4-Hydroxy-7-methyl-3-phenylcoumarin Suppresses Aflatoxin Biosynthesis via Downregulation of aflK Expressing Versicolorin B Synthase in Aspergillus flavus
Abstract
:1. Introduction
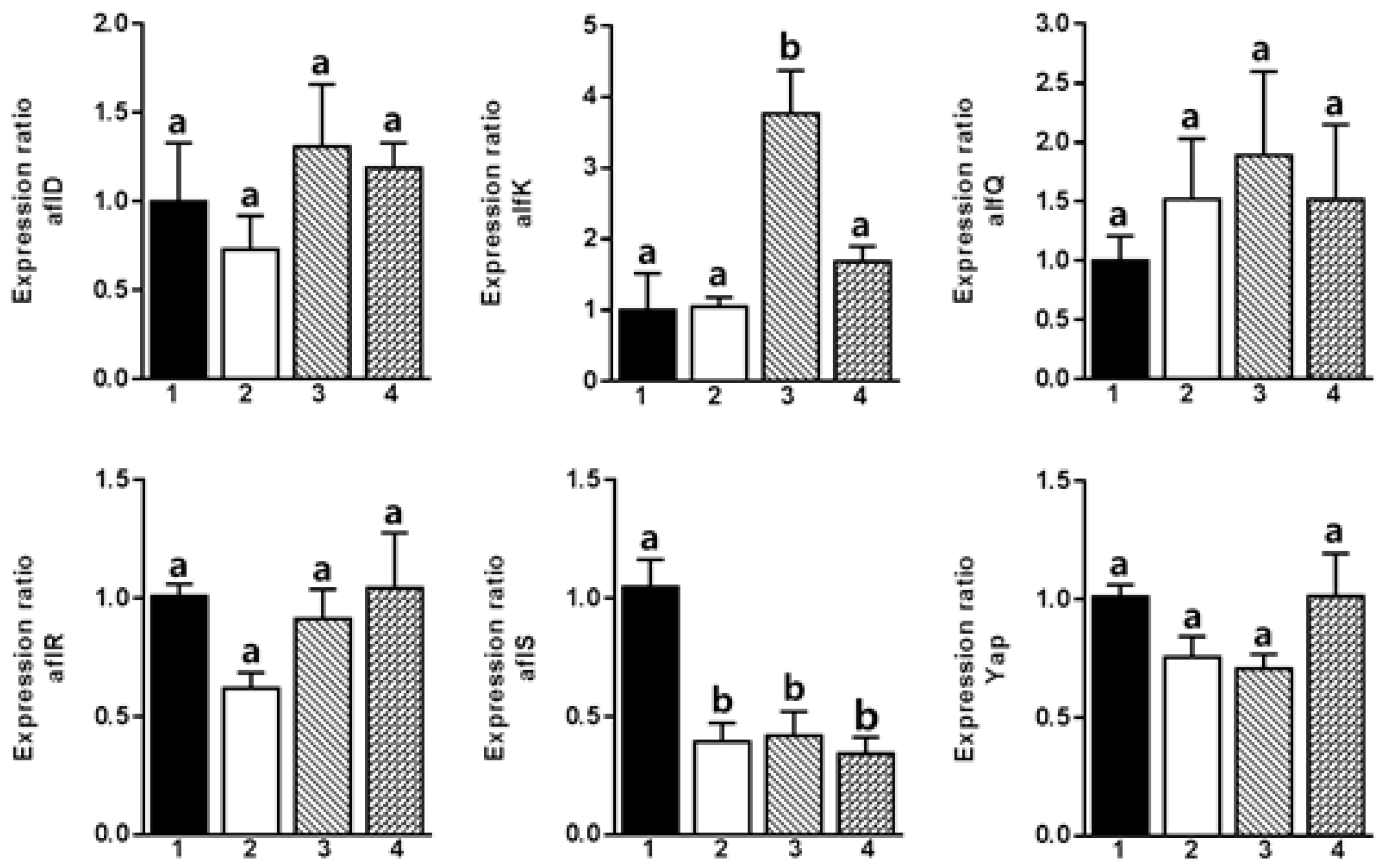
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Microorganisms and Preparation of the Spore Solution
3.3. Aflatoxin Analysis Using an HPLC-FLD
3.4. Real-Time qPCR (RT-qPCR) after Isolation of Total RNA
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Stroka, J.; Anklam, E. Analysis of aflatoxins in various food and feed matrices-results of international validation studies. Mycotoxin Res. 2000, 16 (Suppl. S2), 224–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Jiao, X.; Hu, Y.; Lu, X.; Gao, W. Mycobiota and mycotoxins in traditional medicinal seeds from China. Toxins 2015, 7, 3858–3875. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.C.; Scully, B.T.; Ni, X.Z.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Atehnkeng, J.; Donner, M.; Ojiambo, P.S.; Ikotun, B.; Augusto, J.; Cotty, P.J.; Bandyopadhyay, R. Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategy to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb. Biotechnol. 2015, 9, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Diedhiou, P.M.; Bandyopadhyay, R.; Atehnkeng, J.; Ojiambo, P.S. Aspergillus colonization and aflatoxin contamination of maize and sesame kernels in two agro-ecological zones in Senegal. J. Phytopathol. 2011, 159, 268–275. [Google Scholar] [CrossRef]
- Calori-Domingues, M.A.; Fonseca, H. Laboratory of chemical control of aflatoxin production in unshelled peanuts (Arachis hypogaea L.). Food Addit. Contam. 1995, 12, 347–350. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Macdonald, A.M.C.; Postel, D.; Dijksma, W.T.P.; Dujardin, A.; Plancinta, C.M. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur. J. Plant Path. 1998, 104, 741–751. [Google Scholar]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unraveled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Yuan, Q.; Yan, Y. Combinatorial biosynthesis of plant-specific coumarins in bacteria. Metab. Eng. 2013, 18, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Y.; Li, S.; Ding, W. Resvertrol and coumarin: Novel agricultural antibacterial agent against Ralstonia solanacearum in vitro and in vivo. Molecules 2016, 21, 1501. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Li, J.; Qu, D.; Hou, Z.; Yang, X.H.; Zhang, Z.D.; Wang, Y.K.; Luo, X.X.; Li, M.K. Synthesis and pharmacological evaluations of 4-hydroxycoumarin derivatives as a new class of anti-Staphylococcus aureus agent. J. Pharm. Pharmacol. 2015, 67, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Ge, Z.; Ge, Z.; Chen, K.; Wu, H.; Liu, X.; Huang, Y.; Yuan, L.; Yang, X.; Liao, F. Synthesis and biological evaluation of novel phosphoramidate derivatives of coumarin as chitin synthase inhibitors and antifungal agents. Eur. J. Med. Chem. 2016, 108, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hua, Z.; Song, Y.; Feng, C. Monoterpenoid indole alkaloids from Alstonia rupestris with cytotoxic, antibacterial and antifungal activities. Fitoterapia 2014, 97, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Laivenieks, M.; Hong, S.Y.; Wee, J.; Wong, S.S.; Vanos, B.; Awad, D.; Ehrlich, K.C.; Linz, J.E. Aflatoxin Biosynthesis Is a Novel Source of Reactive Oxygen Species—A Potential Redox Signal to Initiate Resistance to Oxidative Stress? Toxins 2015, 7, 1411–1430. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.K.; Priyanka; Singh, V.; Katiyar, D. Design, synthesis and biological evaluation of some new coumarin derivatives as potential antimicrobial agents. Med. Chem. 2015, 11, 128–134. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, R.S.A.; Guerra, F.Q.S.; de, O.; Lima, E.; de Simone, C.A.; Tavares, J.F.; Scotti, L.; Scotti, M.T.; de Aquino, T.M.; de Moura, R.O.; Mendonça, F.J.B., Jr.; et al. Synthesis, structure–activity relationships (SAR) and in Silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013, 14, 1293–1309. [Google Scholar]
- Hussein, K.A.; Joo, J.H. Isolation and characterization of rhizomicrobial isolates for phosphate solubilization and indole acetic acid production. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 847–855. [Google Scholar] [CrossRef]
- Moon, Y.S.; Choi, W.S.; Park, E.S.; Bae, I.K.; Choi, S.D.; Paek, O.; Kim, S.H.; Chun, H.S.; Lee, S.E. Antifungal and antiaflatoxigenic methylenedioxy-containing compounds and piperine-like synthetic compounds. Toxins 2016, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Mahoney, N.E.; Campbell, B.C. Inhibition of aflatoxin B1 biosynthesis by piperlongumine isolated from Piper longum L. J. Microbiol. Biotechnol. 2002, 12, 679–682. [Google Scholar]
- Kohiyama, C.Y.; Yamamoto, R.M.M.; Mossini, S.A.; Bando, E.; Bomfim Nda, S.; Nerilo, S.B.; Rocha, G.H.; Grespan, R.; Mikcha, J.M.; Machinski, M., Jr. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015, 173, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Bae, I.K.; Kim, H.J.; Lee, S.E. Novel regulation of aflatoxin B1 biosynthesis in Aspergillus flavus by piperonal. Nat. Prod. Res. 2016, 30, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Paster, N.; Juven, B.J.; Harshemesh, H. Antimicrobial activity and inhibition of aflatoxin B1 formation by olive plant tissue constituents. J. Appl. Bacteriol. 1988, 64, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.S.; El-Shayeb, N.M.A. Inhibition of aflatoxin production in Aspergillus flavus by natural coumarins and chromones. World J. Microbiol. Biotechnol. 1992, 8, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Bmicrobiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.A. Inhibition of aflatoxin B1 synthesis by Aspergillus flavus. Phytopathology 1997, 87, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Jamali, M.; Karimipour, M.; Shams-Ghahfarokhi, M.; Amani, A.; Razzaghi-Abyaneh, M. Expression of aflatoxin genes aflO (omtB) and aflQ (ordA) differentiates levels of aflatoxin production by Aspergillus flavus strains from soils of pistachio orchards. Res. Microbiol. 2013, 164, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.Z.; Zhang, H.; Wu, Q.H.; Hu, L.B. Inhibitory effects of tea extract on aflatoxin production by Aspergillus flavus. Lett. Appl. Microbiol. 2013, 56, 462–466. [Google Scholar] [PubMed]
- Scherm, B.; Palomba, M.; Serra, D.; Marcello, A.; Migheli, Q. Detection of transcripts of the aflatoxin genes aflD, aflO, and aflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus parasiticus. Int. J. Food Microbiol. 2005, 98, 201–210. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS User’s Guide, 4th ed.; SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
Sample Availability: Samples of the tested compounds are available from the authors. |
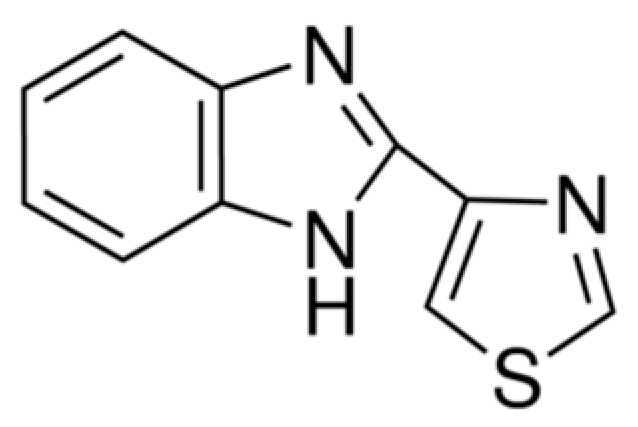

| Compounds | Treated Concentration (μg/mL) | Mycelial Growth (mg) |
|---|---|---|
| Thiabendazole (Positive control) | 10 | 1.2 ± 2.1 (1.0%) |
| 5 | 6.3 ± 10.1 (5.0%) | |
| 1 | 96.3 ± 2.4 (77.0%) | |
| 4-Hydroxy-6,7-dimethylcoumarin | 1000 | 0.0 ± 0.00 (0%) |
| 100 | 22.5 ± 3.7 (18.0%) | |
| 10 | 73.9 ± 17.4 (59.1%) | |
| 4-Hydroxy-7-methoxy-3-phenylcoumarin | 1000 | 0.0 ± 0.00 (0%) |
| 100 | 41.1 ± 27.1 (32.8%) | |
| 10 | 61.9 ± 14.1 (49.5%) | |
| 6,7-Dimethoxycoumarin | 1000 | 0.0 ± 0.00 (0%) |
| 100 | 63.0 ± 44.3 (50.4%) | |
| 10 | 93.7 ± 8.3 (74.9%) | |
| 2,3-dihydrobenzofuran | 1000 | 0.0 ± 0.00 (0%) |
| 100 | 152.3.0 ± 45.1 (124%) | |
| 4-(Bromomethyl)-6,7-dimethoxycoumarin | 1000 | 0.0 ± 0.00 (0%) |
| 100 | 46.4 ± 3.7 (37.1%) | |
| 10 | 120.9 ± 18.5 (96.7%) |
| Compounds | Treated Conc. (μg/mL) | Aflatoxin Production (ng/mL) | |||
|---|---|---|---|---|---|
| Aflatoxin B1 | Aflatoxin B2 | Aflatoxin G1 | Aflatoxin G2 | ||
| Control | - | 1928.9 ± 403.4 a | 37.2 ± 6.3 a | 184.3 ± 66.5 a | 29.5 ± 5.3 a |
| Thiabendazole | 5 | ND *,b | ND b | 64.4 ± 2.6 b | ND b |
| 1 | − | − | 239.2 ± 65.7 a | − | |
| 3-Acetyl-6-bromocoumarin | 10 | ND b | ND b | 92.6 ± 25.7 b | ND b |
| 1 | − | − | − | − | |
| 4-Hydroxy-6,7-dimethyl-coumarin | 10 | ND b | ND b | ND b | ND b |
| 1 | − | ND b | − | ND b | |
| 4-Hydroxy-7-methoxy-3-phenyl-coumarin | 100 | ND b | ND b | ND b | ND b |
| 10 | 158.8 ± 25.6 c | ND b | 192.6 ± 32.4 a | ND b | |
| 1 | 1025.4 ± 329.9 d | 19.3 ± 3.4 c | 137.8 ± 39.0 a | 5.3 ± 1.0 c | |
| Dihydrocoumarin | 1000 | ND b | ND b | ND b | ND b |
| 10 | 1397.5 ± 675.9 a | 28.8 ± 7.8 b | 99.6 ± 62.0 b | ND b | |
| 1 | 2517.9 ± 199.8 a | 49.7 ± 36.5 a | 164.6 ± 120.8 a | 5.1 ± 1.7 c | |
| 2,3-Dihydrobenzofuran | 1000 | ND b | ND b | ND b | ND b |
| 100 | ND b | ND b | ND b | ND b | |
| 1 | 1140.0 ± 342.1 c | 21.8 ± 5.1 c | 123.3 ± 33.7 c | 5.8 ± 2.6 c | |
| 4-(Bromomethyl)-6,7-dimehtoxycoumarin | 1000 | ND b | ND b | ND b | ND b |
| 100 | ND b | ND b | ND b | ND b | |
| 10 | − | − | − | ND b | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.-S.; Kim, L.; Chun, H.S.; Lee, S.-E. 4-Hydroxy-7-methyl-3-phenylcoumarin Suppresses Aflatoxin Biosynthesis via Downregulation of aflK Expressing Versicolorin B Synthase in Aspergillus flavus. Molecules 2017, 22, 712. https://doi.org/10.3390/molecules22050712
Moon Y-S, Kim L, Chun HS, Lee S-E. 4-Hydroxy-7-methyl-3-phenylcoumarin Suppresses Aflatoxin Biosynthesis via Downregulation of aflK Expressing Versicolorin B Synthase in Aspergillus flavus. Molecules. 2017; 22(5):712. https://doi.org/10.3390/molecules22050712
Chicago/Turabian StyleMoon, Young-Sun, Leesun Kim, Hyang Sook Chun, and Sung-Eun Lee. 2017. "4-Hydroxy-7-methyl-3-phenylcoumarin Suppresses Aflatoxin Biosynthesis via Downregulation of aflK Expressing Versicolorin B Synthase in Aspergillus flavus" Molecules 22, no. 5: 712. https://doi.org/10.3390/molecules22050712







