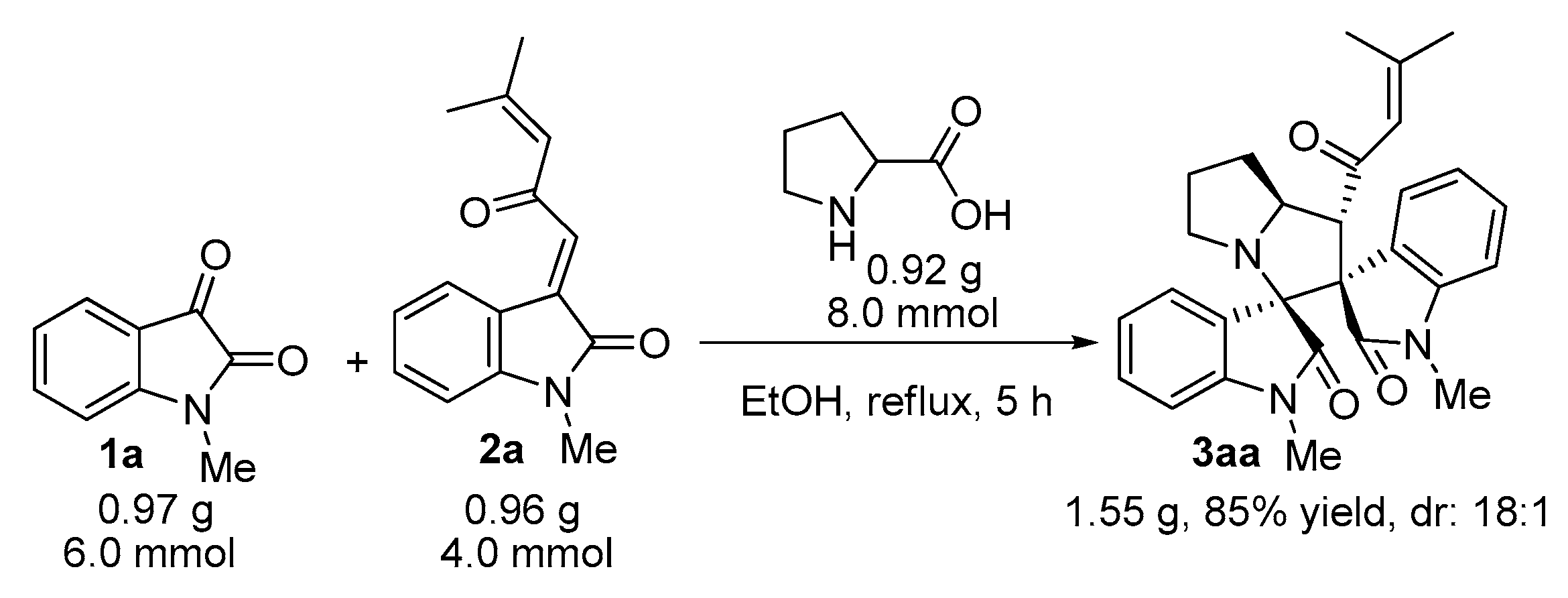
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. General Experimental Procedures for the Synthesis of Compounds 3–5
3.3. Characterization Data of Compounds 3–5













































3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Nicolaou, K.C.; Vourloumis, D.; Winssinger, N.; Baran, P.S. The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century. Angew. Chem. Int. Ed. 2000, 39, 44–122. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Gaich, T.; Baran, P.S. Aiming for the Ideal Synthesis. J. Org. Chem. 2010, 75, 4657–4673. [Google Scholar] [CrossRef] [PubMed]
- Arun, Y.; Bhaskar, G.; Balachandran, C.; Ignacimuthu, S.; Perumal, P.T. Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line. Bioorg. Med. Chem. Lett. 2013, 23, 1839–1945. [Google Scholar] [CrossRef] [PubMed]
- Velikorodov, A.V.; Ionova, V.A.; Degtyarev, O.V.; Sukhenko, L.T. Synthesis and antimicrobial and antifungal activity of carbamate-functionized spiro compounds. Pharm. Chem. J. 2013, 46, 715–719. [Google Scholar] [CrossRef]
- Babu, A.R.S.; Raghunathan, R.; Mathivanan, N.; Omprabha, G.; Velmurugan, D.; Raghu, R. Synthesis, Characterisation, Anti-Microbial Activity and Docking Studies of Novel Dispiro-Oxindolopyrrolidines. Curr. Chem. Biol. 2008, 2, 312–320. [Google Scholar] [CrossRef]
- Zhao, K.; Zhi, Y.; Li, X.; Puttreddy, R.; Rissanen, K.; Enders, D. Asymmetric synthesis of 3,3′-pyrrolidinyl-dispirooxindoles via a one-pot organocatalytic Mannich/deprotection/aza-Michael sequence. Chem. Commun. 2016, 52, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Ganesh Kumar, S. A highly stereoselective, catalytic four-component synthesis of dispiroindolo-pyrrolidines/-imidazolidines via azomethine ylides. Tetrahedron 2016, 72, 2392–2401. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, X.-L.; Wu, Q.; Shi, F.; Tu, S.-J. Diastereo- and Enantioselective Construction of 3,3′-Pyrrolidinyldispirooxindole Framework via Catalytic Asymmetric 1,3-Dipolar Cycloadditions. J. Org. Chem. 2015, 80, 5737–5744. [Google Scholar] [CrossRef] [PubMed]
- Suman, K.; Thennarasu, S. Acetic acid promoted tandem cyclization of in situ generated 1,3-dipoles: Stereoselective synthesis of dispiroimidazolidinyl and dispiropyrrolidinyl oxindoles with multiple chiral stereocenters. RSC Adv. 2015, 5, 79413–79422. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Periyasami, G.; Ghabbour, H.A.; Fun, H.-K. A Novel One-Pot Green Synthesis of Dispirooxindolo-pyrrolidines via 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides. Molecules 2015, 20, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Cai, T.; Zhou, J.; Tian, F.; Xu, X.-Y.; Wang, L.-X. An unprecedented base-promoted domino reaction of methyleneindolinones and N-tosyloxycarbamates for the construction of bispirooxindoles and spiroaziridine oxindoles. Chem. Commun. 2015, 51, 10726–10729. [Google Scholar] [CrossRef] [PubMed]
- Suman, K.; Srinu, L.; Thennarasu, S. Lewis Acid Catalyzed Unprecedented [3+2] Cycloaddition Yields 3,3′-Pyrrolidinyldispirooxindoles Containing Four Contiguous Chiral Stereocenters with Two Contiguous Quaternary Spirostereocenters. Org. Lett. 2014, 16, 3732–3735. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Jain, A.K.; Laxkar, A.K.; Bhati, D.S. Synthesis and stereochemical investigation of highly functionalized novel dispirobisoxindole derivatives via [3+2] cycloaddition reaction in ionic liquid. Tetrahedron 2013, 2062–2069. [Google Scholar] [CrossRef]
- Xiao, J.-A.; Zhang, H.-G.; Liang, S.; Ren, J.-W.; Yang, H.; Chen, X.-Q. Synthesis of Pyrrolo(spiro-[2.3′]-oxindole)-spiro-[4.3″]-oxindole via 1,3-Dipolar Cycloaddition of Azomethine Ylides with 3-Acetonylideneoxindole. J. Org. Chem. 2013, 78, 11577–11583. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-L.; Xue, Z.-Y.; Tao, H.-Y.; Wang, C.-J. Catalytic asymmetric 1,3-dipolar cycloaddition of N-unprotected 2-oxoindolin-3-ylidene derivatives and azomethine ylides for the construction of spirooxindole-pyrrolidines. Org. Biomol. Chem. 2011, 9, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-S.; Wang, W.-B.; Yuan, B.-B.; Li, Y.-N.; Wang, Q.-L.; Bu, Z.-W. A DBU-catalyzed Michael-Pinner-isomerization cascade reaction of 3-hydroxyoxindoles with isatylidene malononitriles: Access to highly functionalized bispirooxindoles containing a fully substituted dihydrofuran motif. Org. Biomol. Chem. 2017, 15, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Hanhan, N.V.; Ball-Jones, N.R.; Tran, N.T.; Franz, A.K. Catalytic Asymmetric [3+2] Annulation of Allylsilanes with Isatins: Synthesis of Spirooxindoles. Angew. Chem. Int. Ed. 2012, 51, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Dugal-Tessier, J.; O‘Bryan, E.A.; Schroeder, T.B.H.; Cohen, D.T.; Scheidt, K.A. An N-Heterocyclic Carbene/Lewis Acid Strategy for the Stereoselective Synthesis of Spirooxindole Lactones. Angew. Chem. Int. Ed. 2012, 51, 4963–4967. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-L.; Liang, T.; Wojtas, L.; Antilla, J.C. An Asymmetric Diels–Alder Reaction Catalyzed by Chiral Phosphate Magnesium Complexes: Highly Enantioselective Synthesis of Chiral Spirooxindoles. Angew. Chem. Int. Ed. 2013, 52, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.-J.; Jiang, H.; Li, J.-H.; Gschwend, B.; Li, Q.-Z.; Yin, X.; Grouleff, J.; Chen, Y.-C.; Jorgensen, K.A. Trienamines in Asymmetric Organocatalysis: Diels-Alder and Tandem Reactions. J. Am. Chem. Soc. 2011, 133, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-B.; Zhao, H.; Liu, Z.-M.; Liu, G.-G.; Tao, J.-C.; Wang, X.-W. Chiral Counteranion Synergistic Organocatalysis under High Temperature: Efficient Construction of Optically Pure Spiro[cyclohexanone-oxindole] Backbone. Org. Lett. 2011, 13, 4866–4869. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Candeias, N.R.; Barbas, C.F., III. Core-Structure-Motivated Design of a Phosphine-Catalyzed [3+2] Cycloaddition Reaction: Enantioselective Syntheses of Spirocyclopenteneoxindoles. J. Am. Chem. Soc. 2011, 133, 4672–4675. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Hernndez-Torres, G.; Barbas, C.F., III. Highly Efficient Hydrogen-Bonding Catalysis of the Diels-Alder Reaction of 3-Vinylindoles and Methyleneindolinones Provides Carbazole spirooxindole Skeletons. J. Am. Chem. Soc. 2011, 133, 12354–12357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-K.; Nappi, M.; Arceo, E.; Vera, S.; Melchiorre, P. Asymmetric Catalysis of Diels–Alder Reactions with in Situ Generated Heterocyclic ortho-Quinodimethanes. J. Am. Chem. Soc. 2011, 133, 15212–15218. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Zeng, X.-F.; Leong, W.W.Y.; Shi, Z.-G.; Barbas, C.F., III.; Zhong, G.-F. Core Structure-Based Design of Organocatalytic [3+2]-Cycloaddition Reactions: Highly Efficient and Stereocontrolled Syntheses of 3,3′-Pyrrolidonyl Spirooxindoles. Chem. Eur. J. 2012, 18, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Noole, A.; Jrving, I.; Werner, F.; Lopp, M.; Malkov, A.; Kanger, T. Organocatalytic Asymmetric Synthesis of 3-Chlorooxindoles Bearing Adjacent Quaternary-Tertiary Centers. Org. Lett. 2012, 14, 4922–4925. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-S.; Zhu, G.-M.; Wu, C.-Y.; Hong, L.; Wang, R. An Organocatalytic Cascade Strategy for the Enantioselective Construction of Spirocyclopentane Bioxindoles Containing Three Contiguous Stereocenters and Two Spiro Quaternary Centers. Chem. Eur. J. 2012, 18, 6737–6741. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-T.; Jia, W.-Q.; Ye, S. Catalytic [4+2]Cyclization of α,β-Unsaturated Acyl Chlorides with 3-Alkylenyloxindoles: Highly Diastereo- and Enantioselective Synthesis of Spirocarbocyclic Oxindoles. Angew. Chem. Int. Ed. 2013, 52, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Dong, G.; Miao, Z.; Zhang, W.; Sheng, C. Meeting Organocatalysis with Drug Discovery: Asymmetric Synthesis of 3,3′-Spirooxindoles Fused with Tetrahydrothiopyrans as Novel p53-MDM2 Inhibitors. Org. Lett. 2016, 18, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Duan, S.-W.; Zhang, R.; Liu, Y.-H.; Chen, J.-R.; Xiao, W.-J. Base-catalyzed controllable reaction of 3-ylideneoxindoles with O-Boc hydroxy carbamates for the synthesis of amid acrylates and spiroaziridine oxindoles. Org. Biomol. Chem. 2016, 14, 5224–5228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-L.; Du, D.-M. Organocatalytic cascade Michael/Michael reaction for the asymmetric synthesis of spirooxindoles containing five contiguous stereocenters. Chem. Commun. 2016, 52, 6162–6165. [Google Scholar] [CrossRef] [PubMed]
- Cerisoli, L.; Lombardo, M.; Trombini, C.; Quintavalla, A. The First Enantioselective Organocatalytic Synthesis of 3-Spiro-α-Alkylidene-γ-Butyrolactone Oxindoles. Chem. Eur. J. 2016, 22, 3865–3872. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Du, F. Diastereo- and Enantioselective Construction of Dihydroiso coumarin-Based Spirooxindole Frameworks via Organocatalytic Tandem Reactions. Adv. Synth. Catal. 2016, 358, 2777–2790. [Google Scholar] [CrossRef]
- Zhu, Q.-N.; Zhang, Y.-C.; Xu, M.-M.; Sun, X.-X.; Yang, X.; Shi, F. Enantioselective Construction of Tetrahydroquinolin-5-one-Based Spirooxindole Scaffold via an Organocatalytic Asymmetric Multicomponent [3+3] Cyclization. J. Org. Chem. 2016, 81, 7898–7907. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Jiang, Y.; Xu, Q.; Tang, X.-Y.; Shi, M. Enantioselective [3+2] Cyclization of 3-Isothiocyanato Oxindoles with Trifluoromethylated 2-Butenedioic Acid Diesters. ChemCatChem 2015, 7, 1366–1371. [Google Scholar] [CrossRef]
- Rajesh, R.; Suresh, M.; Selvam, R.; Raghunathan, R. Synthesis of acridinedione derived mono spiro-pyrrolidine/pyrrolizidine derivatives—A facile approach via intermolecular [3+2] cycloaddition reaction. Tetrahedron Lett. 2014, 55, 4047–4053. [Google Scholar] [CrossRef]
- Senthil Kumar, G.; Satheesh kumar, R.; Kaminsky, W. A facile regioselective 1,3-dipolar cycloaddition protocol for the synthesis of new class of quinolinyl dispiro heterocycles. Tetrahedron Lett. 2014, 55, 5475–5480. [Google Scholar] [CrossRef]
- Peng, C.; Ren, J.; Xiao, J.-A.; Zhang, H.; Yang, H.; Luo, Y. Additive-assisted regioselective 1,3-dipolar cycloaddition of azomethine ylides with benzylideneacetone. Beilstein J.Org. Chem. 2014, 10, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lanka, S.; Thennarasu, S.; Perumal, P.T. Facile synthesis of novel dispiroheterocyclic derivatives through cycloaddition of azomethine ylides with acenaphthenone-2-ylidine ketones. Tetrahedron Lett. 2012, 53, 7052–7055. [Google Scholar] [CrossRef]
- Al Mamari, K.; El Mokhtar, E. Synthesis of novel dispiro-oxindoles via 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Lett. 2012, 53, 2328–2331. [Google Scholar] [CrossRef]
- Raj, A.A.; Raghunathan, R. A novel entry into a new class of spiroheterocyclic framework: Regioselective synthesis of dispiro[oxindole-cyclohexanone]pyrrolidines and dispiro[oxindole-hexahydroindazole]pyrrolidines. Tetrahedron 2001, 57, 10293–10298. [Google Scholar]
- Ranjith, K.R.; Perumal, S.; Senthilkumar, P.; Yogeeswari, P.; Sriram, D. A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines. Eur. J. Med. Chem. 2009, 44, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Coulter, T.; Grigg, R.; Malone, J.F.; Sridharan, V. Chiral induction in cycloaddition reactions of azomethine ylides derived from secondary α-amino acids by the decarboxylative route. Tetrahedron Lett. 1991, 32, 5417–5420. [Google Scholar] [CrossRef]
- Naga Siva Rao, J.; Raghunathan, R. A tactical 1,3-dipolar cycloaddition approach for the synthesis of carbohydrate derived polycyclic spiro heterocycles. Tetrahedron Lett. 2015, 56, 1539–1544. [Google Scholar]
- Arumugam, N.; Periyasami, G.; Raghunathan, R.; Kamalraj, S.; Muthumary, J. Synthesis and antimicrobial activity of highly functionalised novel β-lactam grafted spiropyrrolidines and pyrrolizidines. Eur. J. Med. Chem. 2011, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, S.; Prasanna, R.; Raghunathan, R. Regioselective synthesis of spiropyrrolidine/spiropyrrolizidine/spirothiazolidine-grafted macrocycles through 1,3-dipolar cycloaddition methodology. Tetrahedron 2013, 69, 9742–9750. [Google Scholar] [CrossRef]
- Santos, M.M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Hong, L.; Wang, R. Recent Advances in Asymmetric Organocatalytic Construction of 3,3′-Spirocyclic Oxindoles. Adv. Synth. Catal. 2013, 355, 1023–1052. [Google Scholar] [CrossRef]
- Han, W.-Y.; Zhao, J.-Q.; Zuo, J.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Recent Advances of α-Isothiocyana to Compounds in the Catalytic Asymmetric Reaction. Adv. Synth. Catal. 2015, 357, 3007–3031. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.L.; Zhou, J. Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Synth. Catal. 2010, 352, 1381–1407. [Google Scholar] [CrossRef]
- Ball-Jones, N.R.; Badillo, J.J.; Franz, A.K. Strategies for the enantioselective synthesis of spirooxindoles. Org. Biomol. Chem. 2012, 10, 5165–5181. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; Desta, Z.Y. Isatins as Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- El-Ahl, A.-A.S. Three-component 1,3-dipolar cycloaddition reactions in synthesis of spiro[pyrrolidine-2,3′-oxindoline] derivatives. Heteroat. Chem. 2002, 13, 324–329. [Google Scholar] [CrossRef]
- Hazra, A.; Paira, P.; Sahu, K.B.; Naskar, S.; Saha, P.; Paira, R.; Mondal, S.; Maity, A.; Luger, P.; Weber, M.; et al. Chemistry of andrographolide: Formation of novel di-spiropyrrolidino and di-spiropyrrolizidino-oxindole adducts via one-pot three-component [3+2] azomethine ylide cycloaddition. Tetrahedron Lett. 2010, 51, 1585–1588. [Google Scholar] [CrossRef]
- Babu, S.R.; Raghunathan, R. An easy access to novel steroidal dispiropyrrolidines through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett. 2008, 49, 4618–4620. [Google Scholar] [CrossRef]
- Dandia, A.; Jain, A.K.; Bhati, D.S. Direct construction of novel dispiro heterocycles through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett. 2011, 52, 5333–5337. [Google Scholar] [CrossRef]
- Naga Siva Rao, J.; Raghunatha, R. An expedient diastereoselective synthesis of pyrrolidinyl spirooxindoles fused to sugar lactone via [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett. 2012, 53, 854–858. [Google Scholar] [CrossRef]
- Poornachandran, M.; Raghunathan, R. Synthesis of Spirooxindolo/Spiroindano Nitro Pyrrolizidines through Regioselective Azomethine Ylide Cycloaddition Reaction. Synth. Commun. 2007, 37, 2507–2517. [Google Scholar] [CrossRef]
- Pavlovskaya, T.L.; Yaremenko, F.G.; Lipson, V.V.; Shishkina, S.V.; Shishkin, O.V.; Musatov, V.I.; Karpenko, A.I. The regioselective synthesis of spirooxindolo pyrrolidines and pyrrolizidines via three-component reactions of acrylamides and aroylacrylic acids with isatins and α-amino acids. Beilstein J. Org. Chem. 2014, 10, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Rajesh, S.M.; Perumal, S.; Banerjee, D.; Yogeeswari, P.; Sriram, D. Novel three-component domino reactions of ketones, isatin and amino acids: Synthesis and discovery of antimycobacterial activity of highly functionalised novel dispiropyrrolidines. Eur. J. Med. Chem. 2010, 45, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Balamurugan, K.; Perumal, S.; Yogeeswari, P.; Sriram, D. A regio- and stereoselective 1,3-dipolar cycloaddition for the synthesis of novel spiro-pyrrolothiazolyloxindoles and their antitubercular evaluation. Eur. J. Med. Chem. 2010, 45, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R.S. Anti-Inflammatory Compounds of Plant Origin. Part I. Action on Arachidonic Acid Pathway, Nitric Oxide and Nuclear Factor κ B (NF-κB). Planta Med. 2003, 69, 973–983. [Google Scholar] [PubMed]
- Hong, C.H.; Noh, M.S.; Lee, W.Y.; Lee, S.K. Inhibitory effects of natural sesquiterpenoids isolated from the rhizomes of Curcuma zedoaria on prostaglandin E2 and nitric oxide production. Planta Med. 2002, 68, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshiya, J.; Miyajima, M.; Hirakawa, K.; Kakurai, T. Dimethyl (2-oxo-4-methyl-3-pentenyl)phosphonate as a precursor of.alpha.,.alpha.′-dienones. Short syntheses of (.+-.)-.alpha.-atlantone and (.+-.)-ar-turmerone. J. Org. Chem. 1985, 50, 1326–1327. [Google Scholar] [CrossRef]
- Prete, D.D.; Millán, E.; Pollastro, F.; Chianese, G.; Luciano, P.; Collado, J.A.; Munoz, E.; Appendino, G.; Taglialatela-Scafati, O. Turmeric Sesquiterpenoids: Expeditious Resolution, Comparative Bioactivity, and a New Bicyclic Turmeronoid. J. Nat. Prod. 2016, 79, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Y.; Li, S.-W.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. 3-Isothiocyanato Oxindoles Serving as Powerful and Versatile Precursors to Structurally Diverse Dispirocyclic Thiopyrrolidineoxindoles through a Cascade Michael/Cyclization Process with Amino-Thiocarbamate Catalysts. Chem. Eur. J. 2013, 19, 5551–5556. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Y.; Chen, W.-B.; Han, W.-Y.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Highly Efficient and Stereoselective Construction of Dispiro-[oxazolidine-2-thione]bisoxindoles and Dispiro[imidazolidine-2-thione]bisoxindoles. Org. Lett. 2012, 14, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Han, W.-Y.; Zhang, X.-M.; Yuan, W.-C. Highly Efficient and Stereocontrolled Construction of 3,3′-Pyrrolidonyl Spirooxindoles via Organocatalytic Domino Michael/Cyclization Reaction. Org. Lett. 2013, 12, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1530052, CCDC 1530053 and 1530563 contain the supplementary crystallographic datas for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Mosman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor celllines using amicro culture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 3–5 are available from the authors. |

| Entry a | Solvent | T (°C) | Time (h) | Yield b (%) | Dr c |
|---|---|---|---|---|---|
| 1 | toluene | 80 | 5 | 67 | 10:1 |
| 2 | DCE | reflux | 5 | 61 | 15:1 |
| 3 | EtOAc | reflux | 5 | 48 | 12:1 |
| 4 | EtOH | reflux | 5 | 83 | 18:1 |
| 5 | THF | reflux | 5 | 71 | 16:1 |
| 6 | H2O | 80 | 5 | 39 | 11:1 |
| 7 | CH3CN | reflux | 5 | 82 | 19:1 |
| 8 | EtOH | 40 | 48 | 52 | 17:1 |
| 9 d | EtOH | reflux | 3 | 89 | 20:1 |
| 10 e | EtOH | reflux | 5 | 57 | 16:1 |
| 11 f | EtOH | reflux | 3 | 72 | 19:1 |



| Compound | 3le | 3fa | 3la | 3ia | 3fd | 4da | 5ia | 5ba | 5oa | 5pa | Cisplatin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K562 IC50 (μM) | 43.5 | 51.3 | 33.8 | 77.8 | 62.3 | 38.5 | 49.4 | 79.0 | 22.3 | 27.6 | 21.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Zhou, G.; Gong, Y.; Wei, Q.-D.; Tian, M.-Y.; Liu, X.-L.; Feng, T.-T.; Zhou, Y.; Yuan, W.-C. Molecular Hybridization-Guided One-Pot Multicomponent Synthesis of Turmerone Motif-Fused 3,3′-Pyrrolidinyl-dispirooxindoles via a 1,3-Dipolar Cycloaddition Reaction. Molecules 2017, 22, 645. https://doi.org/10.3390/molecules22040645
Lin B, Zhou G, Gong Y, Wei Q-D, Tian M-Y, Liu X-L, Feng T-T, Zhou Y, Yuan W-C. Molecular Hybridization-Guided One-Pot Multicomponent Synthesis of Turmerone Motif-Fused 3,3′-Pyrrolidinyl-dispirooxindoles via a 1,3-Dipolar Cycloaddition Reaction. Molecules. 2017; 22(4):645. https://doi.org/10.3390/molecules22040645
Chicago/Turabian StyleLin, Bing, Gen Zhou, Yi Gong, Qi-Di Wei, Min-Yi Tian, Xiong-Li Liu, Ting-Ting Feng, Ying Zhou, and Wei-Cheng Yuan. 2017. "Molecular Hybridization-Guided One-Pot Multicomponent Synthesis of Turmerone Motif-Fused 3,3′-Pyrrolidinyl-dispirooxindoles via a 1,3-Dipolar Cycloaddition Reaction" Molecules 22, no. 4: 645. https://doi.org/10.3390/molecules22040645