The Effect of Deoxynivalenol on Selected Populations of Immunocompetent Cells in Porcine Blood—A Preliminary Study
Abstract
:1. Introduction
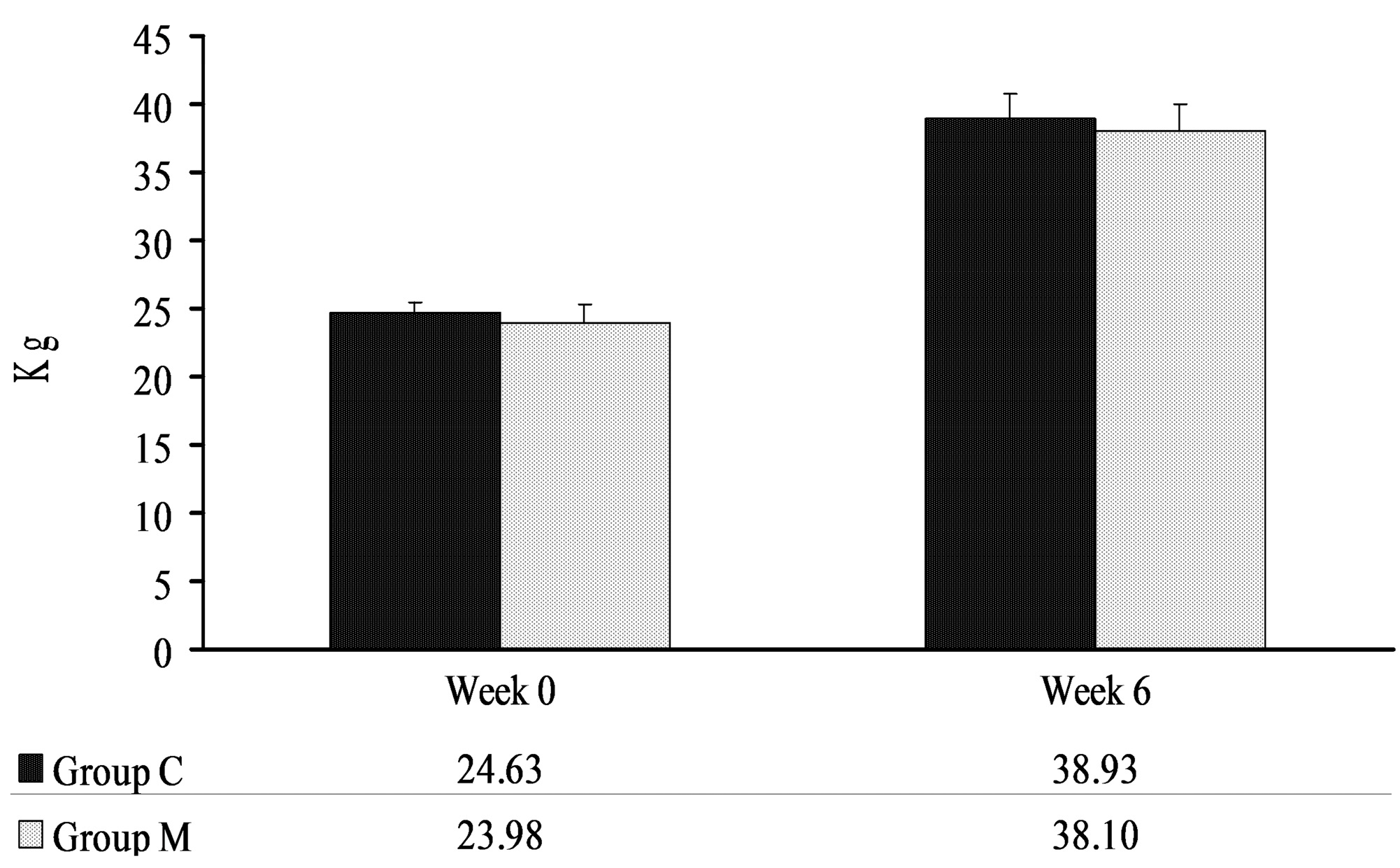
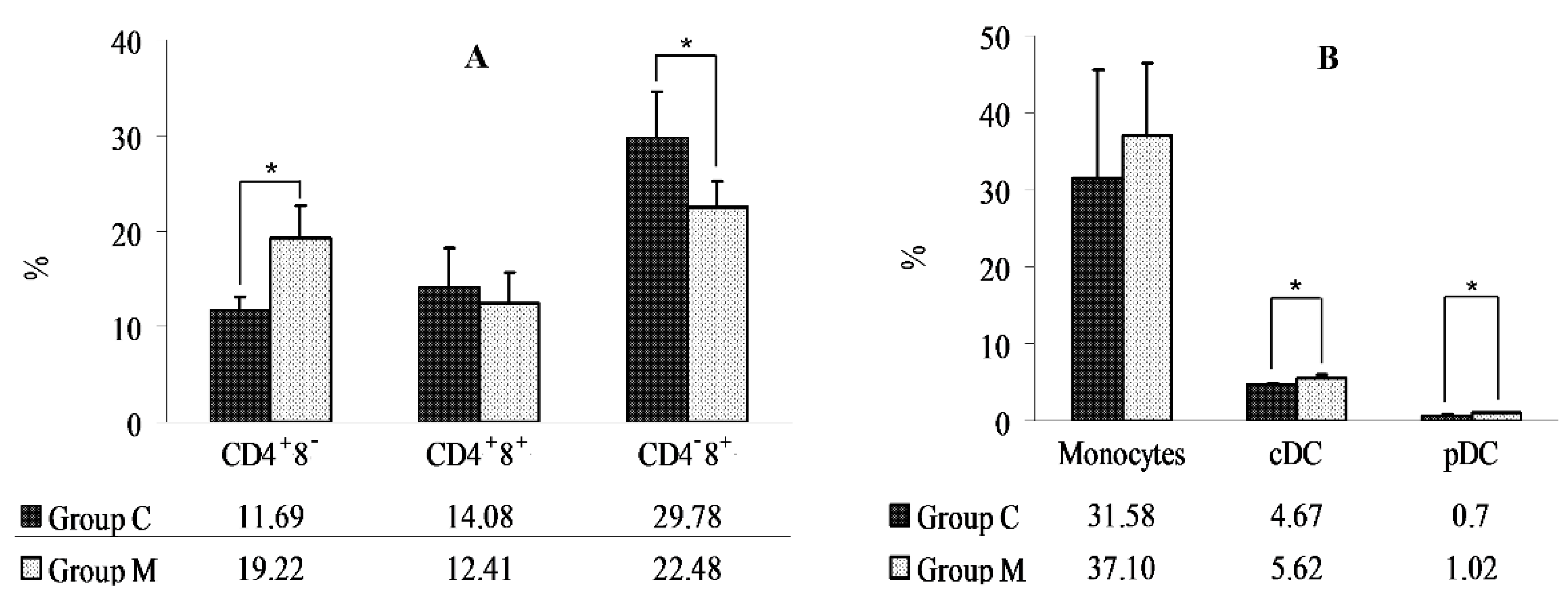
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Body Weights of Pigs
4.3. Mycotoxin Levels in Feed
4.4. Proximate Chemical Composition of Feed
4.5. Hematology Tests
4.6. Cytometric Analysis
Determination of the Percentages of Lymphocytes (CD4+8−, CD4−8+, CD4+8+), Monocytes, and DCs
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zielonka, Ł.; Wiśniewska, M.; Gajęcka, M.; Obremski, K.; Gajęcki, M. Influence of deoxynivalenol at low doses administered per os on histopathological image of selected organs of pigs. Pol. J. Vet. Sci. 2009, 12, 89–95. [Google Scholar] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, H.L.; Thompson, B.K.; Foster, B.C.; Charmley, L.L.; Hartin, K.E.; Coppock, R.W.; Albassam, M.A. Effects of feeding diets containing Fusarium (naturally) contaminated wheat or pure deoxynivalenol (DON) in growing pigs. Can. J. Anim. Sci. 1994, 74, 361–369. [Google Scholar] [CrossRef]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003, 72, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.L.; Azcona-Olivera, J.I.; Murtha, J.; Pestka, J.J. Vomitoxin-mediated IL-2, IL-4, and IL-5 superinduction in murine CD4+ T cells stimulated with phorbol ester calcium ionophore: Relation to kinetics of proliferation. Toxicol. Appl. Pharmacol. 1996, 138, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Drochner, W.; Schollenberger, M.; Piepho, H.P.; Götz, S.; Lauber, U.; Tafaj, M.; Klobasa, F.; Weiler, U.; Claus, R.; Steffl, M. Serum IgA-promoting effects induced by feed loads containing isolated deoxynivalenol (DON) in growing piglets. J. Toxicol. Environ. Health A 2004, 67, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, F.; Pinton, P.; Callu, P.; Oswald, I. Effets de la consommation par le porcelet sevré d’aliment contenant dublé naturellement fusarié. Journees De La Recherche Porcine En France 2007, 39, 427–428. [Google Scholar]
- Monbaliu, S.; van Poucke, C.; Detavernier, C.L.; Dumoulin, F.D.R.; van De Velde, M.; Schoeters, E.; van Dyck, S.; Averkieva, O.; van Peteghem, C.; de Saeger, S. Occurrence ofmycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Naehrer, K. Prevalence of mycotoxins in feedstuffs and feed surveyed worldwide in 2009 and 2010. Phytopathol. Mediterr. 2012, 51, 175–192. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Report of EFSA-Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- European Commission (EC). Commision recomendation on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 73, 1–42. [Google Scholar]
- Jackson, P.G.G.; Cockcroft, P.D. Appendix 2: Laboratory reference values: Haematology. In Clinical Examination of Farm Animals; Blackwell Science Ltd.: Oxford, UK, 2002; p. 302. [Google Scholar]
- Zielonka, Ł.; Gajęcka, M.; Tarasiuk, M.; Gajęcki, M. The effects of dietary deoxynivalenol (DON) on selected blood biochemical and hematological parameters in pre-pubertal gilts. Pol. J. Vet. Sci. 2015, 18, 223–231. [Google Scholar] [CrossRef]
- Modra, H.; Blahova, J.; Marsalek, P.; Banoch, T.; Fictum, P.; Svoboda, M. The effects of mycotoxin deoxynivalenol (DON) on haematological and biochemical parameters and selected parameters of oxidative stress in piglets. Neuro Endocrinol. Lett. 2013, 34 (Suppl. 2), 84–89. [Google Scholar] [PubMed]
- Accensi, F.; Pinton, P.; Callu, P.; Abella-Bourges, N.; Guelfi, J.F.; Grosjean, F.; Oswald, I.P. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets. J. Anim. Sci. 2006, 84, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.V.; Smith, T.K.; MacDonald, E.J.; Karrow, N.A.; Woodward, B.; Boermans, H.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2003, 81, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A.; Thompson, B.K.; Lessard, M.; Trenholm, H.L.; Tryphonas, H. Influence of low-level exposure to Fusarium mycotoxins on selected immunological and hematological parameters in young swine. Fundam. Appl. Toxicol. 1994, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Waché, Y.; Laffitte, J.; Taranu, I.; Saeedikouzehkonani, N.; Mori, Y.; Oswald, I.P. Deoxynivalenol impairs the immune functions of neutrophils. Mol. Nutr. Food Res. 2013, 57, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, M.; Obremski, K.; Gajęcka, M.; Gajęcki, M.T.; Zielonka, Ł. Changes in the Subpopulations of Porcine Peripheral Blood Lymphocytes Induced by Exposure to Low Doses of Zearalenone (ZEN) and Deoxynivalenol (DON). Molecules 2016, 21, 557. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Cantoni, A.M.; Borghetti, P.; De Angelis, E.; Corradi, A. Cellular immune response and immunotoxicity induced by DON (deoxynivalenol) in piglets. Vet. Res. Commun. 2009, 33, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Goyarts, T.; Rothkötter, H.; Dänicke, S. Effects of deoxynivalenol on immunohistological parameters in pigs. Mycotoxin Res. 2006, 22, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Levkut, M.; Revajova, V.; Slaminkova, Z.; Levkutova, M.; Borutova, R.; Gresakova, L.; Leng, L. Lymphocyte subpopulations in blood and duodenal epithelium of broilers fed diets contaminated with deoxynivalenol and zearalenone. Anim. Feed Sci. Technol. 2011, 165, 210–217. [Google Scholar] [CrossRef]
- Kubosaki, A.; Aihara, M.; Park, B.J.; Sugiura, Y.; Shibutani, M.; Hirose, M.; Suzuki, Y.; Takatori, K.; Sugita-Konishi, Y. Immunotoxicity of nivalenol after subchronic dietary exposure to rats. Food Chem. Toxicol. 2008, 46, 253–258. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.C.; Smits, H.H.; Kapsenberg, M.L. Dendritic cell-mediated T cell polarization. Springer Semin. Immun. 2005, 26, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.J. Development of dendritic-cell lineages. Immunity 2007, 26, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hymery, N.; Sibiril, Y.; Parent-Massin, D. In vitro effect of trichotecenes on human dendritic cells. Toxicol. In Vitro 2006, 20, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Loungo, D.; Severino, L.; Bergamo, P.; D’Arienzo, R.; Rossi, M. Trichotecenes NIV and DON modulate the maturation of murine dendritic cells. Toxicon 2010, 55, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bimczok, D.; Döll, S.; Rau, H.; Goyarts, T.; Wundrack, N.; Naumann, M.; Dänicke, S.; Rothkötter, H.J. The Fusarium toxin deoxynivalenol disrupts phenotype and function of monocyte-derived cells in vivo and in vitro. Immunobiology 2007, 212, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Meky, F.A.; Hardie, L.J.; Evans, S.W.; Wild, C.P. Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem. Toxicol. 2001, 39, 827–836. [Google Scholar] [CrossRef]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food. Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Basak, S.K.; Harui, A.; Stolina, M.; Sharma, S.; Mitani, K.; Dubinett, S.M.; Roth, M.D. Increased dendritic cell number and function following continuous in vivo infusion of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood 2002, 99, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Jeong, S.H.; Cho, J.H.; Shin, H.S.; Son, S.W.; Yeo, Y.K.; Kang, H.G. Effects of oral deoxynivalenol exposure on immune-related parameters in lymphoid organs and serum of mice vaccinated with porcine parvovirus vaccine. Mycotoxin Res. 2013, 29, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Tang, H.; Manicassamy, S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat. Immunol. 2010, 11, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Zwierzchowski, W.; Gajęcki, M.; Obremski, K.; Zielonka, Ł.; Baranowski, M. The occurrence of zearalenone and its derivatives in standard and therapeutic feeds for companion animals. Pol. J. Vet. Sci. 2004, 7, 289–293. [Google Scholar] [PubMed]
- Wiśniewska-Dmytrow, H.; Kozak, A.; Żmudzki, J. Determination of fumonisins B1 and B2 in corn and fodder by liquid chromatography. Med. Weter. 1999, 55, 114–117. [Google Scholar]
- Czerwiecki, L.; Czyżyk, K.; Kwiecińska, A.; Wilczyńska, G. Immunoaffinity columns and determination of ochratoxin A in cereals by HPLC Part I.: Evaluation of extraction using methanol/water. Roczniki Panstwowego Zakladu Higieny 2004, 55, 133–138. [Google Scholar] [PubMed]
- Ravlo, K.; Koefoed-Nielsen, P.; Secher, N.; Søndergaard, P.; Keller, A.K.; Petersen, M.S.; Møldrup, U.; Østraat, E.Ø.; Bibby, B.M.; Jørgensen, T.M.; et al. Effect of remote ischemic conditioning on dendritic cell number in blood after renal transplantation—Flow cytometry in a porcine model. Transpl. Immunol. 2012, 26, 146–150. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Groups | C | M | |
|---|---|---|---|
| Parameter | |||
| Ash (g/kg) | 50.60 | 51.20 | |
| Crude fiber (g/kg) | 45.95 | 43.09 | |
| Starch (g/kg) | 456.90 | 441.50 | |
| Protein (g/kg) | 162.95 | 167.10 | |
| Fat (g/kg) | 41.90 | 43.00 | |
| Moisture content (%) | 11.97 | 12.01 | |
| Parameter | WBC 103/μL | NEUT 103/μL | LYMPH 103/μL | MONO 103/μL | EOS 103/μL | BASO 103/μL | LUC 103/μL | PLT 103/μL | MPV fL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | ||||||||||
| C | Mean | 14.40 | 3.97 | 9.43 | 0.60 | 0.09 | 0.10 | 0.21 | 310.25 | 9.08 |
| SD | 3.52 | 0.51 | 3.40 | 0.14 | 0.05 | 0.04 | 0.14 | 65.06 | 1.51 | |
| M | Mean | 11.65 | 3.04 | 7.82 | 0.42 | 0.09 | 0.07 | 0.22 | 346.75 | 9.00 |
| SD | 1.68 | 0.98 | 0.86 | 0.05 | 0.09 | 0.04 | 0.16 | 96.73 | 1.32 |
| Parameter | RBC 106/μL | HGB g/dL | HCT % | MCV fL | MCH pg | MCHC g/dL | RDW % | HDW g/dL | |
|---|---|---|---|---|---|---|---|---|---|
| Groups | |||||||||
| C | Mean | 6.88 | 11.50 | 36.58 | 53.15 | 16.78 | 31.53 | 16.80 | 1.60 |
| SD | 1.01 | 1.62 | 6 | 1.83 | 1.16 | 1.65 | 1.47 | 0.15 | |
| M | Mean | 6.89 | 11.33 | 36.80 | 53.40 | 16.48 | 30.88 | 15.40 | 1.57 |
| SD | 0.97 | 1.43 | 5.32 | 0.68 | 0.64 | 1.04 | 0.92 | 0.10 |
| INGREDIENTS | Composition of the Complete Diet, Declared by the Manufacturer (%) |
|---|---|
| Soybean meal | 16 |
| Wheat | 35 |
| Barley | 42.3 |
| Wheat bran | 4.0 |
| Limestone | 0.2 |
| Mineral-vitamin premix 1 | 2.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, M.; Jakimiuk, E.; Baranowski, M.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M.T. The Effect of Deoxynivalenol on Selected Populations of Immunocompetent Cells in Porcine Blood—A Preliminary Study. Molecules 2017, 22, 691. https://doi.org/10.3390/molecules22050691
Dąbrowski M, Jakimiuk E, Baranowski M, Gajęcka M, Zielonka Ł, Gajęcki MT. The Effect of Deoxynivalenol on Selected Populations of Immunocompetent Cells in Porcine Blood—A Preliminary Study. Molecules. 2017; 22(5):691. https://doi.org/10.3390/molecules22050691
Chicago/Turabian StyleDąbrowski, Michał, Ewa Jakimiuk, Mirosław Baranowski, Magdalena Gajęcka, Łukasz Zielonka, and Maciej Tadeusz Gajęcki. 2017. "The Effect of Deoxynivalenol on Selected Populations of Immunocompetent Cells in Porcine Blood—A Preliminary Study" Molecules 22, no. 5: 691. https://doi.org/10.3390/molecules22050691





