Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
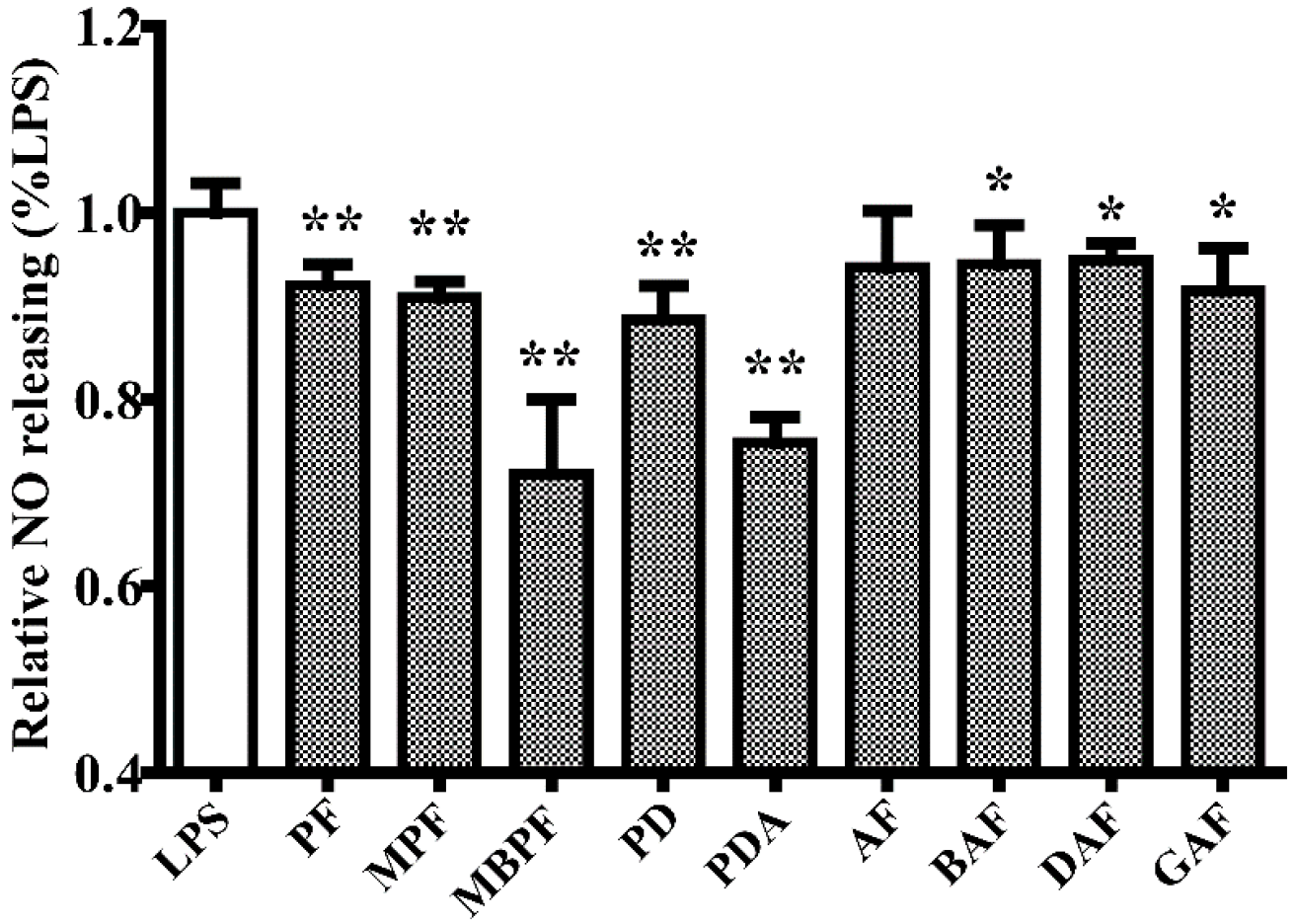
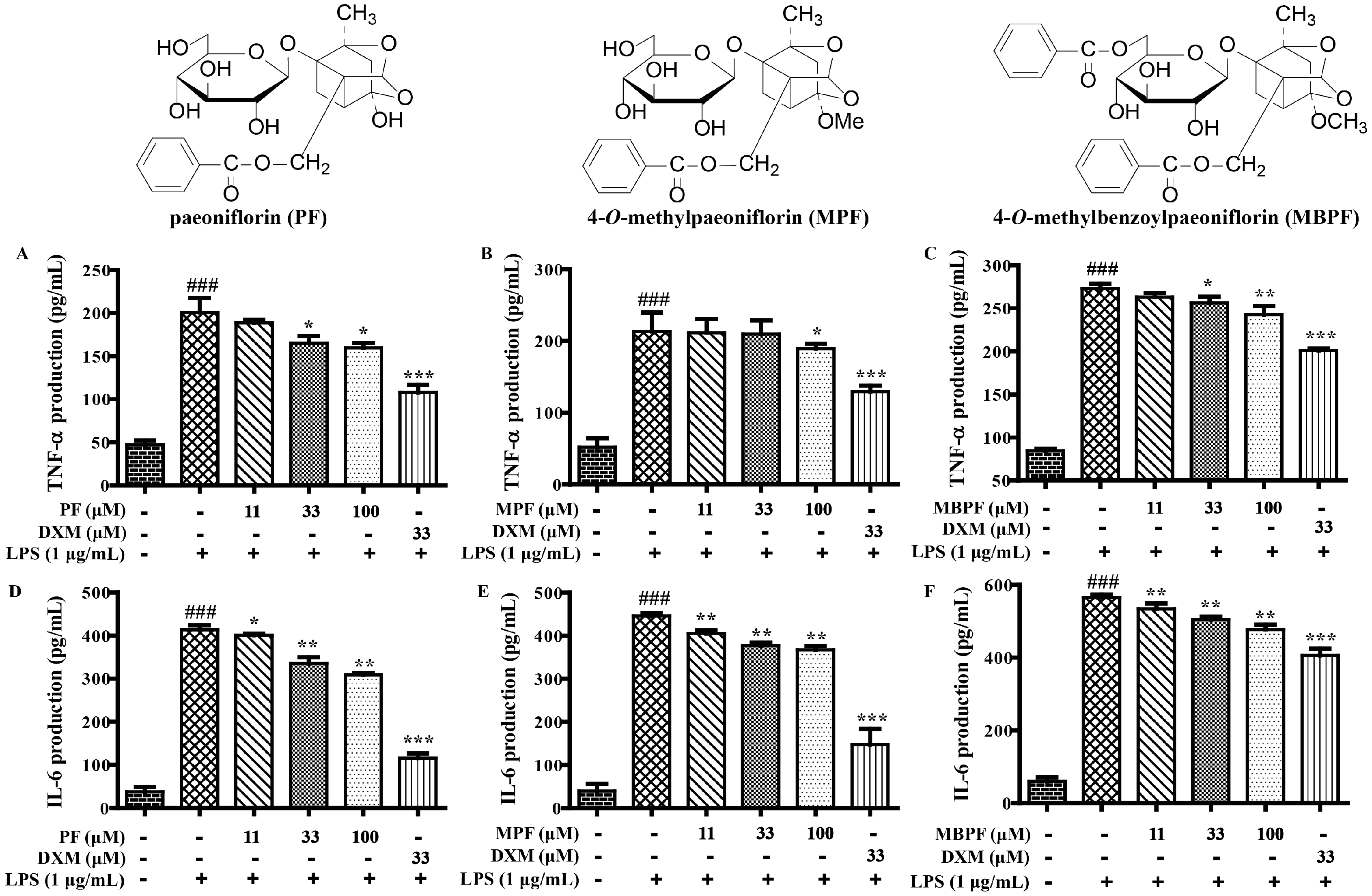
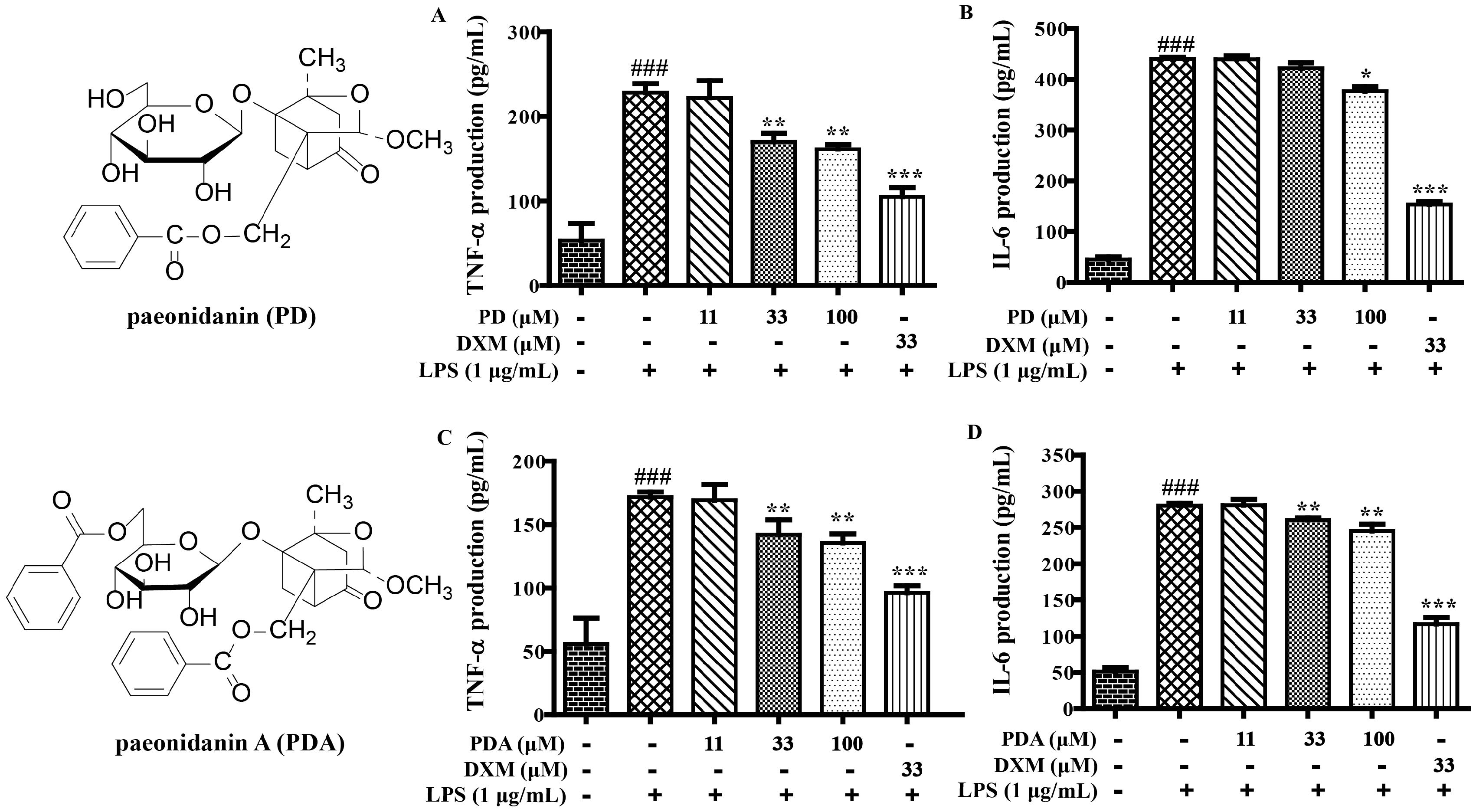
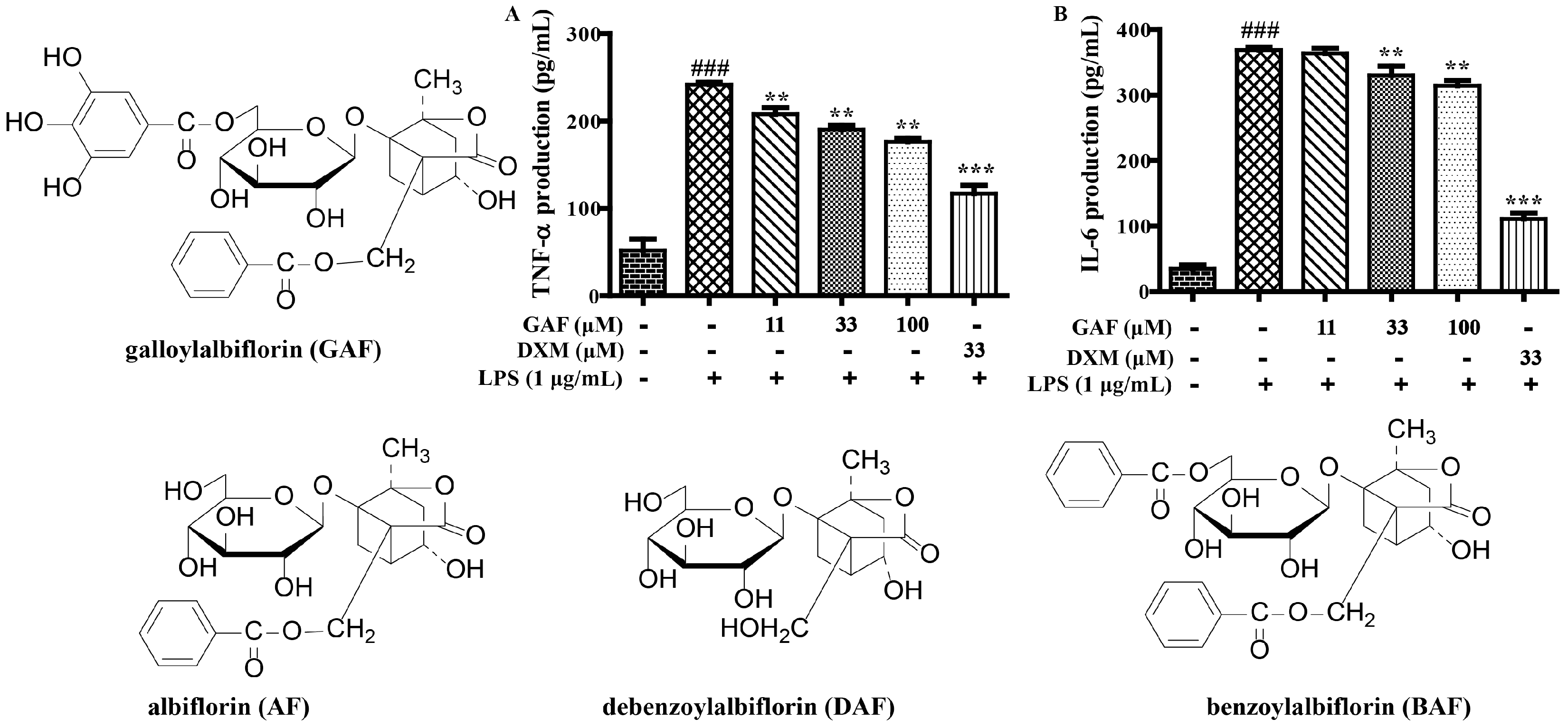
2.1. SAR and Effect of Monoterpenoids on Anti-Inflammation In Vitro
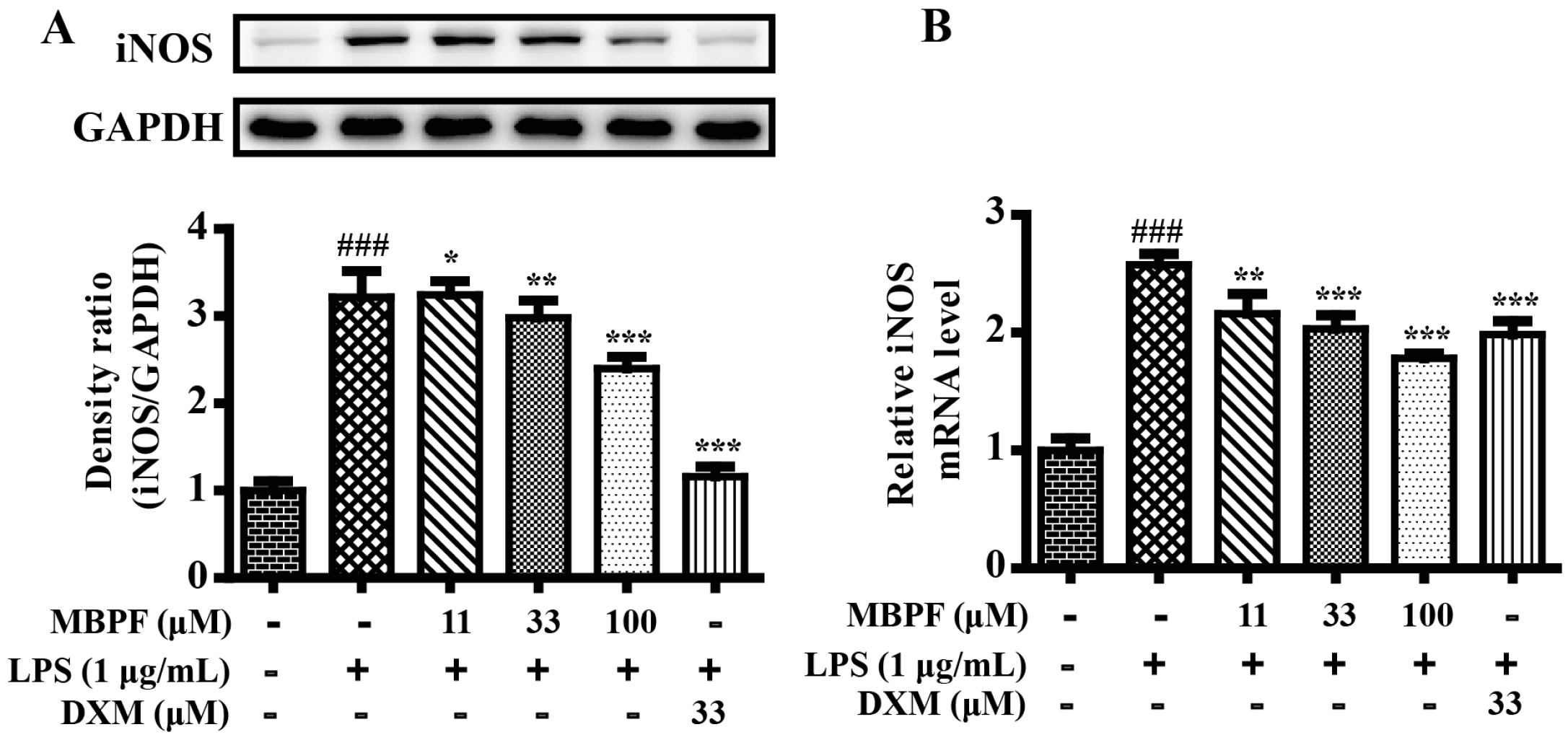
2.2. MBPF Inhibits Expression of iNOS in LPS-Induced RAW 264.7 Cells
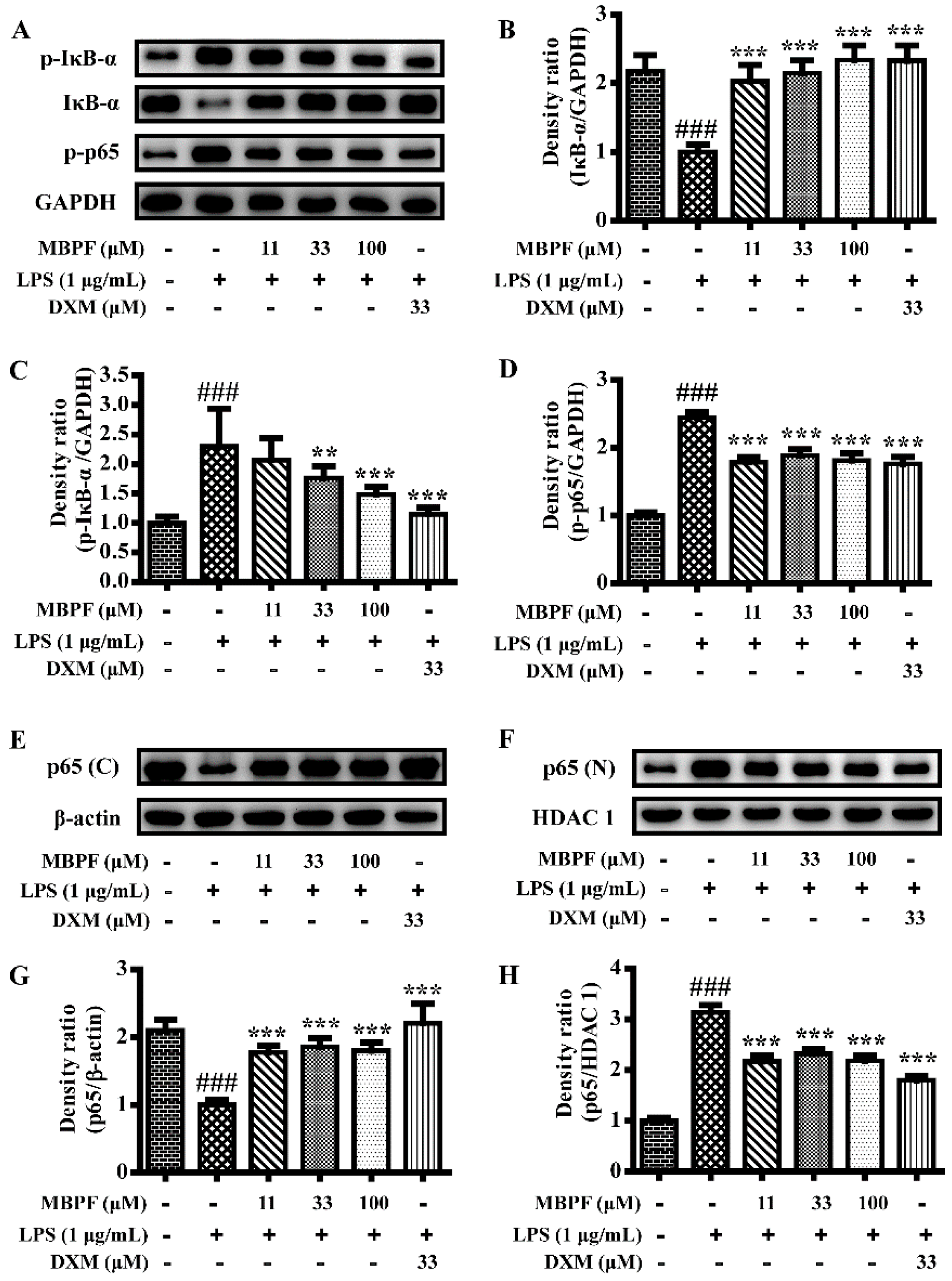
2.3. MBPF Inhibits the Activation of MAPK, PI3K/Akt and NF-κB Signaling Pathway in LPS-Induced RAW 264.7 Cells
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Cell Culture and Cytotoxic Assay
3.3. Determination of Nitric Oxide (NO) Production
3.4. Measurement of Cytokine (IL-6 and TNF-α)
3.5. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
3.6. Western Blot Analyses
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, D.; Tan, Q.R.; Zhang, Z.J. Neuroprotective effects of paeoniflorin, but not the isomer albiflorin, are associated with the suppression of intracellular calcium and calcium/calmodulin protein kinase II in PC12 cells. J. Mol. Neurosci. 2013, 51, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Cheng, Z.H.; Chen, D.F. Anticomplement monoterpenoid glucosides from the root bark of Paeonia suffruticosa. J. Nat. Prod. 2013, 77, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, Y.; Hou, X.; Xu, J.; Mu, X.; Chen, W. Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol. 2011, 137, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.J.; Yang, J.Y.; Chen, L.X.; Zhang, L.J.; Jiang, Z.H.; Cai, X.D.; Zhang, X.; Qiu, F. Monoterpenes from Paeonia albiflora and their inhibitory activity on nitric oxide production by lipopolysaccharide-activated microglia. J. Nat. Prod. 2009, 72, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhao, F.; Chen, L.; Jiang, Z.; Liu, Y.; Li, Z.; Qiu, F.; Yao, X. New monoterpene glycosides from Paeonia suffruticosa Andrews and their inhibition on NO production in LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2012, 22, 7243–7247. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Yu, T.; Yuan, H.M.; Song, Y.; Zou, L. Paeonidanins F-H: Three new dimeric monoterpene glycosides from Paeonia lactiflora and their anti-inflammatory activity. Phytochem. Lett. 2015, 13, 386–389. [Google Scholar] [CrossRef]
- He, X.; Han, L.; Huang, X. A new phenolic glucoside from Paeonia lactiflora. Chin. Herbal Med. 2011, 3, 84–86. [Google Scholar]
- Zhao, D.D.; Jiang, L.L.; Li, H.Y.; Yan, P.F.; Zhang, Y.L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Gao, T.; Cui, Y.L.; Gao, L.N.; Jiang, H.L. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol. 2014, 52, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, W.; Zhang, E.; Sun, A.; Ding, L.; Wei, X.; Chou, G.; Mani, S.; Wang, Z. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol.-Gastrointestinal Liver Physiol. 2014, 306, G27–G36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Yang, L.; Zhang, A.L.; May, B.H.; Yu, J.J.; Guo, X.; Lu, C.; Xue, C.C. Is oral Chinese herbal medicine beneficial for psoriasis vulgaris? A meta-analysis of comparisons with acitretin. J. Alterna. Complement. Med. 2016, 22, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Zhou, X.; Wong, H.L.; Ng, C.F.; Fu, W.M.; Leung, P.C.; Peng, G.; Ko, C.H. In vivo and in vitro anti-inflammatory effects of Zao-Jiao-Ci (the spine of Gleditsia sinensis Lam.) aqueous extract and its mechanisms of action. J. Ethnopharmacol. 2016, 192, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, N.; Rivest, S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R.G.; Lee, K.T.; Lee, H.J.; Choi, Y.H.; Jeong, J.W.; Kim, G.Y. Anti-inflammatory effects of beta-hydroxyisovalerylshikonin in BV2 microglia are mediated through suppression of the PI3K/Akt/NF-kB pathway and activation of the Nrf2/HO-1 pathway. Food Chem. Toxicol. 2014, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, K.H.; Park, J.W.; Kwon, T.K. LY294002 inhibits LPS-induced NO production through a inhibition of NF-κB activation: Independent mechanism of phosphatidylinositol 3-kinase. Immunol. Lett. 2005, 99, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.; Harnett, M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Yu, X.; Sun, X.; Meng, D.; Jia, J.M. A new steroidal saponin, furotrilliumoside from Trillium tschonoskii inhibits lipopolysaccharide-induced inflammation in Raw264. 7 cells by targeting PI3K/Akt, MARK and Nrf2/HO-1 pathways. Fitoterapia 2016, 115, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Kim, M.Y.; Cho, J.Y. MAPK/AP-1-Targeted anti-inflammatory activities of Xanthium strumarium. Am. J. Chin. Med. 2016, 44, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Hou, X.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264. 7 macrophages. Mediators Inflammation 2015, 2015, 380218. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Jin, K.S.; Lee, Y.W.; Song, Y.S. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Niu, J.; Schmidt, C.; Sclabas, G.M.; Peng, B.; Uwagawa, T.; Li, Z.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. NF-κB and AP-1 connection: mechanism of NF-κB-dependent regulation of AP-1 activity. Mol. Cell. Biol. 2004, 24, 7806–7819. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds PF, MPF, MBPF, PD, PDA, AF, BAF, DAF, and GAF are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, X.; Han, L.; Qu, T.; Mu, Y.; Guan, P.; Qu, X.; Wang, Z.; Huang, X. Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells. Molecules 2017, 22, 715. https://doi.org/10.3390/molecules22050715
Bi X, Han L, Qu T, Mu Y, Guan P, Qu X, Wang Z, Huang X. Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells. Molecules. 2017; 22(5):715. https://doi.org/10.3390/molecules22050715
Chicago/Turabian StyleBi, Xiaoxu, Li Han, Tiange Qu, Yu Mu, Peipei Guan, Xiaodan Qu, Zhanyou Wang, and Xueshi Huang. 2017. "Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells" Molecules 22, no. 5: 715. https://doi.org/10.3390/molecules22050715




