Evaluation of Tanshinone IIA Developmental Toxicity in Zebrafish Embryos
Abstract
:1. Introduction
2. Results
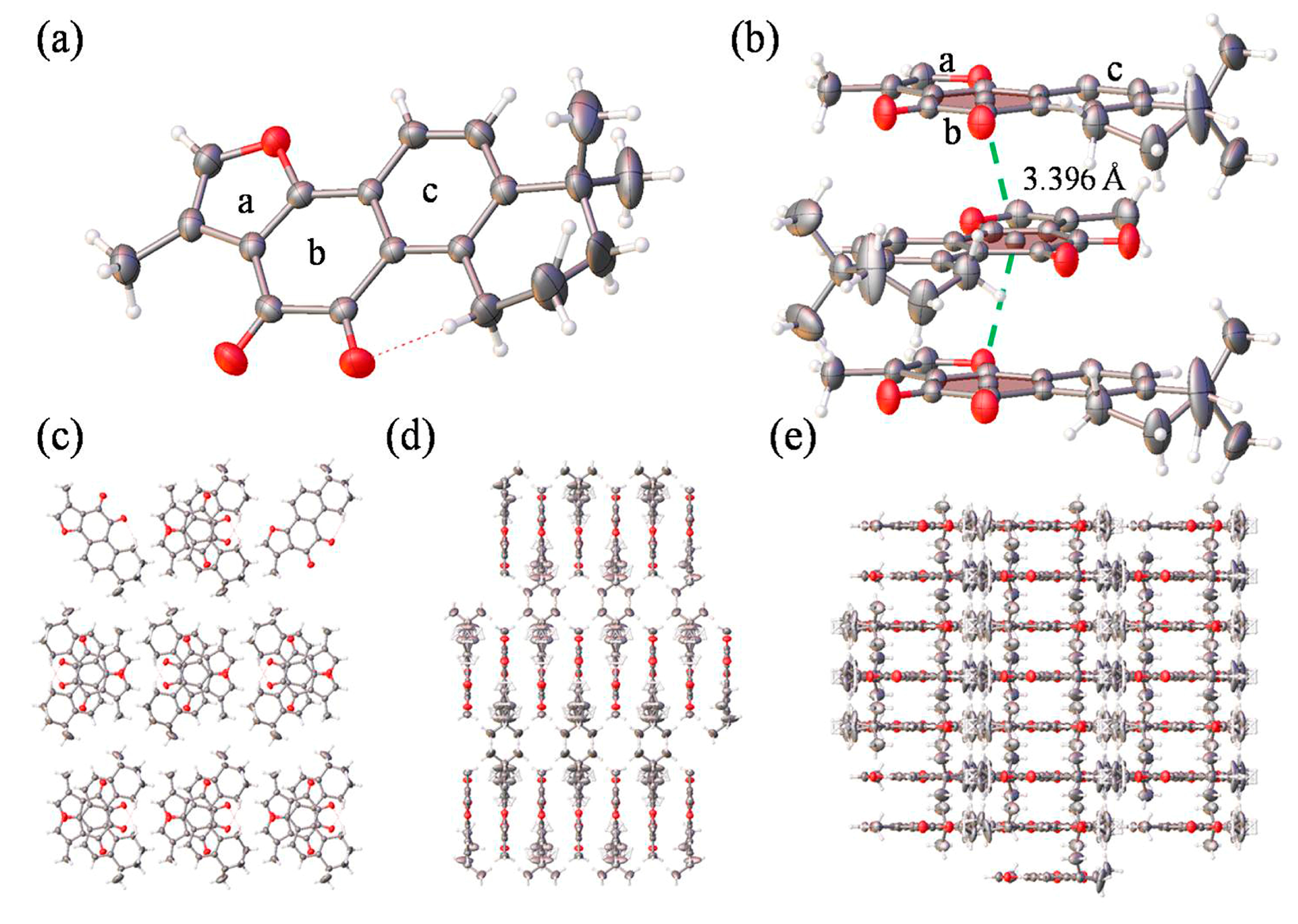
2.1. The Crystal Structure of Tan-IIA
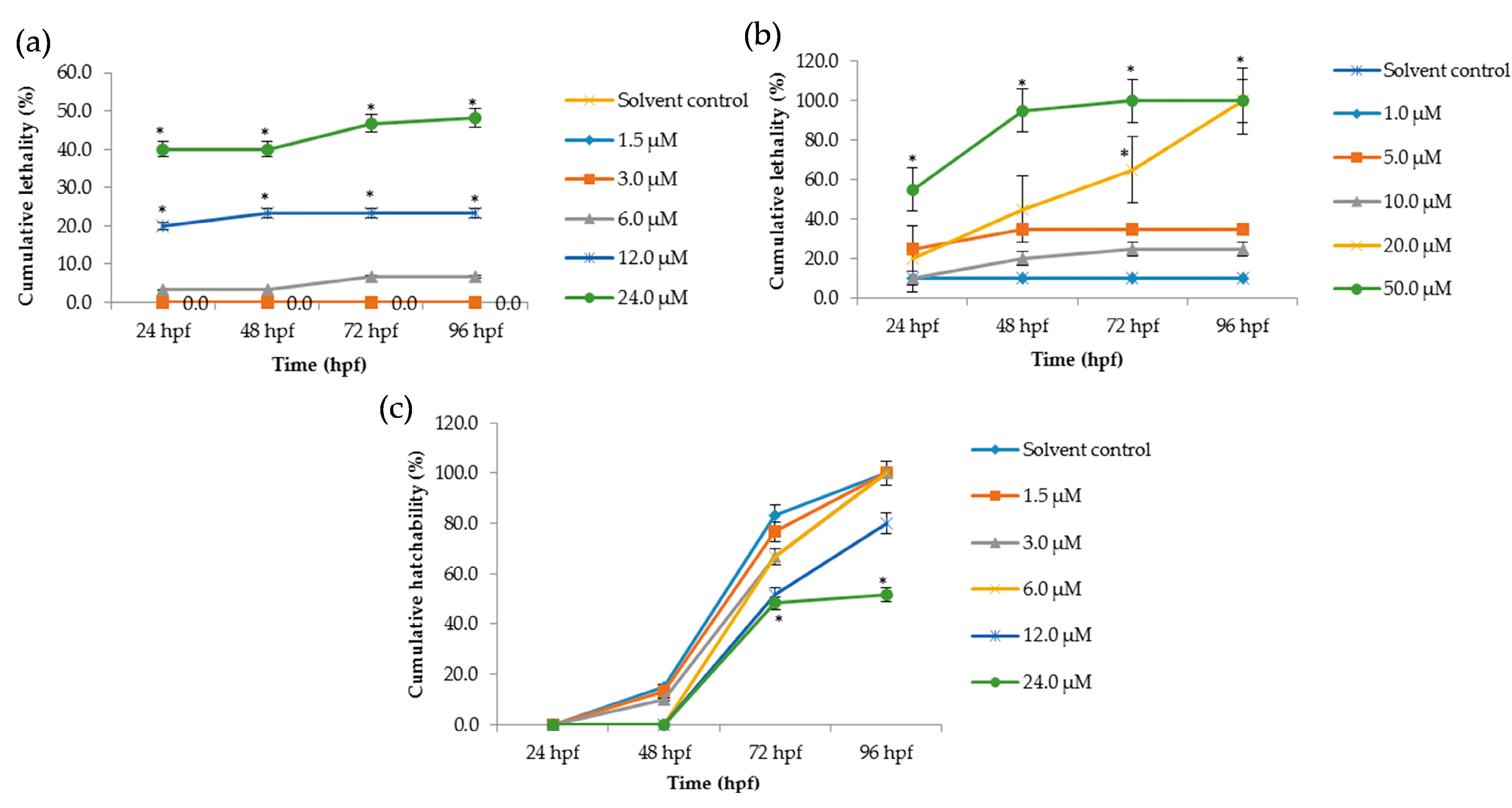
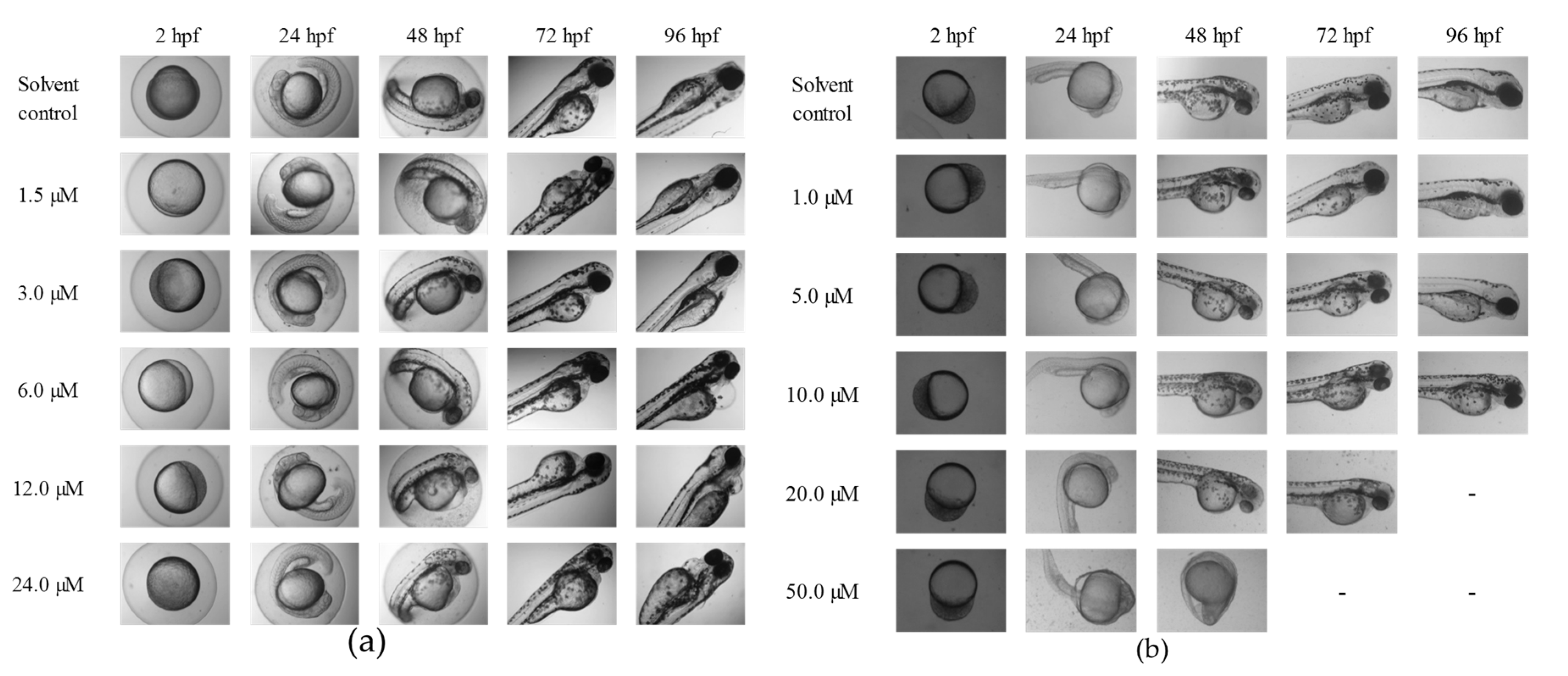
2.2. The Lethal Effects of Tan-IIA on Embryos
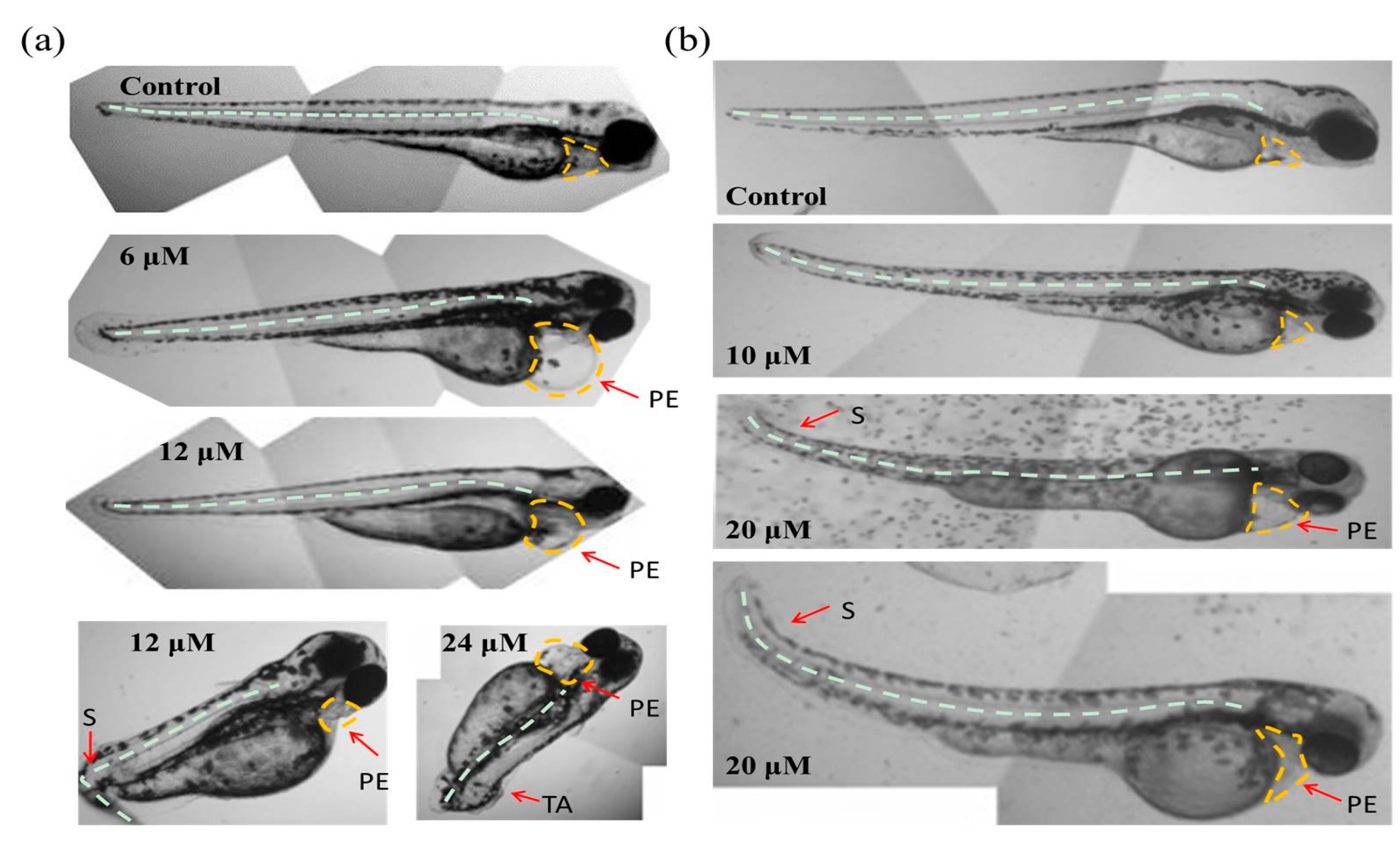
2.3. The Teratogenic Effects of Tan-IIA on Embryos
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Brood Zebrafish Maintenance and Egg Production
4.3. The Developmental Toxicity Assay
4.3.1. Removal of Chorion
4.3.2. Embryo Exposure
4.3.3. Observation of Mortal and Teratogenic Effects
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, S.; Liu, Z.P.; Li, H.; Little, P.J.; Liu, P.Q.; Xu, S.W. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 2012, 220, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Liu, P.Q. Tanshinone II-A: New perspectives for old remedies. Expert. Opin. Ther. Pat. 2013, 23, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Xing, C.Y.; Zhang, M.Y. Tanshinone IIA represses inflammatory response and reduces radiculopathic pain by inhibiting IRAK-1 and NF-κB/p38/JNK signaling. Int. Immunopharmacol. 2015, 28, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Tang, F.T.; Xie, B.L.; Chen, S.R.; Huang, H.Q.; Liu, P.Q. Amelioration of atherosclerosis by tanshinone IIA in hyperlipidemic rabbits through attenuation of oxidative stress. Eur. J. Pharmacol. 2012, 674, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Hu, X.R.; Hung, Z.A.; Huang, D.D.; Zhang, S. Effects of tanshinone IIA on fibrosis in a rat model of cirrhosis through heme oxygenase-1, inflammation, oxidative stress and apoptosis. Mol. Med. Rep. 2016, 13, 3036–3042. [Google Scholar] [PubMed]
- Wang, P.; Zhou, S.G.; Xu, L.P.; Lu, Y.; Yuan, X.; Zhang, H.J.; Li, R.F.; Fang, J.; Liu, P.Q. Hydrogen peroxide-mediated oxidative stress and collagen synthesis in cardiac fibroblasts: Blockade by tanshinone IIA. J. Ethnopharmacol. 2013, 145, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, P.X.; Ye, M.; Kim, S.H.; Jiang, C.; Lü, J.X. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.; Li, C.Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug. Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebei, U.; Muller, F.; Strahle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- McCollum, C.W.; Ducharme, N.A.; Bondesson, M.; Gustafsson, J.A. Developmental toxicity screening in zebrafish. Birth. Defects Res. C. Embryo. Today 2011, 93, 67–114. [Google Scholar] [CrossRef] [PubMed]
- McClain, R.M.; Keller, D.; Casciao, D.; Fu, P.; MacDonald, J.; Popp, J.; Sagarta, J. Neonatal mouse model: Review of methods and results. Toxicol. Pathol. 2001, 29, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.H.; Carney, E.W. The rabbit as a model for reproductive and developmental toxicity studies. Reprod. Toxicol. 2000, 14, 477–493. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.P.; Chen, L.; Gao, H.W.; Wu, L.L. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ. Sci. Pollut. Res. Int. 2016, 23, 18832–18841. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhou, D.J.; Dong, J.; Jiang, F.C.; Chen, W. Acute toxicity of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), on zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2016, 133, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bugel, S.M.; Bonventre, J.A.; Tanguay, R.L. Comparative developmental toxicity of flavonoids using an integrative zebrafish system. Toxicol. Sci. 2016, 154, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.C.; Ho, J.C.; Cheung, A.H.; Lai, K.P.; William, K.F. Developmental toxicity of the common UV filter, benophenone-2, in zebrafish embryos. Chemosphere 2016, 164, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Li, Y.F.; Coelhan, M.; Chan, H.M.; Ma, W.L.; Liu, L.Y. Relative developmental toxicity of short-chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2016, 219, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tu, W.Q.; Deng, M.; Jin, Y.X.; Lu, B.; Zhang, C.N.; Lin, C.M.; Wu, Y.M.; Liu, W.P. Stereoselective induction of developmental toxicity and immunotoxicity by acetochlor in the early life stage of zebrafish. Chemosphere 2016, 164, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.D.; Chen, Z.S.; Wang, C.X.; Zeng, R.F.; Wang, M.C.; Mei, W.J.; Zhang, W.D. Toxic effect of dimethyl sulfoxide on zebrafish embryo. J. Guangdong. Pharm. Univ. 2014, 30, 636–650. [Google Scholar]
- Xiong, X.Q.; Luo, S.; Wu, B.L.; Wang, J.W. Comparative developmental toxicity and stress protein responses of dimethyl sulfoxide to rare minnow and zebrafish embryos/larvae. Zebrafish 2017, 14, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.M.; Chen, X.M.; Sun, W.L. Safety of sodium tanshinone IIA sulfonate injection. J. Med. Postgrad. 2010, 23, 474–476. [Google Scholar]
- Kim, D.H.; Hwang, C.N.; Sun, Y.; Lee, S.H.; Kim, B.; Neson, B.J. Mechanical analysis of chorion softening in prehatching stages of zebrafish embryos. IEEE. Trans. Nanobioscience 2006, 5, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sun, S.; Kim, B.; Hwang, C.N.; Lee, S.H.; Nelson, B.J. Mechanical property characterization of the zebrafish embryo chorion. Conf. Proc. IEEE. Eng. Med. Biol. Soc. 2004, 7, 5061–5064. [Google Scholar] [PubMed]
- Ong, K.J.; Zhao, X.X.; Thistle, M.E.; MacCormack, T.J.; Clark, R.J.; Ma, G.B.; Martinez-Rubi, Y.; Simard, B.; Loo, J.S.C.; Veinot, J.G.; et al. Mechanistic insights into the effect of nanoparticles on zebrafish hatch. Nanotoxicology 2014, 8, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Sano, K.; Nagata, K.; Yasumasu, S.; Ohtsuka, J.; Yamamura, A.; Kubota, K.; Iuchi, I.; Tanokura, M. Crystal structure of zebrafish hatching enzyme 1 from the zebrafish Danio rerio. J. Mol. Biol. 2010, 402, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, J.; Maruyama, K.; Kawazu, K.; Kaneko, T.; Ohtani-Kaneko, R.; Yasumasu, S. Structure and developmental expression of hatching enzyme genes of the Japanese eel Anguilla japonica: An aspect of the evolution of fish hatching enzyme gene. Dev. Genes. Evol. 2004, 214, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book, 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000; pp. 1–196. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Haldi, M.; Harden, M.; D’Amico, L.; DeLise, A.; Seng, W.L. Developmental toxicity assessment in zebrafish. In Zebrafish: Methods for Assessing Drug Safety and Toxicity, 1st ed.; McGrath, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 15–25. [Google Scholar]
- McGrath, P. Use of emerging models for developmental toxicity testing. In Zebrafish: Methods for Assessing Drug Safety and Toxicity, 1st ed.; McGrath, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 27–44. [Google Scholar]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Li, J.X.; Xu, M.D.; Deng, X.Q.; Yue, G.H.; Zheng, J.H. Meta-analysis on effectiveness and safety of tanshinone IIa sulfonate sodium injection in treatment of unstable angina pectoris. World Sci. Technol. Mod. Tradit. Chin. Med. 2015, 17, 1766–1774. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, C.; Wu, Q.; Zheng, K.; Chen, J.; Lan, Y.; Qin, Y.; Mei, W.; Wang, B. Evaluation of Tanshinone IIA Developmental Toxicity in Zebrafish Embryos. Molecules 2017, 22, 660. https://doi.org/10.3390/molecules22040660
Wang T, Wang C, Wu Q, Zheng K, Chen J, Lan Y, Qin Y, Mei W, Wang B. Evaluation of Tanshinone IIA Developmental Toxicity in Zebrafish Embryos. Molecules. 2017; 22(4):660. https://doi.org/10.3390/molecules22040660
Chicago/Turabian StyleWang, Tao, Chengxi Wang, Qiong Wu, Kangdi Zheng, Jiaojiao Chen, Yutao Lan, Yao Qin, Wenjie Mei, and Baoguo Wang. 2017. "Evaluation of Tanshinone IIA Developmental Toxicity in Zebrafish Embryos" Molecules 22, no. 4: 660. https://doi.org/10.3390/molecules22040660





