Anthocyanin Profiles in Flowers of Grape Hyacinth
Abstract
:1. Introduction
2. Results
2.1. Anthocyanin Components in Grape Hyacinth
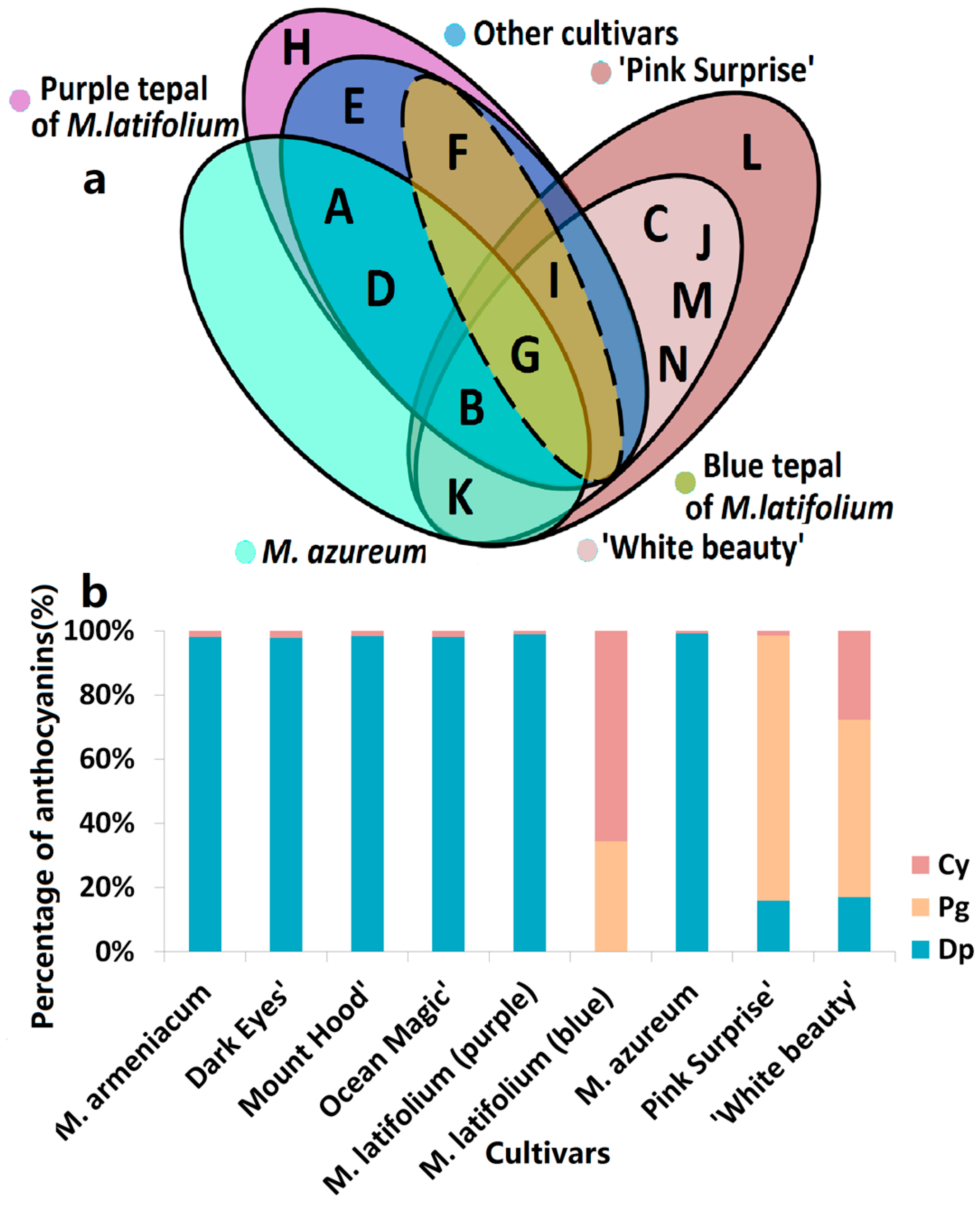
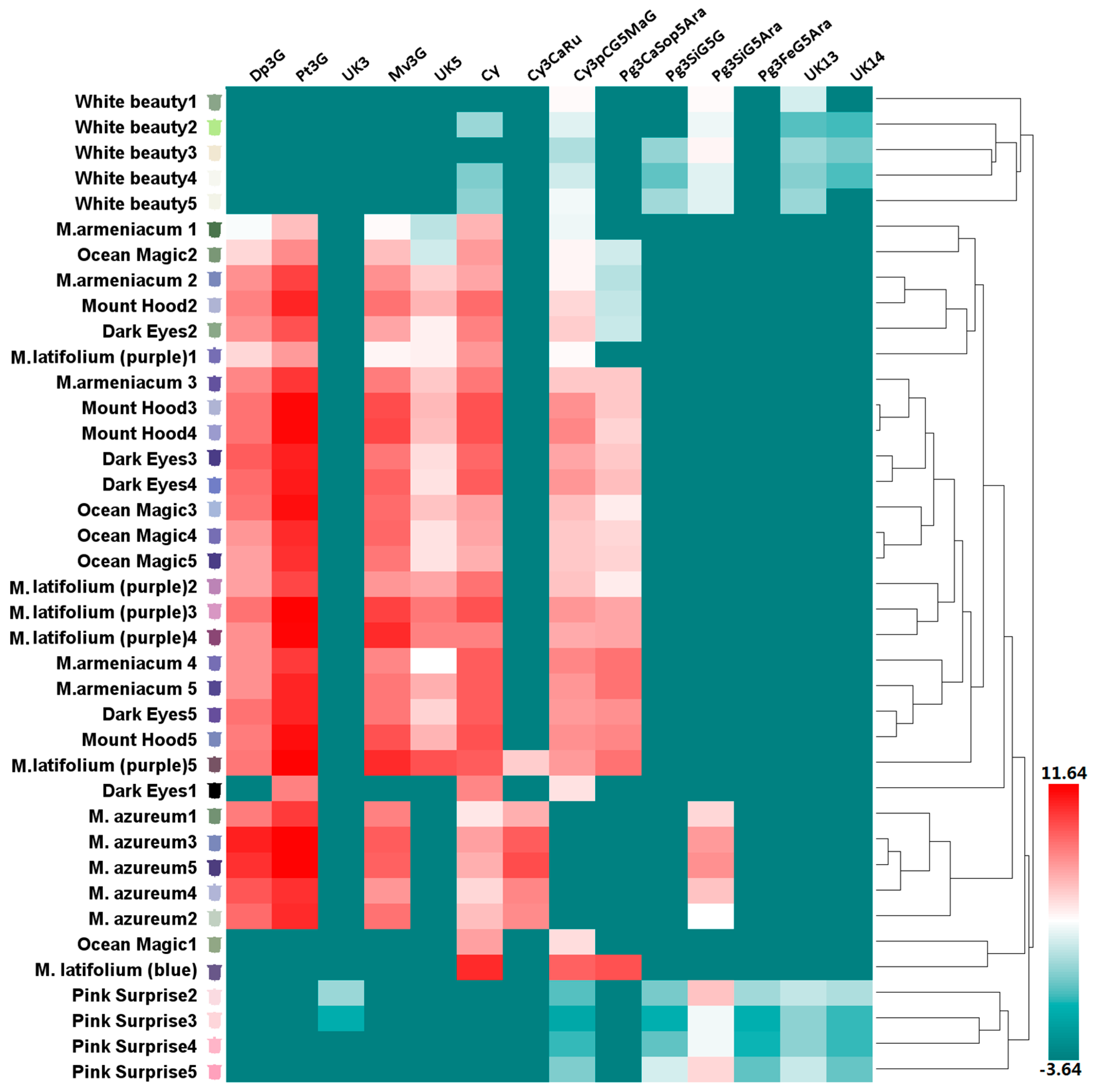
2.2. Specific Accumulation and Distribution of Anthocyanins in Grape Hyacinth
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. HPLC Analysis
4.3. High-Performance Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nakayama, T. Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol. 2014, 56, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Brugliera, F. Flower color and cytochromes P450. Philos. Trans. R. Soc. B 2013, 368, 20120432. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Miki, N.; Momonoi, K.; Kawachi, M.; Katou, K.; Okazaki, Y.; Uozumi, N.; Maeshima, M.; Kondo, T. Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc. Jpn. Acad. B-Phys. 2009, 85, 187–197. [Google Scholar] [CrossRef]
- Shoji, K.; Momonoi, K.; Tsuji, T. Alternative expression of vacuolar iron transporter and ferritin genes leads to blue/purple coloration of flowers in Tulip cv. ‘Murasakizuisho’. Plant Cell Physiol. 2010, 51, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Asano, S.; Kobayashi, H.; Nakano, M. Analyses of anthocyanidins and anthocyanins in flowers of Muscari spp. Ngt. Dgk. Ngk. Knkyu. Hkk. 2002, 55, 13–18. [Google Scholar]
- Lou, Q.; Liu, Y.; Qi, Y.; Jiao, S.; Tian, F.; Jiang, L.; Wang, Y. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower color in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Aoki, H.; Kameda, K.; Kondo, T. Structure of muscarinin A, an acylated anthocyanin, from purplish blue spicate flower petals of Muscari arumeniacum. ITE Lett. Batter New Technol. Med. 2002, 1, 35–38. [Google Scholar]
- Muscari—Wikipedia. Available online: https://en.wikipedia.org/wiki/Muscari (accessed on 11 February 2017).
- Qi, Y.; Lou, Q.; Li, H.; Yue, J.; Liu, Y.; Wang, Y. Anatomical and biochemical studies of bicolored flower development in Muscari latifolium. Protoplasma 2013, 250, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.; Schwikkard, S.; Crouch, N. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165–1210. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yuan, Y.; Xu, Y.; Shi, Y.; Tang, D. Anthocyanin profiles in petals of different Hyacinthus orientalis. Acta Hortic. Sin. 2015, 42, 301–310. [Google Scholar]
- Uddin, A.; Hashimoto, F.; Nishimoto, S.; Shimizu, K.; Sakata, Y. Flower growth, coloration, and petal pigmentation in four Lisianthus cultivars. J. Jpn. Soc. Hortic. Sci. 2002, 71, 40–47. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Level, A.; Hugueney, P.; Verriès, A.; Ageorges, A. Genetic mechanisms underlying the methylation level of anthocyanins in grape (Vitis vinifera L.). BMC Plant Biol. 2011, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, S.; Yin, X.; Wang, K.; Liu, Y.; Li, S.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, X.; Gao, F.; Wang, L.; Zhang, H.; Xu, Y.; Lia, C.; Ge, Y.; Zhang, J.; Zhang, J. Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry. J. Chromatogr. A 2009, 1216, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, J.; Ji, K.; Zeng, Q.; Bhuiya, M.; Su, S.; Shu, Q.; Ren, H.; Liu, Z.; Wang, L. Methylation mediated by an anthocyanin, O-methyltransferase, is involved in purple flower coloration in Paeonia. J. Exp. Bot. 2015, 66, 6563–6577. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Aoki, N.; Tsunematsu, S.; Nishikouri, H.; Johjima, T. Petal coloration and pigmentation of tree peony bred and selected in Daikon Island (Shimane Prefecture). J. Jpn. Soc. Hortic. Sci. 1995, 64, 351–357. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Yasunaga, M.; Okamoto, E.; Okamoto, M.; Hirose, Y.; Yamaguchi, M.; Ozeki, Y.; Sasaki, N. p-Hydroxybenzoyl-glucose is a zwitter donor for the biosynthesis of 7-polyacylated anthocyanin in delphinium. Plant Cell 2013, 25, 4150–4165. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, Y.; Sasaki, N.; Yasunaga, M.; Miyahara, T.; Okamoto, E.; Okamoto, M.; Hirose, Y.; Ozeki, Y. Identification of the glucosyltransferase gene that supplies the p-hydroxybenzoyl-glucose for 7-polyacylation of anthocyanin in delphinium. J. Exp. Bot. 2014, 65, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kondo, T.; Tamura, H.; Imagawa, H.; Iino, A.; Takeda, K. Structure of gentiodelphin, an acylated anthocyanin isolated from Gentiana Makinoi, that is stable in dilute aqueous solution. Tetrahedron Lett. 1982, 23, 3695–3698. [Google Scholar] [CrossRef]
- Brandt, K.; Kondo, T.; Aoki, H.; Goto, T. Structure and biosynthesis of anthocyanins in flowers of Campanula. Phytochemistry 1993, 33, 209–212. [Google Scholar] [CrossRef]
- Saito, N.; Toki, K.; Moriyama, H.; Shigihara, A.; Honda, T. Acylated anthocyanins from the blue-violet flowers of Anemone coronaria. Phytochemistry 2002, 60, 365–373. [Google Scholar] [CrossRef]
- Goto, T.; Kondo, T.; Kawai, T.; Tamura, H. Structure of cinerarin, a tetra-acylated anthocyanin isolated from the blue garden cineraria, Senecio cruentus. Tetrahedron Lett. 1984, 25, 6021–6024. [Google Scholar] [CrossRef]
- Saito, N.; Tatsuzawa, F.; Yoda, K.; Yokoi, M.; Kasahara, K.; Iida, S.; Shigihara, A.; Honda, T. Acylated cyanidin glycosides in the violet-blue flowers of Ipomoea purpurea. Phytochemistry 1995, 40, 1283–1289. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Gao, J.; Li, C. Rapid separation and identification of anthocyanins from flowers of Viola yedoensis and V. prionantha by high-performance liquid chromatography-photodiode array detection-electrospray ionisation mass spectrometry. Phytochem. Anal. 2012, 23, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Khaldun, A.; Chen, J.; Zhang, C.; Lv, H.; Yuan, L.; Wang, Y. A R2R3-MYB transcription factor regulates the flavonol biosynthetic pathway in a traditional Chinese medicinal plant, Epimedium sagittatum. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Ishiguro, K.; Tanaka, Y.; Iida, S.; Hoshino, A. Spontaneous mutations of the UDP-glucose: flavonoid 3-O-glucosyltransferase gene confers pale- and dull-colored flowers in the Japanese and common morning glories. Planta 2015, 242, 575–587. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds analyzed in the study are unavailable from the authors due to their isolation on a small scale. They are readily analyzed using the procedures described. |
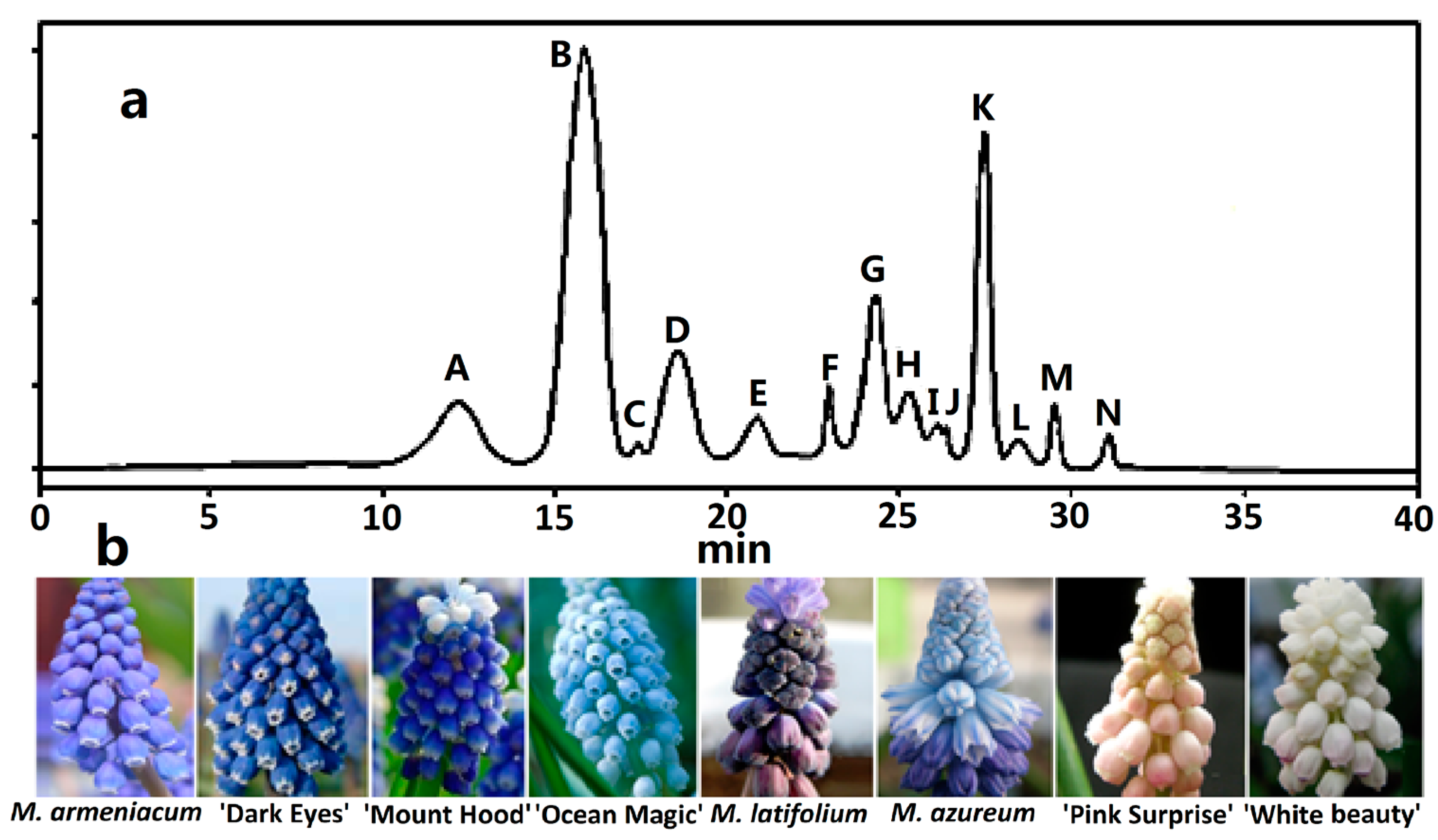
| Peak No. a | Compound | Abbreviations | Retention Time (min) | Molecular Ion (m/z) | Fragment Ions (m/z) |
|---|---|---|---|---|---|
| A | Delphinidin 3-O-glucoside | Dp3G | 12.4 | 465 | 465/303 |
| B | Petunidin 3-O-glucoside | Pt3G | 16.2 | 479 | 479/317 |
| C | Unknown compound | UK3 | 17.8 | - | - |
| D | Malvidin 3-O-glucoside | Mv3G | 18.9 | 493 | 493/331 |
| E | Unknown compound | UK5 | 21.2 | - | - |
| F | Pelargonidin-3-O-caffeoylsophoroside-5-O-arabinoside | Pg3CaSop5Ara | 23.4 | 889 | 889/757/595/271 |
| G | Cyanidin | Cy | 24.6 | 287 | 287 |
| H | Cyanidin-3-O-caffeoylrutinoside | Cy3CaRu | 25.9 | 757 | 757/595/287 |
| I | Cyanidin-3-O-(p-coumaroyl)glucoside-5-O-malonylglucoside | Cy3pCG5MaG | 26.4 | 843 | 843/757/287 |
| J | Pelargonidin-3-O-sinapylglucoside-5-O-glucoside | Pg3SiG5G | 27.3 | 801 | 801/595/433/271 |
| K | Pelargonidin-3-O-sinapylglucoside-5-O-arabinoside | Pg3SiG5Ara | 28.0 | 771 | 771/565/433/271 |
| L | Pelargonidin-3-O-ferulylglucoside-5-O-arabinoside | Pg3FeG5Ara | 29.6 | 741 | 741/565/433/271 |
| M | Unknown compound | UK13 | 30.7 | - | - |
| N | Unknown compound | UK14 | 32.5 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Q.; Wang, L.; Liu, H.; Liu, Y. Anthocyanin Profiles in Flowers of Grape Hyacinth. Molecules 2017, 22, 688. https://doi.org/10.3390/molecules22050688
Lou Q, Wang L, Liu H, Liu Y. Anthocyanin Profiles in Flowers of Grape Hyacinth. Molecules. 2017; 22(5):688. https://doi.org/10.3390/molecules22050688
Chicago/Turabian StyleLou, Qian, Lin Wang, Hongli Liu, and Yali Liu. 2017. "Anthocyanin Profiles in Flowers of Grape Hyacinth" Molecules 22, no. 5: 688. https://doi.org/10.3390/molecules22050688





