Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities
Abstract
:1. Introduction
2. Results and Discussion
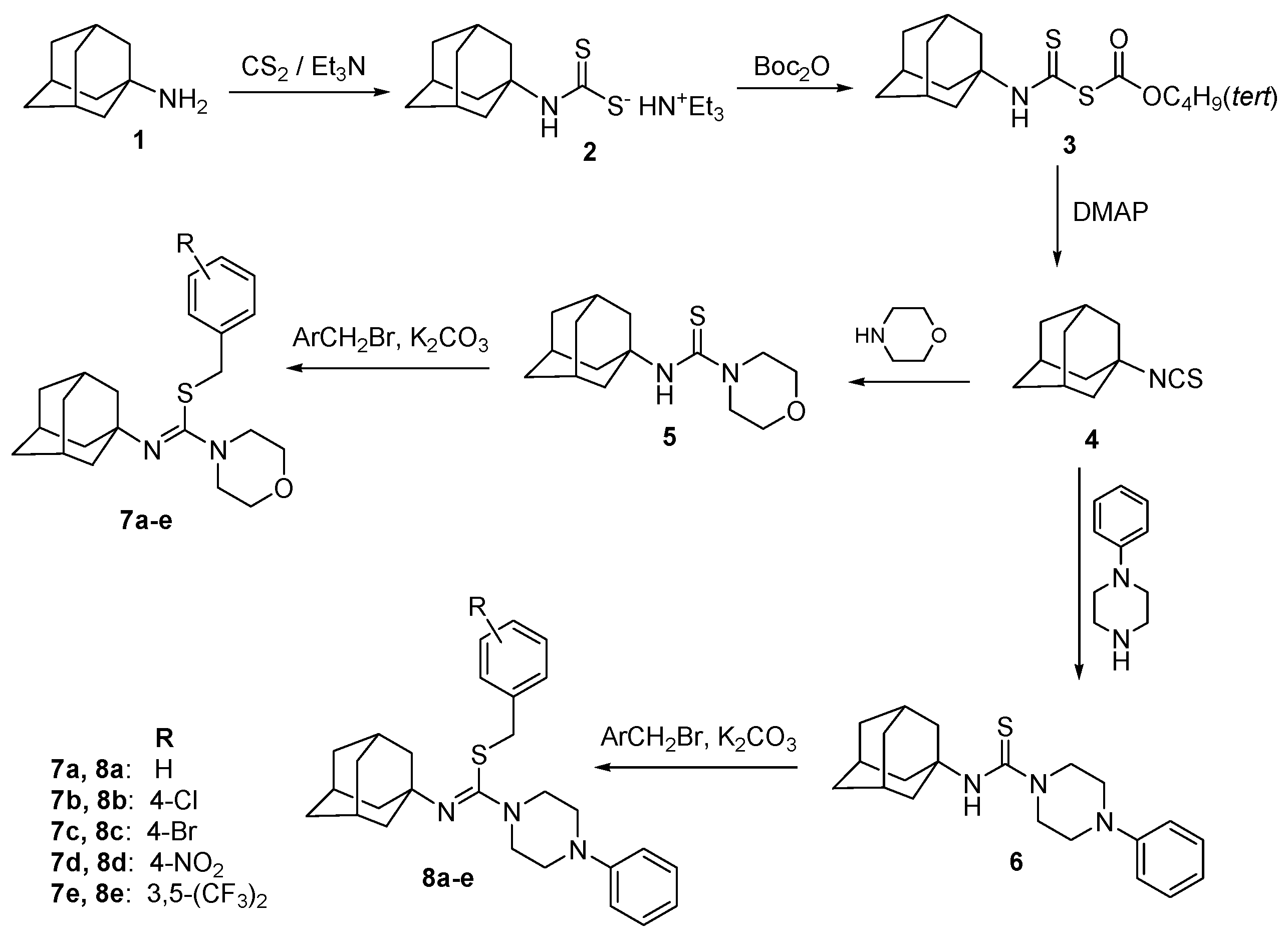
2.1. Chemical Synthesis
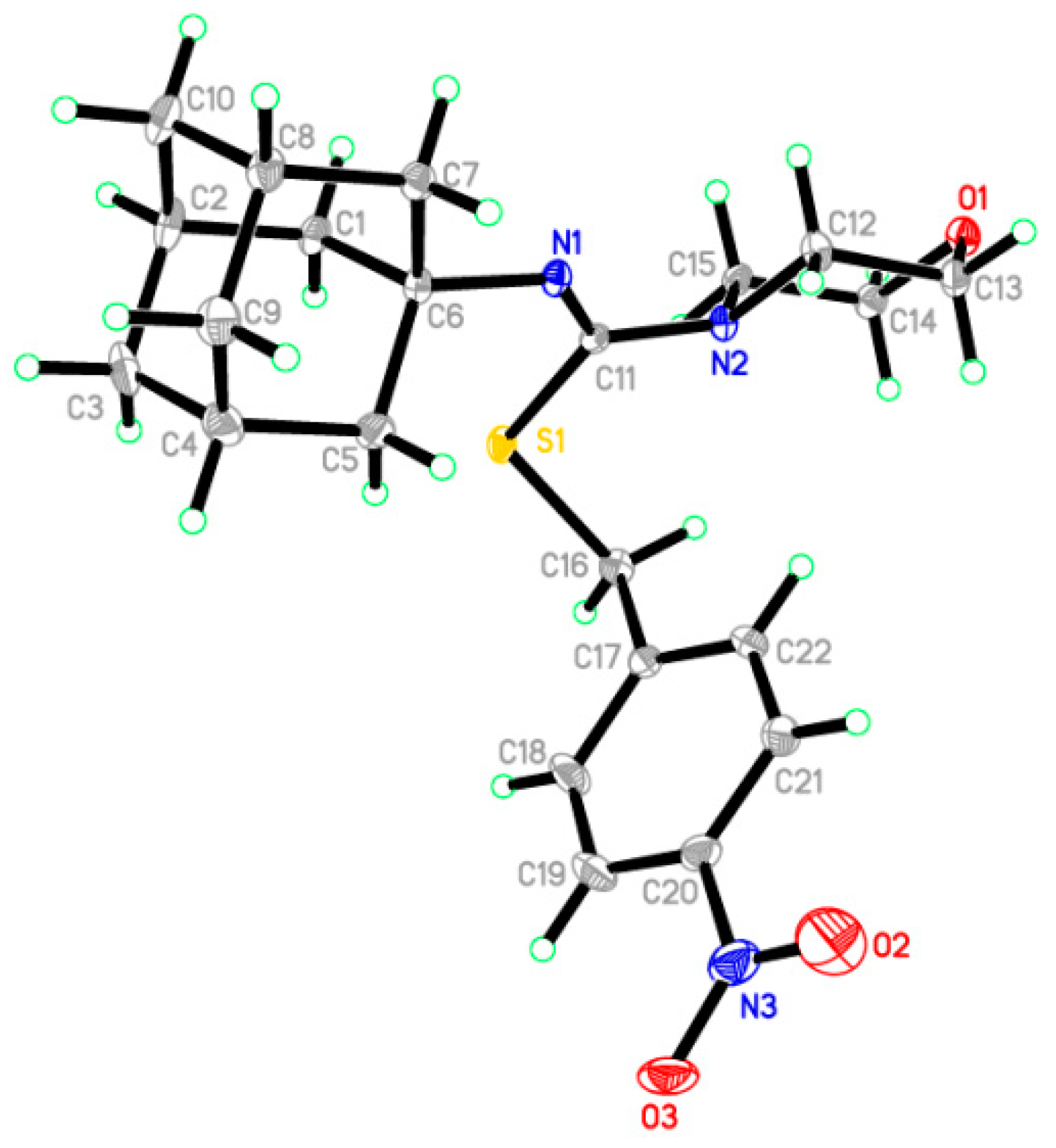
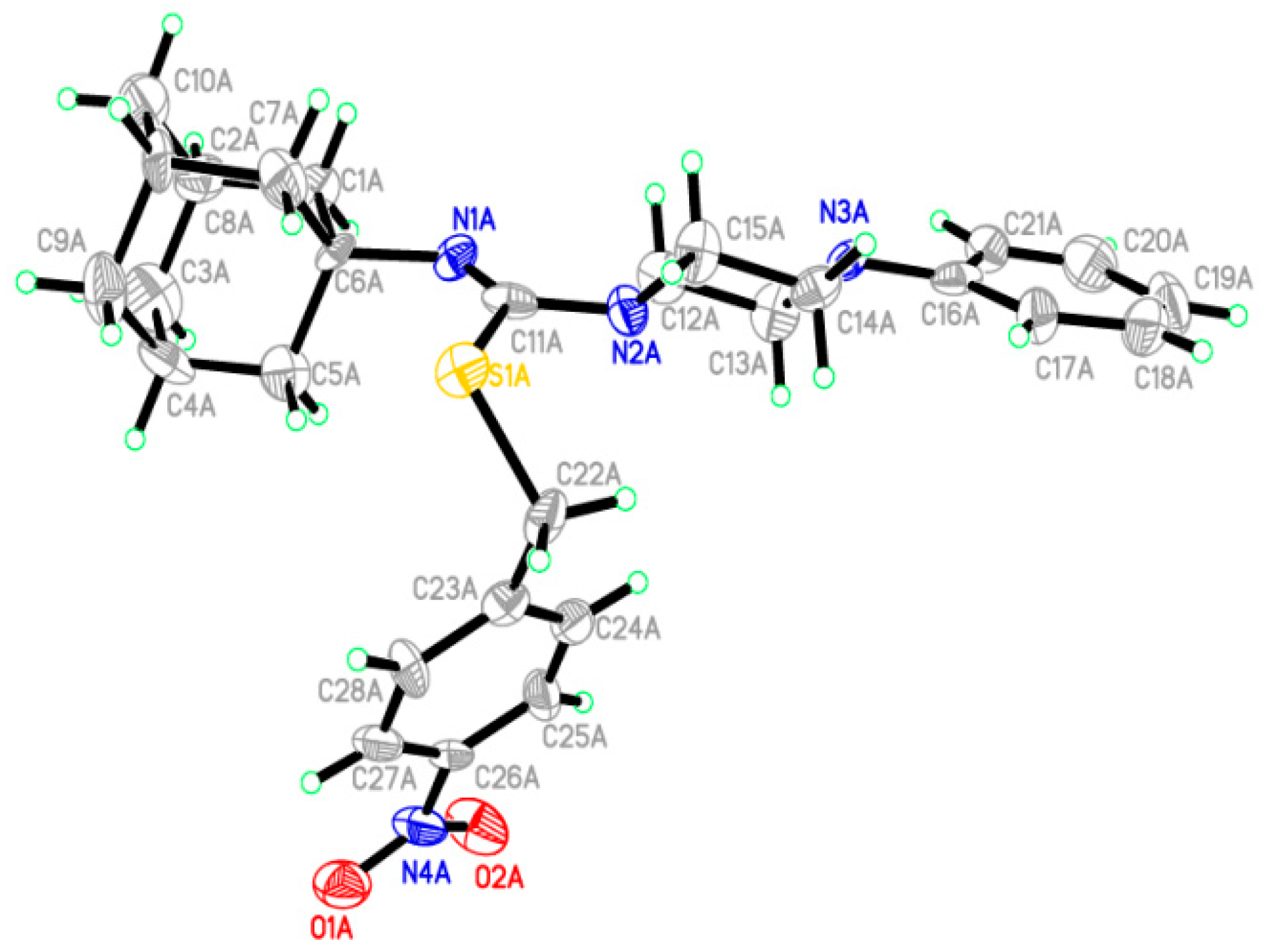
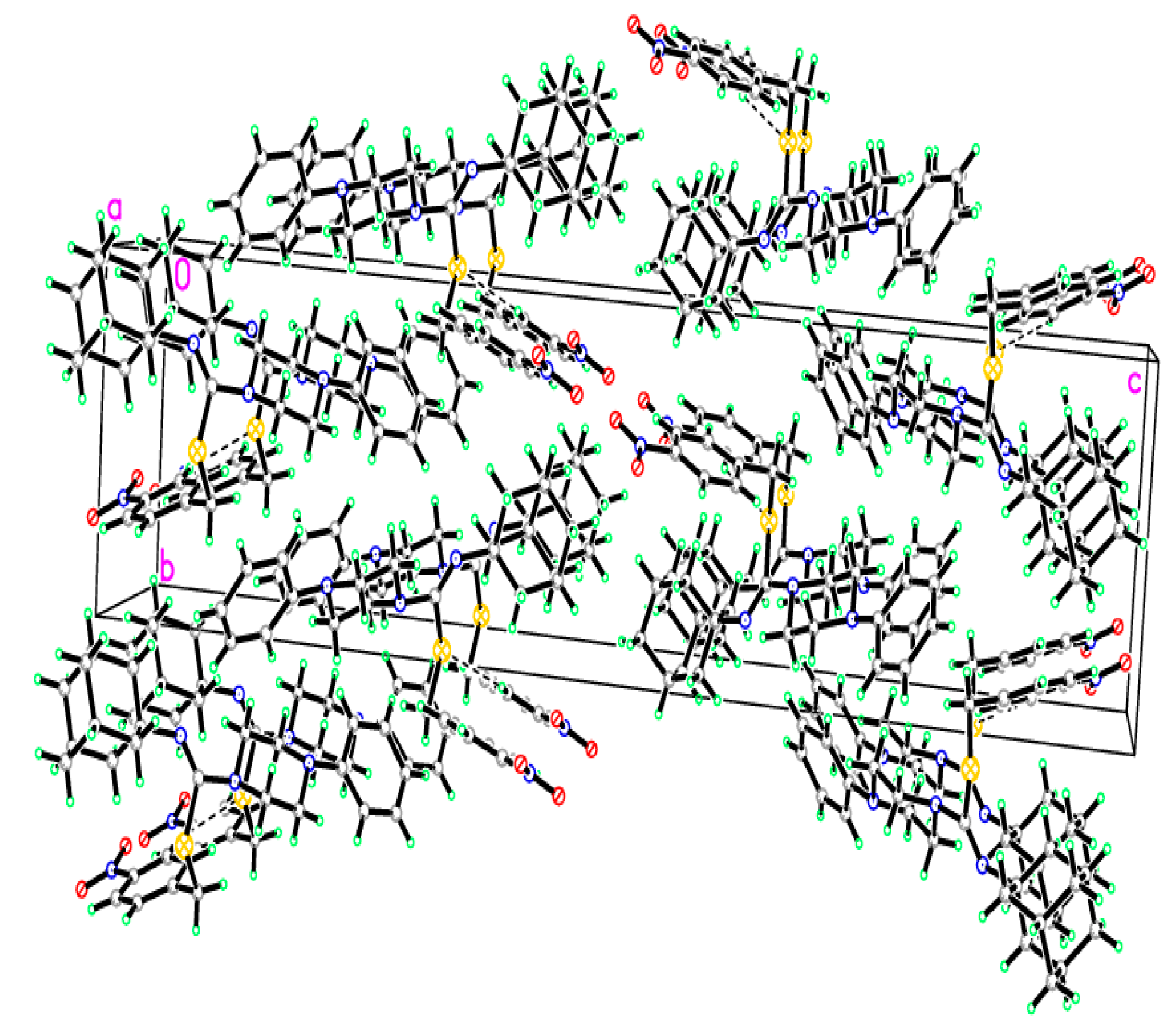
2.2. Crystallographic Studies
2.3. In Vitro Antimicrobial Activity
2.4. In Vivo Hypoglycemic Activity
3. Materials and Methods
3.1. General
3.2. Synthesis of 4-Arylmethyl (Z)-N′-(Adamantan-1-yl)-Morpholine-4-Carbothioimidates 7a–e and 4-Arylmethyl (Z)-N′-(Adamantan-1-yl)-4-Phenylpiperazine-1-Carbothioimidates 8a–e
3.3. Crystal Growth and Single Crystal X-ray Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, G.; Artavia, G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.L.; Grunnert, R.R.; Haff, R.F.; McGahen, J.W.; Neumeyer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantamine (amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Togo, Y.; Hornick, R.B.; Dawkins, A.T. Studies on induced influenza in man. I. Double blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against A2/Rockville/1/65 strain. J. Am. Med. Assoc. 1968, 203, 1089–1094. [Google Scholar] [CrossRef]
- Wendel, H.A.; Snyder, M.T.; Pell, S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Ther. 1966, 7, 38–43. [Google Scholar] [CrossRef]
- Schwab, R.S.; England, A.C.; Poskanzer, D.C.; Young, R.R. Amantadine in the treatment of Parkinson’s disease. J. Am. Med. Assoc. 1969, 208, 1168–1170. [Google Scholar] [CrossRef]
- Rabinovich, S.; Baldini, J.T.; Bannister, R. Treatment of influenza. The therapeutic efficacy of rimantadine HCl in a naturally occurring influenza A2 outbreak. Am. J. Med. Sci. 1969, 257, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S.; Sokol, M.S.; Ingram, R.L.; Subramanian, R.; Fort, R.C. Tromantadine: Inhibitor of early and late events in herpes simplex virus replication. Antimicrob. Agents Chemother. 1982, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Burstein, M.E.; Serbin, A.V.; Khakhulina, T.V.; Alymova, I.V.; Stotskaya, L.L.; Bogdan, O.P.; Manukchina, E.E.; Jdanov, V.V.; Sharova, N.K. Inhibition of HIV-1 replication by newly developed adamantane-containing polyanionic agents. Antiviral Res. 1999, 41, 135–144. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.A.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Orzeszko, B.; Mauri, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Orzeszko-Krzesińska, B.; Maurin, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2- and 3-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2009, 44, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Yue, P.; Chen, X.; Hong, W.K.; Lotan, R. The synthetic retinoid CD437 selectively induces apoptosis in human lung cancer cells while sparing normal human lung epithelial cells. Cancer Res. 2002, 62, 2430–2436. [Google Scholar] [PubMed]
- Jia, L.; Tomaszewski, J.E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 2005, 144, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Protopopova, M.; Hanrahan, C.; Nikonenko, B.; Samala, R.; Chen, P.; Gearhart, J.; Einck, L.; Nacy, C.A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005, 56, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Villhauer, E.B.; Brinkman, J.A.; Naderi, G.B.; Burkey, B.F.; Dunning, B.E.; Prasad, K.; Mangold, B.L.; Russell, M.E.; Hughes, T.E. 1-(3-Hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 2003, 46, 2774–2789. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and preclinical profile of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 5025–5037. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Katsiki, N.; Adamidou, F.; Athyros, V.G.; Karagiann, A.; Kita, M.; Mikhailidis, D.P. 11-Beta-Hydroxysteroid dehydrogenase type 1 inhibitors: Novel agents for the treatment of metabolic syndrome and obesity-related disorders. Metabolism 2013, 62, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.; Aster, S.D.; Brown, K.; Carbin, L.; Graham, D.W.; Hermanowski-Vosatka, A.; LeGrand, C.B.; Mundt, S.S.; Robbins, M.A.; Schaeffer, J.M.; et al. Adamantyl triazoles as selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1. Bioorg. Med. Chem. Lett. 2005, 15, 4359–4362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hoffman, J.; Le, P.; Nair, S.K.; Cripps, S.; Matthews, J.; Smith, C.; Yang, M.; Kupchinsky, S.; Dress, K.; et al. The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Cott, J.S.; Choormun, J. 11β-Hydroxysteroid dehydrogenase Type 1 (11β-HSD-1) inhibitors in development. In New Therapeutic Strategies for Type-2 Diabetes, Small Molecule Approaches; Jones, R.N., Ed.; RSC: Cambridge, UK, 2012; pp. 109–133. [Google Scholar]
- Thoma, G.; Streiff, M.B.; Kovarik, J.; Glickman, F.; Wagner, T.; Beerli, C.; Zerwes, H. Orally bioavailable isothioureas block function of the chemokine receptor CXCR4 in vitro and in vivo. J. Med. Chem. 2008, 51, 7915–7920. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Boyce, C.W.; Ruiz, M.L. Histamine H3 receptor as a drug discovery target. J. Med. Chem. 2011, 54, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Fu, Q.; Shen, Y.; Hu, W.; Zhang, Z.; Timmerman, H.; Leurs, R.; Chen, Z. The histamine H3 receptor antagonist clobenpropit enhances GABA release to protect against NMDA-induced excitotoxicity through the cAMP/protein kinase A pathway in cultured cortical neurons. Eur. J. Pharmacol. 2007, 563, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Bose, D.D.; Feng, W.; Padilla, I.; Pessah, I.N. The Na+/Ca2+ exchange inhibitor 2-(2-(4-(4-nitrobenzyloxy)phenyl)ethyl)isothiourea methane-sulfonate (KBR7943) also blocks ryanodine receptors type 1 (RyR1) and type 2 (RyR2) Channels. Mol. Pharmacol. 2009, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, M.; Romiszewska, A.; Chilmonczyk, Z.; Kazimierczuk, Z. New benzimidazole-derived isothioureas as potential antileukemic agents—Studies in vitro. Med. Chem. 2015, 11, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Perry, J.D.; James, A.L.; Stanforth, S.P.; Carnell, S.; Wilkinson, K.; Anjam Khan, C.M.; De Soyza, A.; Gould, F.K. In vitro activity of S-(3,4-dichlorobenzyl)isothiourea hydrochloride and novel structurally related compounds against multidrug-resistant bacteria, including Pseudomonas aeruginosa and Burkholderia cepacia complex. Int. J. Antimicrob. Agents 2012, 39, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Shearer, B.G.; Lee, S.; Oplinger, J.A.; Frick, L.W.; Garvey, E.P.; Furfine, E.S. Substituted N-phenylisothioureas: Potent inhibitors of human nitric oxide synthase with neuronal isoform selectivity. J. Med. Chem. 1997, 40, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Castaño, T.; Encinas, A.; Pérez, C.; Castro, A.; Campillo, N.E.; Gil, C. Design, synthesis, and evaluation of potential inhibitors of nitric oxide synthase. Bioorg. Med. Chem. 2008, 16, 6193–6206. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Tamimi, A.-M.S.; Al-Omar, M.A.; Alrashood, K.A.; Habib, E.E. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug Des. Dev. Ther. 2014, 8, 505–518. [Google Scholar]
- Al-Abdullah, E.S.; Al-Tuwaijri, H.M.; Hassan, H.M.; Haiba, M.E.; Habib, E.E.; El-Emam, A.A. Antimicrobial and hypoglycemic activities of novel N-Mannich bases derived from 5-(1-adamantyl)-4-substituted-1,2,4- triazoline-3-thiones. Int. J. Mol. Sci. 2014, 15, 22995–23010. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S.; Al-Tuwaijri, H.M.; Hassan, H.M.; Al-Alshaikh, M.A.; Habib, E.E.; El-Emam, A.A. Synthesis, antimicrobial and hypoglycemic activities of novel N-(1-adamantyl)carbothioamide derivatives. Molecules 2015, 20, 8125–8143. [Google Scholar] [CrossRef] [PubMed]
- Woods, G.L.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society of Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Approved Standard Document M-7A; NCCS: Villanova, PA, USA, 1985.
- Olajide, O.A.; Awe, S.O.; Makinde, J.M.; Morebise, O. Evaluation of the antidiabetic property of Morinda lucida leaves in streptozotocin diabetes rats. J. Pharm. Pharmacol. 1999, 51, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Rossini, A.A.; Like, A.A.; Chick, W.L.; Appel, M.C.; Cahill, G.F. Studies of streptozotocin-induced insulitis and diabetes. Proc. Natl. Acad. Sci. USA 1977, 74, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Hassan, H.M.; Abo-Kamar, A.M.; Ghabbour, H.A.; El-Emam, A.A. Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S. Z. Krist. New Cryst. Struct. 2017, 232, 189–191. [Google Scholar] [CrossRef]
- Apex2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems: Madison, WI, USA, 2006.
- Siemens. SMART System, Siemens Analytical X-ray Instruments Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. Short history of SHELX. Acta Crystallogr A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS, Bruker AXS Inc.: Madison, WI, USA, 2007.
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the correspondent author. |


| Comp. No. | R | Cryst. Solv. | M.p. (°C) | Yield (%) | Mol. Formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 7a | H | EtOH/H2O | 108–110 | 91 | C22H30N2OS (370.55) |
| 7b | 4-Cl | EtOH | 92–94 | 76 | C22H29ClN2OS (405.0) |
| 7c | 4-Br | EtOH | 98–100 | 85 | C22H29BrN2OS (449.45) |
| 7d | 4-NO2 | EtOH | 118–120 | 95 | C22H29N3O3S (415.55) |
| 7e | 3,5-(CF3)2 | EtOH/H2O | 106–108 | 72 | C24H28F6N2OS (506.55) |
| 8a | H | EtOH/H2O | 137–139 | 88 | C28H35N3S (445.66) |
| 8b | 4-Cl | EtOH | 153–155 | 90 | C28H34ClN3S (480.11) |
| 8c | 4-Br | EtOH | 140–142 | 92 | C28H34BrN3S (524.56) |
| 8d | 4-NO2 | EtOH | 145–147 | 96 | C28H34N4O2S (490.66) |
| 8e | 3,5-(CF3)2 | EtOH/H2O | 113–115 | 75 | C30H33F6N3S (581.66) |
| Data | Compound 7d | Compound 8d |
|---|---|---|
| Formula | C22H29N3O3S | C28H34N4O2S |
| Formula weight | 415.55 | 490.66 |
| Temperature (K) | 293 | 293 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Orthorhombi |
| Space group | P21/c | P212121 |
| a, b, c (Å) | 6.9204 (5), 29.775 (3), 10.2725 (10) | 6.9426 (9), 9.6472 (12), 39.086 (5) |
| V (Å3) | 2116.7 (3) | 2617.8 (6) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.18 | 0.16 |
| No. of reflections | 11033 | 25091 |
| No. of unique reflections/obs. reflections | 3718/2253 | 4609/1447 |
| No. of parameters | 262 | 318 |
| No. of restraints | 0 | 0 |
| Δρmax, Δρmin (e Å−3) | 0.28, −0.21 | 0.44, −0.40 |
| Tmin, Tmax | 0.939, 0.989 | 0.924, 0.957 |
| Rint | 0.073 | 0.526 |
| Crystal size (mm) | 0.35 × 0.11 × 0.06 | 0.85 × 0.21 × 0.05 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.052, 0.192, 0.65 | 0.128, 0.296, 1.02 |
| CCDC number | 1525183 | 1523432 |
| Comp. No. | Clog P | Diameter of Growth Inhibition Zone (mm) a | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 7a | 5.584 | 22 (2) b | 21 (4) b | 20 (4) b | 18 (16) b | 14 (64) b | - |
| 7b | 6.297 | 24 (4) b | 28 (0.5) b | 22 (4) b | 22 (20) b | 15 (32) | 11 (>128) b |
| 7c | 6.447 | 22 (4) b | 18 (16) b | 14 (64) b | 13 (128) b | 12 (128) b | - |
| 7d | 5.327 | 31 (0.5) b | 32 (0.25) b | 28 (0.5) b | 22 (1) b | 18 (4) b | 14 (32) b |
| 7e | 7.350 | 33 (0.25) b | 34 (0.25) b | 28 (1) b | 24 (2) b | 20 (4) b | - |
| 8a | 7.130 | 18 (8) b | 18 (8) b | 14 (128) b | 12 (>128) b | 10 (>128) b | 10 (>128) b |
| 8b | 7.843 | 21 (8) b | 24 (2) b | 16 (32) b | 16 (64) b | 12 (>128) b | 13 (64) b |
| 8c | 7.993 | 17 (32) b | 19 (8) b | 14 (64) b | 11 (>128) b | 10 (>128) b | 12 (128) b |
| 8d | 6.873 | 24 (1) b | 28 (1) b | 20 (2) b | 18 (2) b | 14 (4) b | 16 (16) b |
| 8e | 8.896 | 28 (1) b | 31 (0.5) b | 22 (2) b | 19 (4) b | 18 (8) b | 14 (64) b |
| Gentamicin sulfate | 27 (1) b | 26 (2) b | 20 (2) b | 22 (0.5) b | 21 (0.5) b | NT | |
| Ampicillin trihydrate | 22 (2) b | 23 (1) b | 20 (2) b | 16 (8) b | 16 (8) b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (4) b | |
| Treatment | Results | ||
|---|---|---|---|
| C0 (mg/dL) a | C24 (mg/dL) a | % Glucose Reduction b | |
| Group 1 c | 302.8 ± 11.64 | 290.2 ± 18.22 | 4.16% |
| Group 1 d | 299.2 ± 16.50 | 171.6 ± 12.32 * | 42.65% |
| 7a (10 mg/kg) | 304.8 ± 13.26 | 212.4 ± 12.16 * | 30.31% (71.08) |
| 7a (20 mg/kg) | 300.6 ± 11.65 | 134.6 ± 9.75 * | 55.22% (64.74) |
| 7b (10 mg/kg) | 288.9 ± 12.15 | 245.2 ± 19.25 * | 15.13% (35.47) |
| 7b (20 mg/kg) | 294.8 ± 9.08 | 201.5 ± 9.60 * | 31.65% (37.13) |
| 7c (10 mg/kg) | 284.8 ± 19.55 | 281.2 ± 7.19 | 1.26% (2.69) |
| 7c (20 mg/kg) | 290.2 ± 21.64 | 286.8 ± 19.02 | 2.75% (1.37) |
| 7d (10 mg/kg) | 278.1 ± 16.24 | 282.2 ± 27.20 | −1.47% |
| 7d (20 mg/kg) | 302.6 ± 22.25 | 299.8 ± 18.80 | 0.93% (1.08) |
| 7e (10 mg/kg) | 306.2 ± 15.20 | 198.7 ± 19.10 * | 35.12 (82.32) |
| 7e (20 mg/kg) | Toxic | ||
| 8a (10 mg/kg) | 294.6 ± 11.30 | 200.2 ± 9.88 * | 32.04% (75.13) |
| 8a (20 mg/kg) | 290.6 ± 8.60 | 108.4 ± 11.05 * | 62.70% (73.50) |
| 8b (10 mg/kg) | 301.4 ± 9.06 | 199.8 ± 10.01 * | 33.71% (79.04) |
| 8b (20 mg/kg) | 296.0 ± 11.02 | 144.6 ± 10.01 * | 51.15% (59.96) |
| 8c (10 mg/kg) | 320.5 ± 22.05 | 277.6 ± 16.20 | 13.39% (31.38) |
| 8c (20 mg/kg) | 313.5 ± 18.60 | 269.9 ± 20.12 | 13.91% (16.30) |
| 8d (10 mg/kg) | 295.0 ± 22.45 | 289.2 ± 25.28 | 1.97% (4.61) |
| 8d (20 mg/kg) | 304.5 ± 27.50 | 309.0 ± 25.95 | −1.48 |
| 8e (10 mg/kg) | 286.6 ± 13.22 | 178.2 ± 16.04 * | 37.82% (88.68) |
| 8e (20 mg/kg) | Toxic | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wahaibi, L.H.; Hassan, H.M.; Abo-Kamar, A.M.; Ghabbour, H.A.; El-Emam, A.A. Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities. Molecules 2017, 22, 710. https://doi.org/10.3390/molecules22050710
Al-Wahaibi LH, Hassan HM, Abo-Kamar AM, Ghabbour HA, El-Emam AA. Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities. Molecules. 2017; 22(5):710. https://doi.org/10.3390/molecules22050710
Chicago/Turabian StyleAl-Wahaibi, Lamya H., Hanan M. Hassan, Amal M. Abo-Kamar, Hazem A. Ghabbour, and Ali A. El-Emam. 2017. "Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities" Molecules 22, no. 5: 710. https://doi.org/10.3390/molecules22050710






