Electrochemical Enhancement of Photocatalytic Disinfection on Aligned TiO2 and Nitrogen Doped TiO2 Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
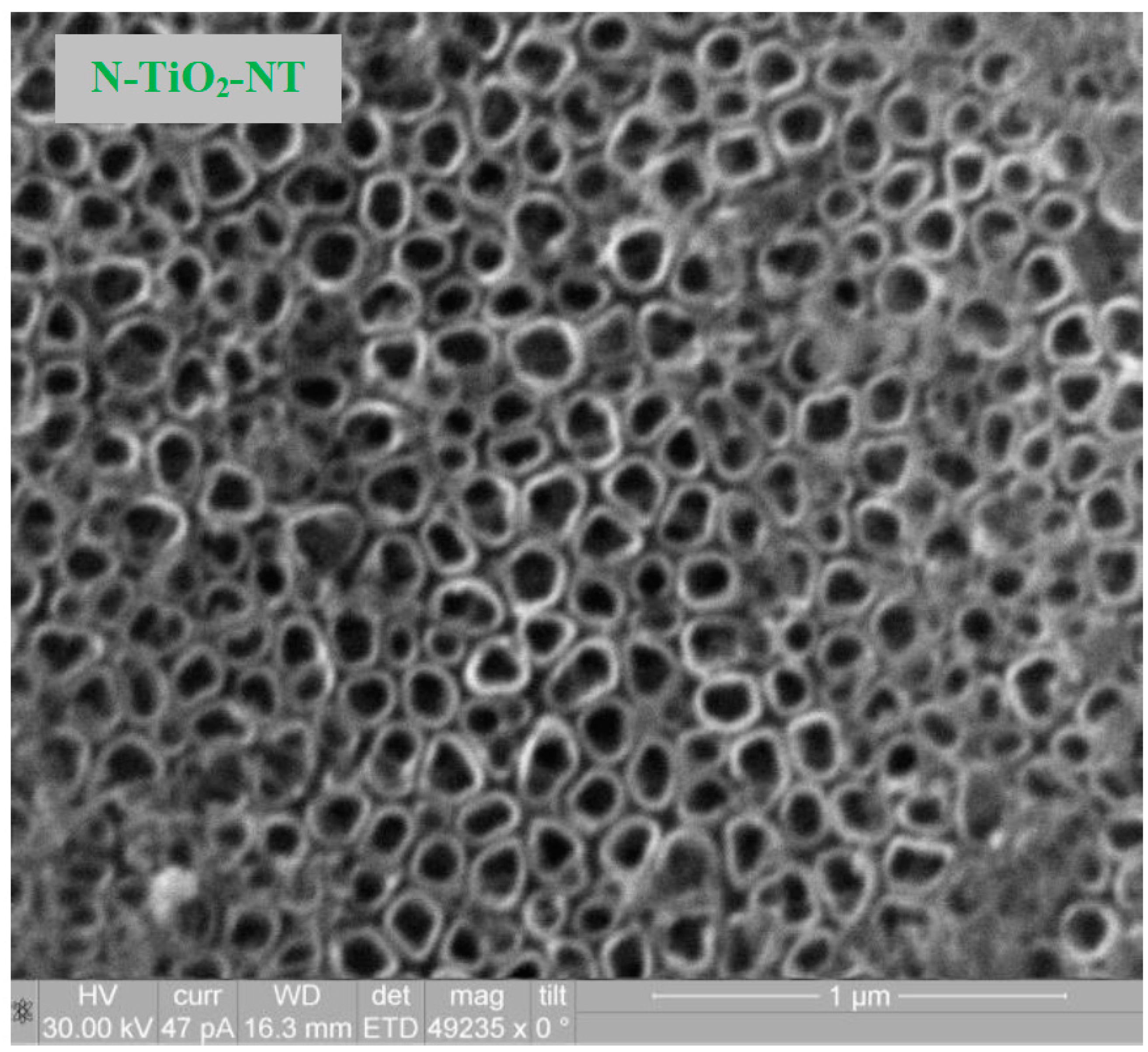
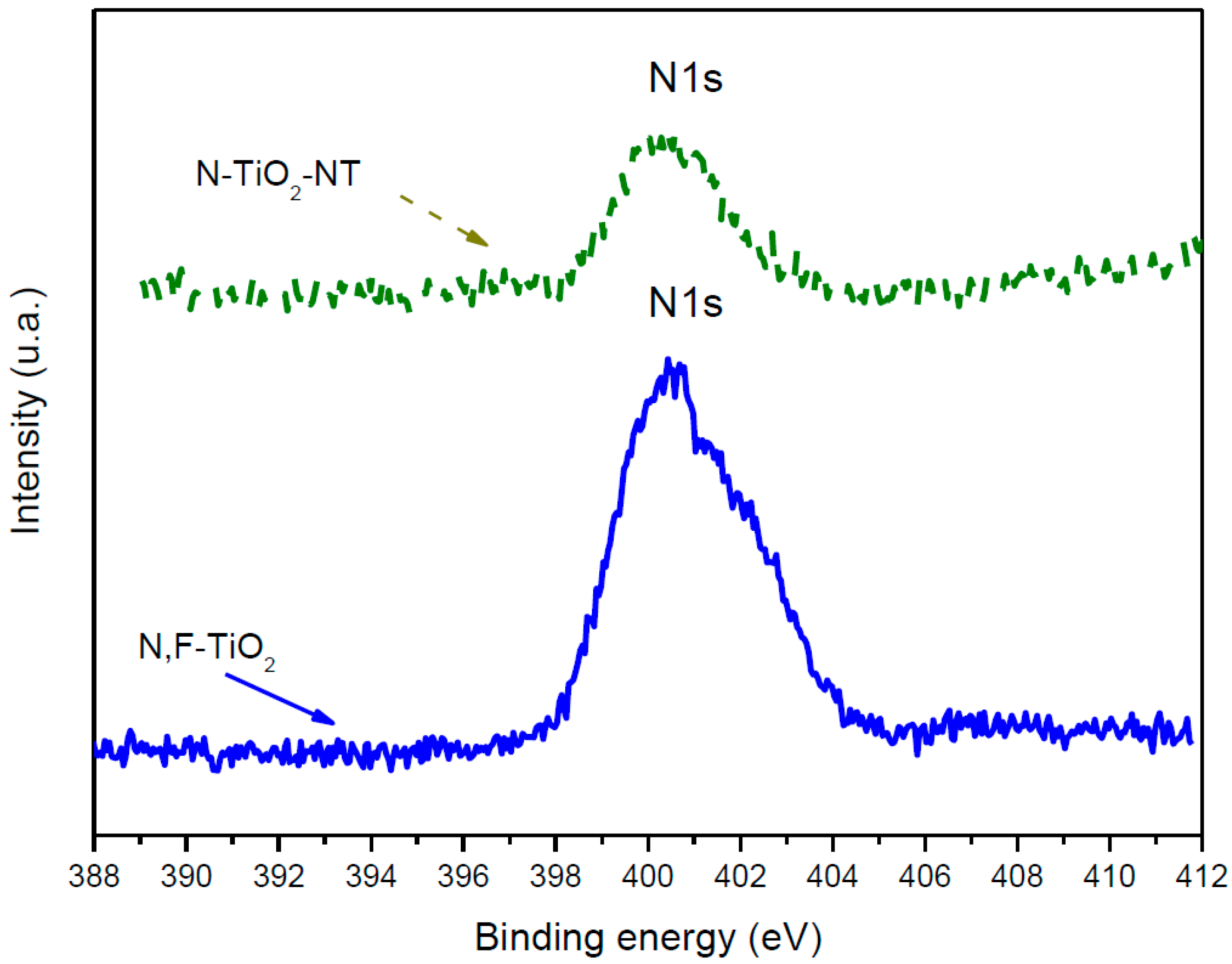
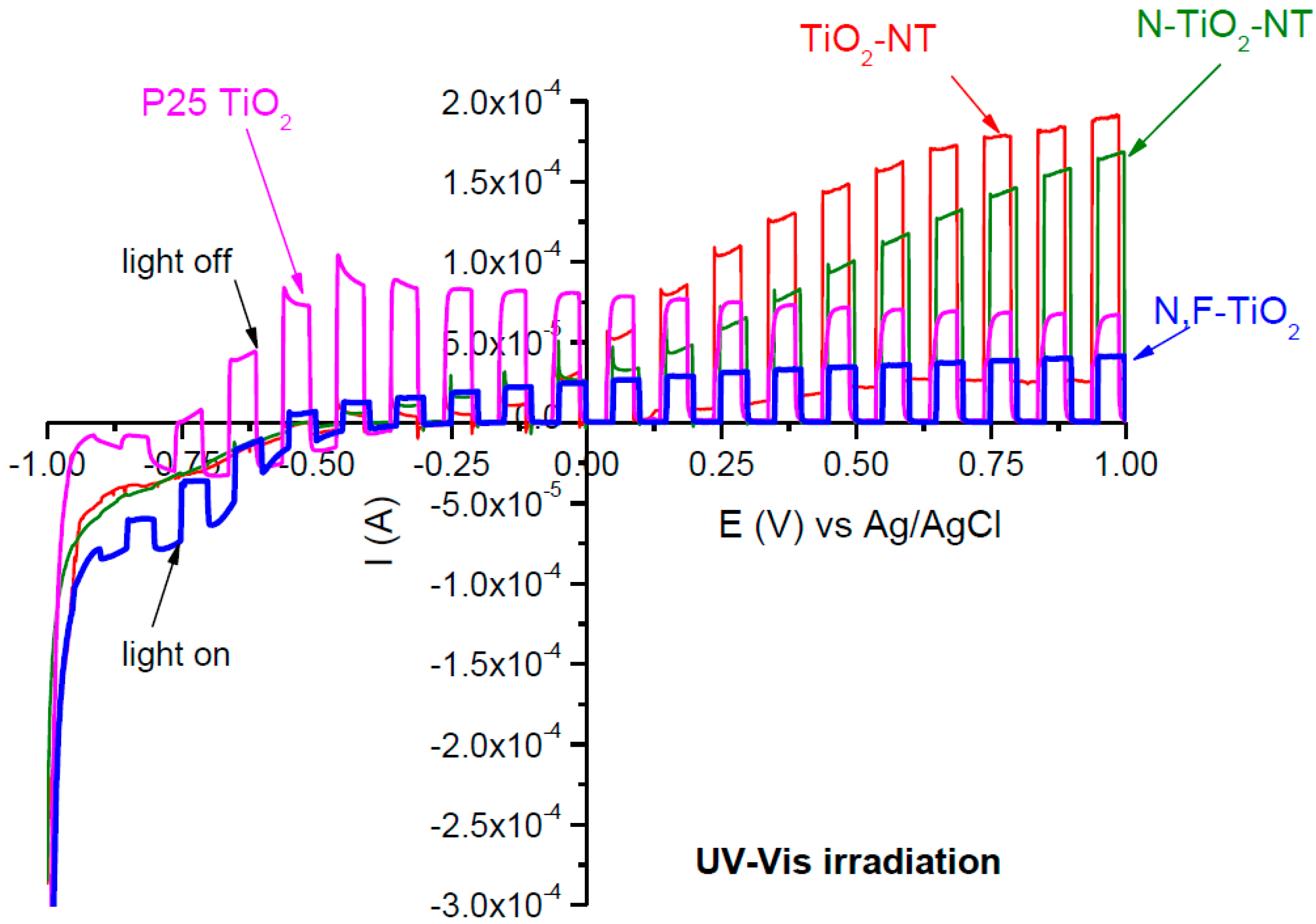
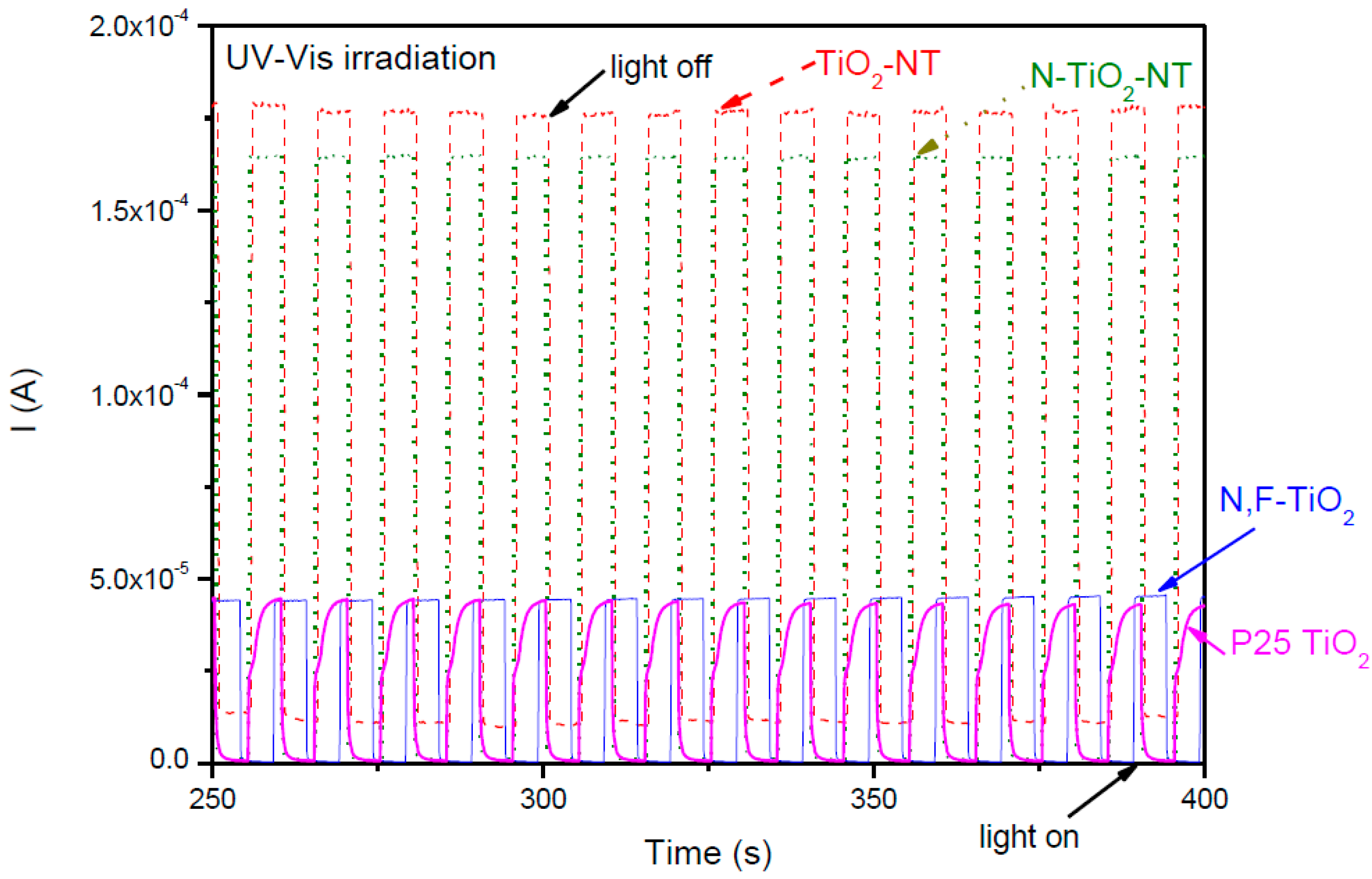
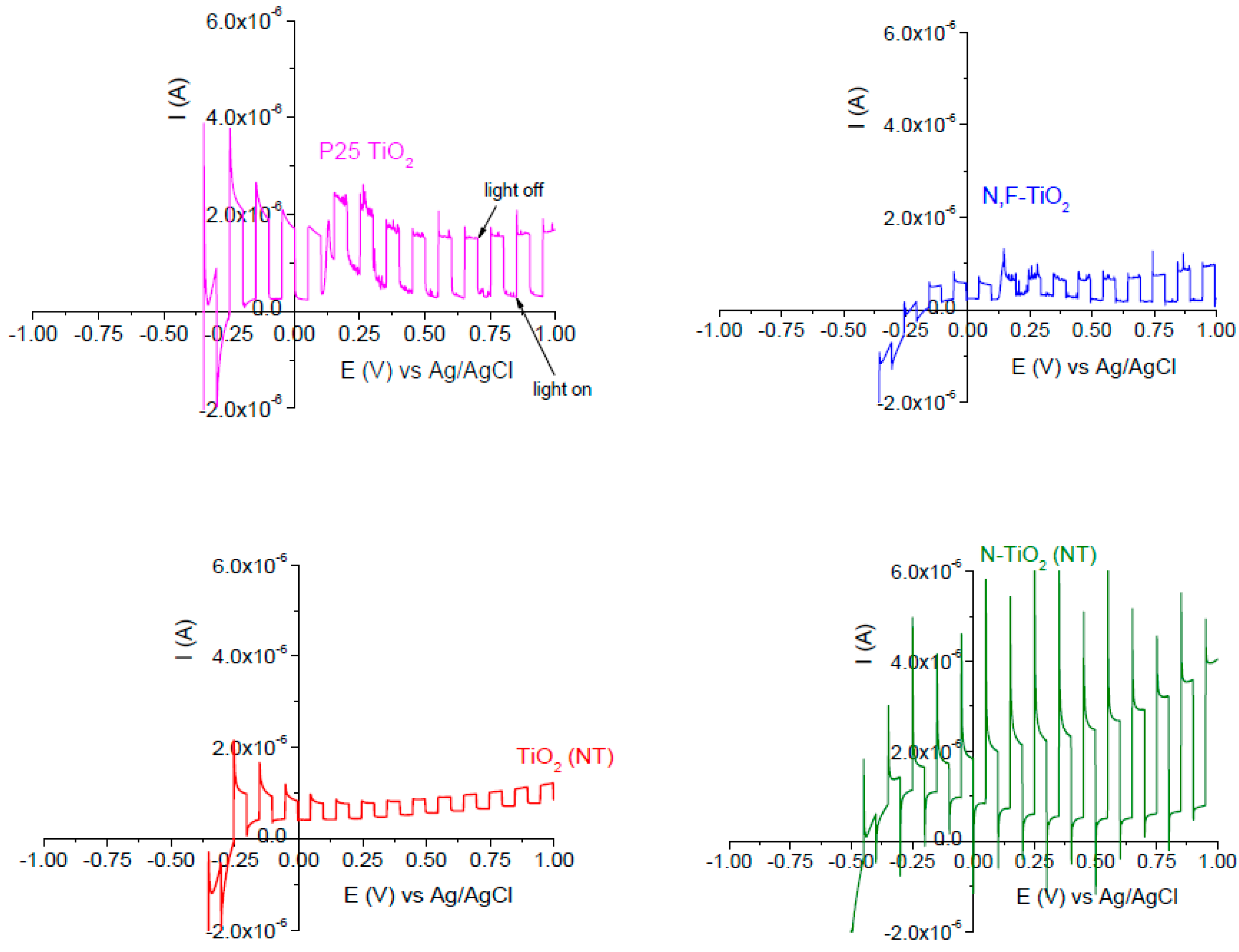
2.1. Electrode Characterization
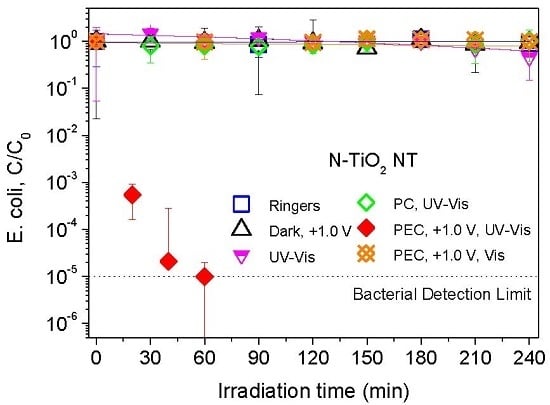
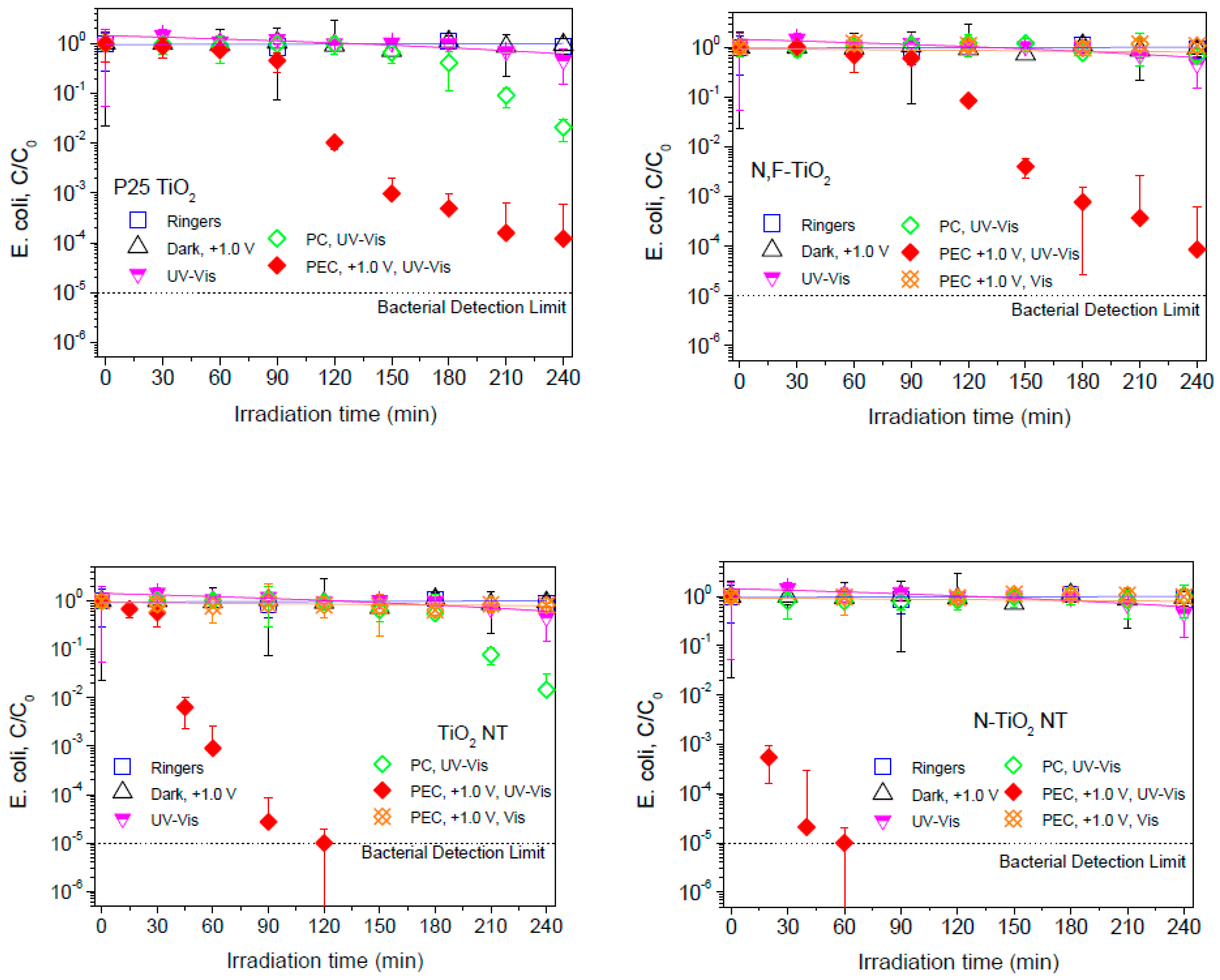
2.2. Electrochemically Assisted Photocatalytic Disinfection
3. Experimental Section
3.1. TiO2 Electrode Preparation
3.2. Materials Characterisation
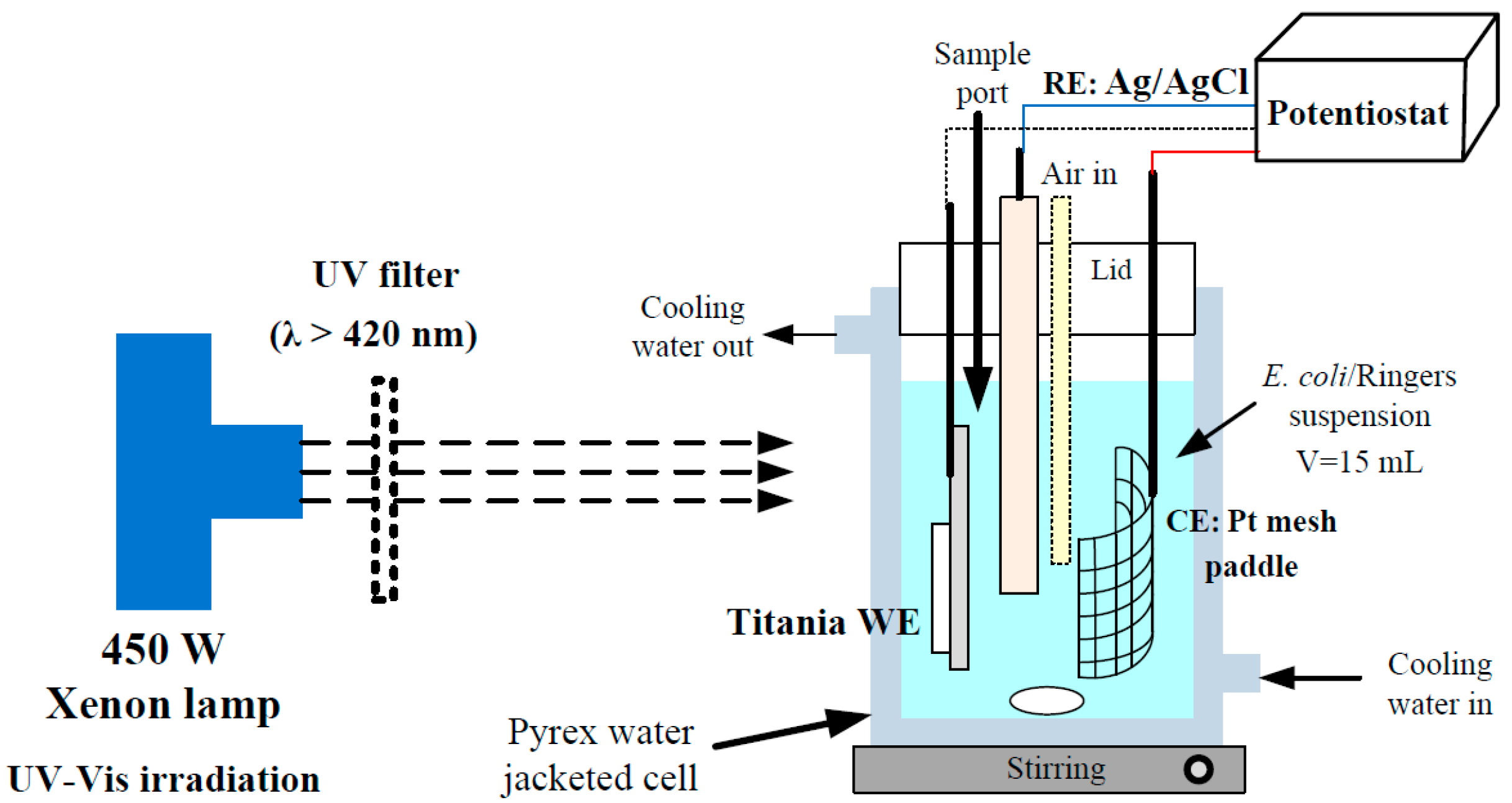
3.3. Photoelectrochemical Analysis
3.4. Photoreactor Configuration
3.5. Bacterial Growth and Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richardson, S.D. Disinfection By-Products and other emerging contaminants in drinking water. Trends Anal. Chem. 2003, 20, 666–684. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–60. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Vinodgopal, K.; Stafford, U.; Gray, K.A.; Kamat, P.V. Electrochemically Assisted Photolysis. II. The Role of Oxygen and Reaction Intermediates in the Degradation of 4-Chlorophenol on Immobilized TiO2 Particulate Films. J. Phys. Chem. 1994, 98, 6797–6803. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Byrne, J.A.; Manga, N.; Eggins, B.R. The photocatalytic removal of bacterial pollutants from drinking water. J. Photochem. Photobiol. A Chem. 2002, 148, 355–563. [Google Scholar] [CrossRef]
- Christensen, P.A.; Curtis, T.P.; Egerton, T.A.; Kosa, S.A.M.; Tinlin, J.R. Photoelectrocatalytic and photocatalytic disinfection of E. coli suspensions by titanium dioxide. Appl. Catal. B Environ. 2003, 41, 371–386. [Google Scholar] [CrossRef]
- Butterfield, I.M.; Christensen, P.A.; Curtis, T.P.; Gunlazuardi, J. Water disinfection using an immobilised titanium dioxide film in a photochemical reactor with electric field enhancement. Water Res. 1997, 31, 675–677. [Google Scholar] [CrossRef]
- Baram, N.; Starosvetsky, D.; Starosvetsky, J.; Epshtein, M.; Armon, R.; Ein-Eli, Y. Enhanced inactivation of E. coli bacteria using immobilized porous TiO2 photoelectrocatalysis. Electrochim. Acta 2009, 54, 3381–3386. [Google Scholar] [CrossRef]
- Cho, M.; Cates, E.L.; Kim, J.H. Inactivation and surface interactions of MS-2 bacteriophage in a TiO2 photoelectrocatalytic reactor. Water Res. 2011, 45, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Zlamal, M.; Macak, J.M.; Schmuki, P.; Krýsa, J. Electrochemically assisted photocatalysis on self-organized TiO2 nanotubes. Electrochem. Commun. 2007, 9, 2822–2826. [Google Scholar]
- Yuan, B.; Wang, Y.; Bian, H.; Shen, T.; Wu, Y.; Chen, Z. Nitrogen doped TiO2 nanotube arrays with high photoelectrochemical activity for photocatalytic applications. Appl. Surf. Sci. 2013, 280, 523–529. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Dale, G.R.; Hamilton, J.W.J.; Dunlop, P.S.M.; Byrne, J.A. Electrochemically assisted photocatalysis on anodic titania nanotubes. Curr. Top. Electrochem. 2009, 14, 89–97. [Google Scholar]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Kang, Q.; Lu, Q.Z.; Liu, S.H.; Yang, L.X.; Wen, L.F.; Luo, S.L.; Cai, Q.Y. A ternary hybrid CdS/Pt–TiO2 nanotube structure for photoelectrocatalytic bactericidal effects on Escherichia coli. Biomaterials 2010, 31, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.C.; Allam, N.K.; El-Sayed, M.A. TiO2 nanotube/CdS hybrid electrodes: Extraordinary enhancement in the inactivation of Escherichia coli. J. Am. Chem. Soc. 2010, 132, 14406–14408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-J.; Chen, M.-L.; Oh, W.-C. Photoelectrocatalytic properties and bactericidal activities of silver-treated carbon nanotube/titania composites. Compos. Sci. Technol. 2011, 71, 658–665. [Google Scholar]
- Brugnera, M.F.; Miyata, M.; Zocolo, G.J.; Queico Fujimura Leite, C.; Valnice Boldrin Zanoni, M. Inactivation and disposal of By-Products from Mycobacterium smegmatis by photoelectrocatalytic oxidation using Ti/TiO2-Ag nanotube electrodes. Electrochim. Acta 2012, 85, 33–41. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Qiu, X.; Burda, C. Bactericidal activity of nitrogen-doped metal oxide nanocatalysts and the influence of bacterial Extracellular Polymeric Substances (EPS). J. Photochem. Photobiol. A Chem. 2007, 190, 94–100. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Subramaniam, V.P.; Gong, D.; Tang, Y.; Highfield, J.; Pehkonen, S.O.; Pichat, P.; Schreyer, M.K.; Chen, Z. Nitrogen-sensitized dual phase titanate/titania for visible-light driven phenol degradation. J. Solid State Chem. 2012, 196, 518–527. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Is the band gap of pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Lin, Y.-S.; Lin, S.-W. Mechanisms of visible light photocatalysis in N-doped anatase TiO2 with oxygen vacancies from GGA + U calculations. Int. J. Photoenergy 2013, 2013, 1–7. [Google Scholar]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for Visible Light Responses in Anodic Photocurrents at N-doped TiO2 Film Electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Emeline, A.V.; Kuznetsov, V.N.; Rybchuk, V.K.; Serpone, N. The case of N-doped TiO2s—Properties and some fundamental issues. Int. J. Photoenergy 2008, 2008, 1–19. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.M.; Seery, K.; Hinder, S.J.; Pillai, S.C. Highly visible light active TiO2-xNx heterojunction photocatalysts. Chem. Mater. 2010, 22, 3843–3853. [Google Scholar] [CrossRef]
- Tafalla, D.; Salvador, P.; Benito, R.M. Kinetic Approach to the Photocurrent Transients in Water Photoelectrolysis at n-TiO2 Electrodes II. Analysis of the Photocurrent-Time Dependence. J. Electrochem. Soc. 1990, 137, 1810–1815. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Walck, S.D.; Yates, J.T. Photochemical activity of nitrogen-doped rutile TiO2(110) in visible light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Rumaiz, A.K.; Woicik, J.C.; Cockayne, E.; Lin, H.Y.; Jaffari, G.H.; Shah, S.I. Oxygen vacancies in N doped anatase TiO2: Experiment and first-principles calculations. Appl. Phys. Lett. 2009, 95, 1–3. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhang, J.; Zhang, Q.; Liu, Z. An N-Doped anatase/rutile TiO2 hybrid from low-temperature direct nitridization: Enhanced photoactivity under UV-/Visible-light. RSC Adv. 2014, 4, 420–427. [Google Scholar] [CrossRef]
- Yang, G.; Wang, T.; Yang, B.; Yan, Z.; Ding, S.; Xiao, T. Enhanced visible-light activity of F-N Co-doped TiO2 nanocrystals via nonmetal impurity, Ti3+ ions and oxygen vacancies. Appl. Surf. Sci. 2013, 287, 135–142. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light Activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Czoska, A.M.; Paganini, M.C.; Giamello, E. Density Functional Theory and Electron Paramagnetic Resonance Study on the Effect of N-F Codoping of TiO2. Chem. Mater. 2008, 20, 3706–3714. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chang, H.-H. Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Falaras, P.; Likodimos, V.; Kontos, A.G.; de la Cruz, A.A.; O’shea, K.; Dionysiou, D. Synthesis, structural characterization and evaluation of sol-gel-based NF-TiO2 films with visible light-photoactivation for the removal of microcystin-LR. Appl. Catal. B Environ. 2010, 99, 378–387. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energ. Mat. Sol. C. 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D. Macro, micro and nanostructure of TiO2 anodised films prepared in a fluorine-containing electrolyte. J. Mater. Sci. 2007, 42, 6729–6734. [Google Scholar] [CrossRef]
- Macak, J.M.; Ghicov, A.; Hahn, R.; Tsuchiya, H.; Schmuki, P. Photoelectrochemical properties of N-doped self-organized titania nanotube layers with different thicknesses. J. Mater. Res. 2006, 21, 2824–2828. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Wang, Y.; Quan, X.; Chen, G. Evaluation of bias potential enhanced photocatalytic degradation of 4-chlorophenol with TiO2 nanotube fabricated by anodic oxidation method. Chem. Eng. J. 2009, 146, 30–35. [Google Scholar] [CrossRef]
- Dale, G.R. Electrochemical Growth and Characterization of Self-Organized Titania Nanotubes. Ph.D. Thesis, Ulster University, Newtownabbey, UK, 2009. [Google Scholar]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; McKinney, B.; Rouse, M. Immobilization of titanium dioxide for the treatment of polluted water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C. TiO2 Nanotube Arrays of 1000 μm Length by Anodization of Titanium Foil: Phenol Red Diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Delegan, N.; Daghrir, R.; Drogui, P.; El Khakani, M.A. Bandgap tailoring of in-situ nitrogen doped TiO2 sputtered films intended for electrophotocatalytic applications under solar light. J. Appl. Phys. 2014, 116, 153510. [Google Scholar] [CrossRef]
- Huang, L.H.; Sun, C.; Liu, Y.L. Pt/N-codoped TiO2 nanotubes and its photocatalytic activity under visible light. Appl. Surf. Sci. 2007, 253, 7029–7035. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Jirsak, T.; Liu, G.; Hrbek, J.; Dvorak, J.; Maiti, A. Chemistry of NO2 on oxide surfaces: formation of NO3 on TiO2(110) and NO2↔O vacancy interactions. J. Am. Chem. Soc. 2001, 123, 9597–9605. [Google Scholar] [CrossRef] [PubMed]
- Navio, J.A.; Cerrillos, C. Photo-induced transformation, upon UV illumination in air, of hyponitrite species N2O22- preadsorbed on TiO2 surface. Real Surf. Interface Anal. 1996, 24, 355–359. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Meyer, G.J.; Searson, P.C. Electron Transport in Porous Nanocrystalline TiO2 Photoelectrochemical Cells. J. Phys. Chem. 1996, 100, 17021–17027. [Google Scholar] [CrossRef]
- Waldner, G.; Krýsa, J.; Jirkovský, J.; Grabner, G. Photoelectrochemical properties of sol-gel and particulate TiO2 layers. Int. J. Photoenergy 2003, 5, 115–122. [Google Scholar] [CrossRef]
- Marugán, J.; Christensen, P.; Egerton, T.; Purnama, H. Synthesis, characterization and activity of photocatalytic sol–gel TiO2 powders and electrodes. Appl. Catal. B Environ. 2009, 89, 273–283. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Electrochemically assisted photocatalysis using nanocrystalline semiconductor thin films. Sol. Energ. Mat. Sol. C 1995, 38, 401–410. [Google Scholar] [CrossRef]
- Byrne, J.A.; Davidson, A.; Dunlop, P.S.M.; Eggins, B.R. Water treatment using nano-crystalline TiO2 electrodes. J. Photochem. Photobiol. A Chem. 2002, 148, 365–374. [Google Scholar] [CrossRef]
- Xie, Y. Photoelectrochemical application of nanotubular titania photoanode. Electrochim. Acta 2006, 51, 3399–3406. [Google Scholar] [CrossRef]
- Liang, H.-C.; Li, X.-Z. Effects of structure of anodic TiO2 nanotube arrays on photocatalytic activity for the degradation of 2,3-dichlorophenol in aqueous solution. J. Hazard. Mater. 2009, 162, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Pang, S.; Lin, Y.; Jiang, T.; Xie, T. A study on photo-generated charges property in highly ordered TiO2 nanotube arrays. Appl. Surf. Sci. 2010, 256, 7217–7221. [Google Scholar] [CrossRef]
- Hamilton, J.W.J.; Byrne, J.A.; Dunlop, P.S.M.; Dionysiou, D.D.; Pelaez, M.; O’Shea, K.; Synnott, D.; Pillai, S.C. Evaluating the Mechanism of Visible Light Activity for N,F-TiO2 Using Photoelectrochemistry. J. Phys. Chem. C 2014, 118, 12206–12215. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Pulgarin, C. Photocatalytic activity of N, S co-doped and N-doped commercial anatase TiO2 powders towards phenol oxidation and E. coli inactivation under simulated solar light irradiation. Sol. Energy 2010, 84, 37–43. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R. Photoelectrochemistry of oxalate on particulate TiO2 electrodes. J. Electroanal. Chem. 1998, 457, 61–72. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Dunlop, P.S.M.; McMurray, T.A.; Byrne, J.A. Photocatalytic inactivation of E. coli in surface water using immobilized nanoparticle TiO2 films. Water Res. 2009, 43, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, P.S.M.; McMurray, T.A.; Hamilton, J.W.J.; Byrne, J.A. Photocatalytic inactivation of Clostridium perfringens spores on TiO2 electrodes. J. Photochem. Photobiol. A Chem. 2008, 196, 113–119. [Google Scholar] [CrossRef]
- Quesada-Cabrera, R.; Sotelo-Vazquez, C.; Darr, J.A.; Parkin, I.P. Critical influence of surface nitrogen species on the activity of N-doped TiO2 thin-films during photodegradation of stearic acid under UV light irradiation. Appl. Catal. B Environ. 2014, 160–161, 582–588. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds TiO2-NT, N-TiO2-NT, N,F-TiO2, and P25 TiO2 are not available. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pablos, C.; Marugán, J.; Van Grieken, R.; Dunlop, P.S.M.; Hamilton, J.W.J.; Dionysiou, D.D.; Byrne, J.A. Electrochemical Enhancement of Photocatalytic Disinfection on Aligned TiO2 and Nitrogen Doped TiO2 Nanotubes. Molecules 2017, 22, 704. https://doi.org/10.3390/molecules22050704
Pablos C, Marugán J, Van Grieken R, Dunlop PSM, Hamilton JWJ, Dionysiou DD, Byrne JA. Electrochemical Enhancement of Photocatalytic Disinfection on Aligned TiO2 and Nitrogen Doped TiO2 Nanotubes. Molecules. 2017; 22(5):704. https://doi.org/10.3390/molecules22050704
Chicago/Turabian StylePablos, Cristina, Javier Marugán, Rafael Van Grieken, Patrick Stuart Morris Dunlop, Jeremy William John Hamilton, Dionysios D. Dionysiou, and John Anthony Byrne. 2017. "Electrochemical Enhancement of Photocatalytic Disinfection on Aligned TiO2 and Nitrogen Doped TiO2 Nanotubes" Molecules 22, no. 5: 704. https://doi.org/10.3390/molecules22050704







_Dionysiou.jpg)