One-Step Synthesis of Silver Nanoparticles on Polydopamine-Coated Sericin/Polyvinyl Alcohol Composite Films for Potential Antimicrobial Applications
Abstract
:1. Introduction
2. Results and Discussion
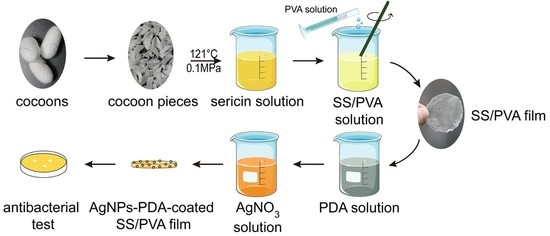
2.1. Preparation of PDA Coated SS/PVA Film
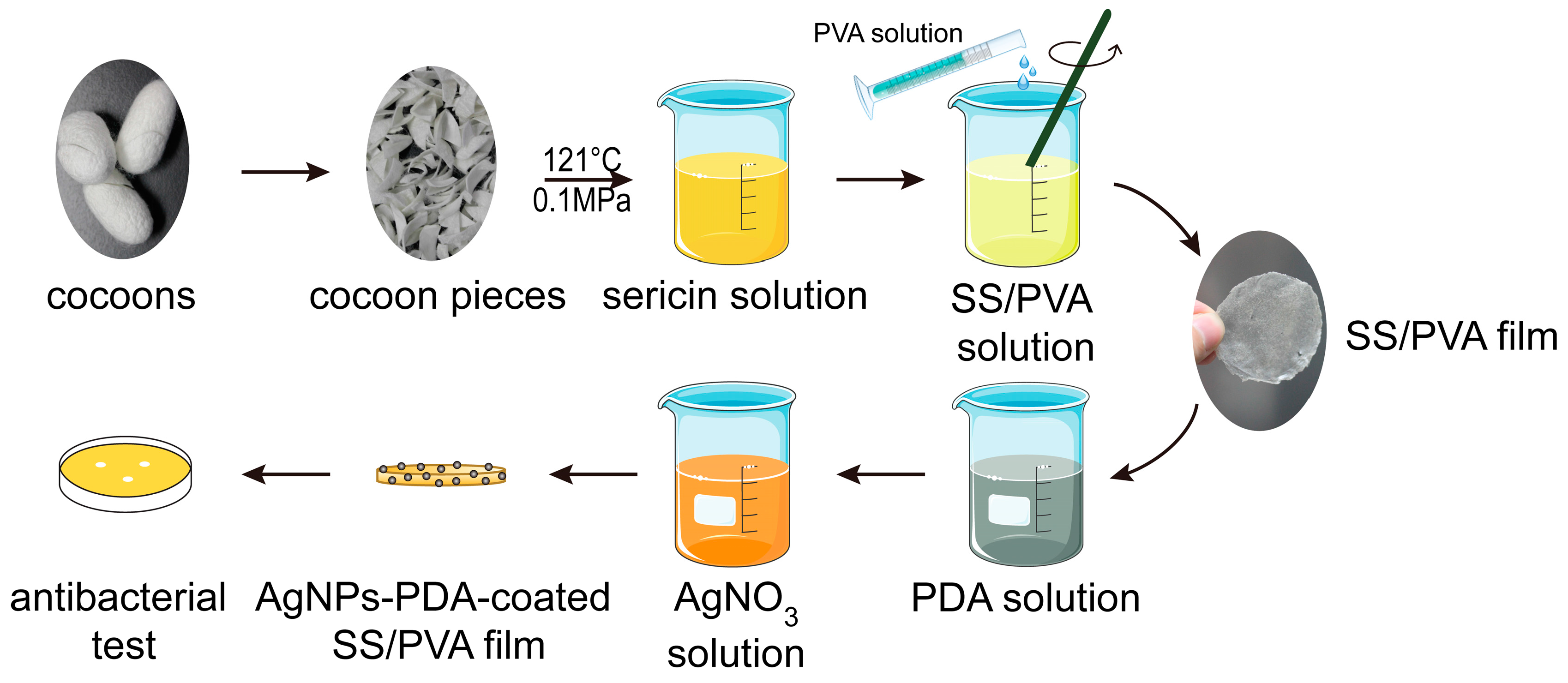
2.2. FESEM, EDS and XRD Analysis
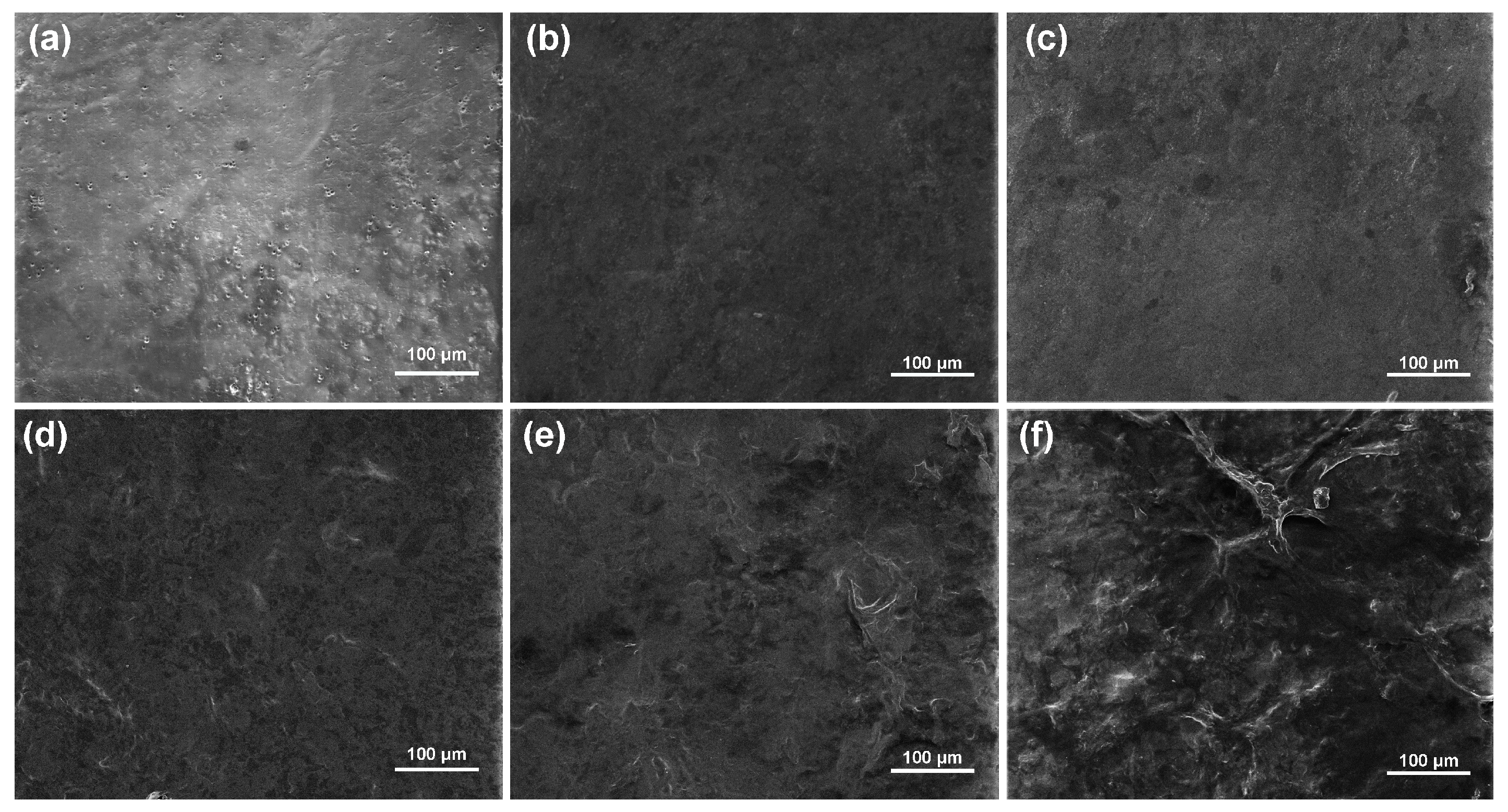
2.3. FT-IR Analysis
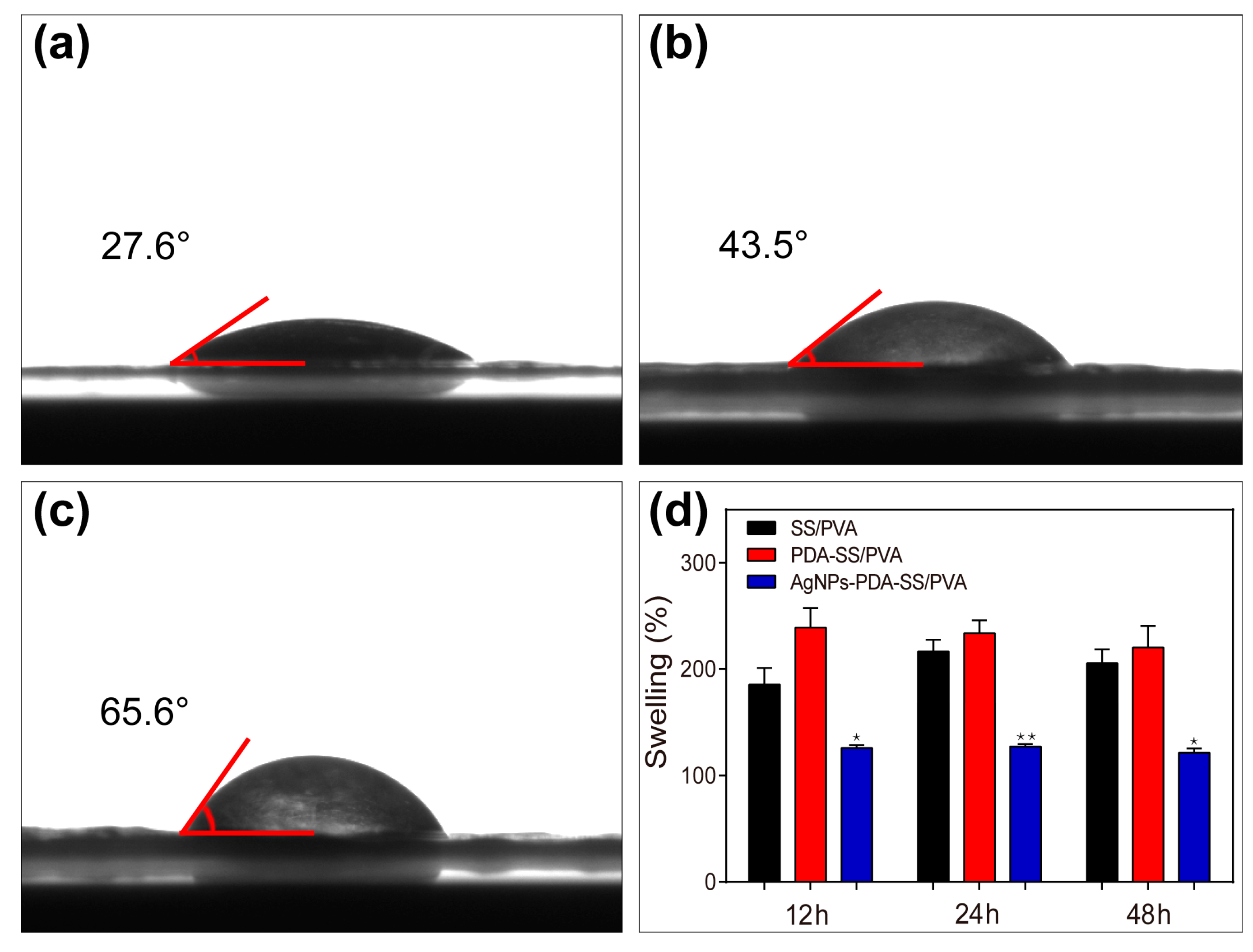
2.4. Wettability and Water Uptake Ability Measurements
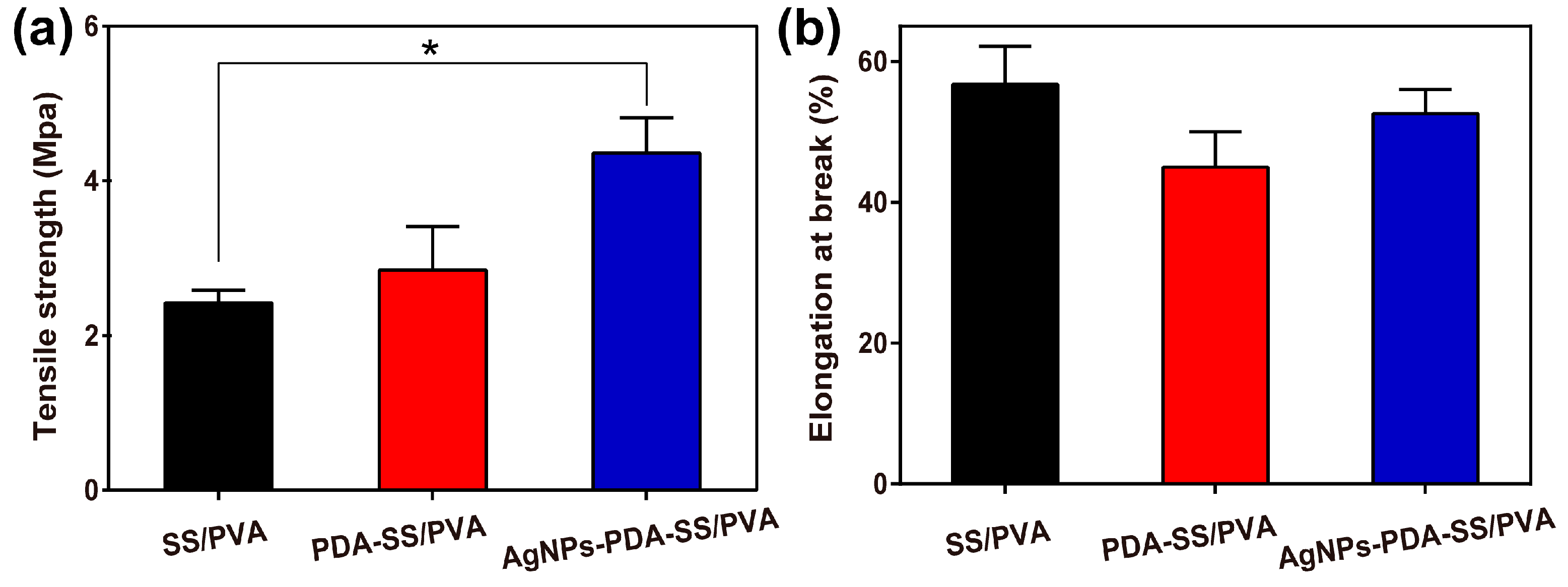
2.5. Mechanical Properties
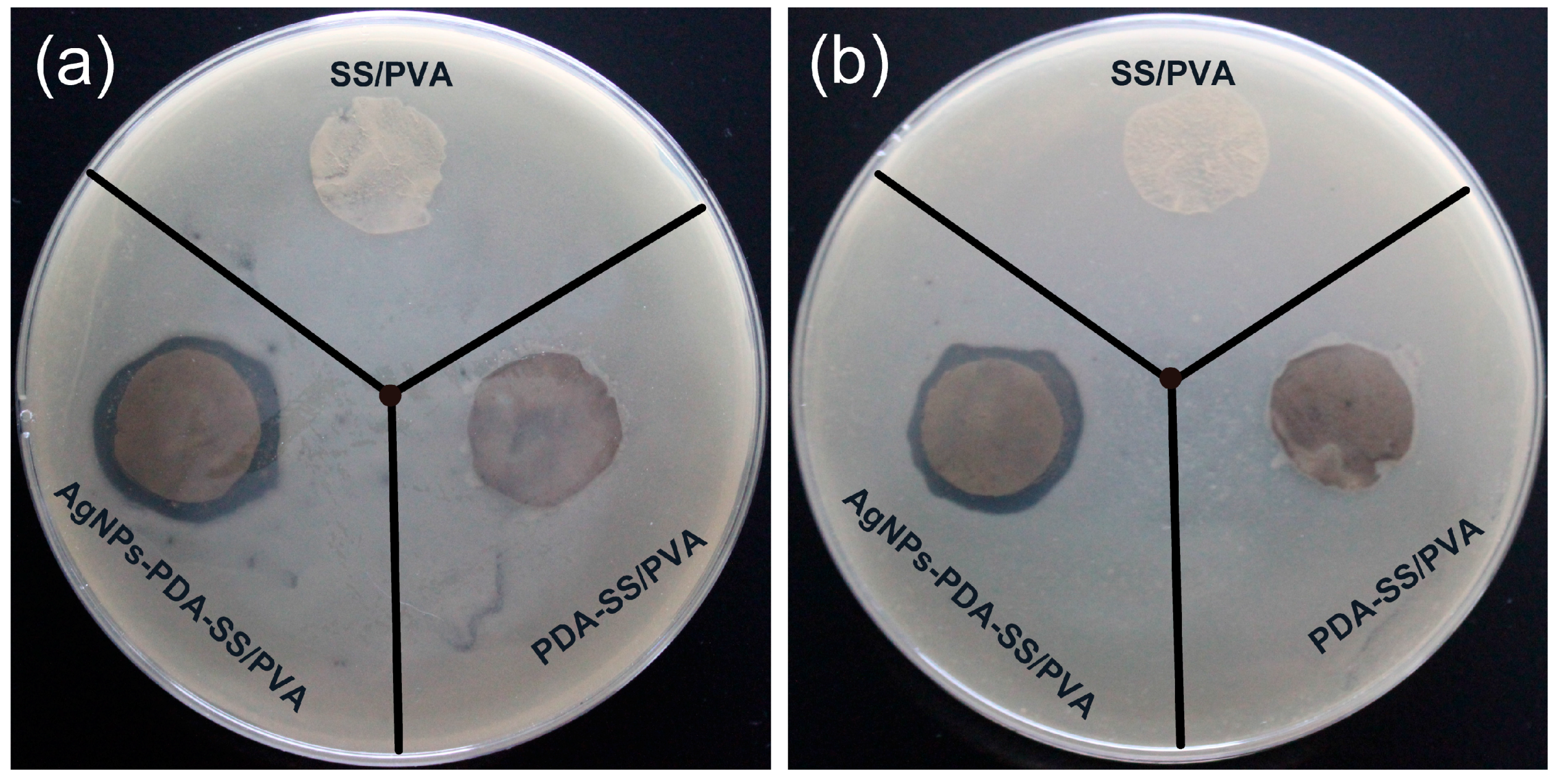
2.6. Inhibition Zone Assays
2.7. Bacterial Growth Curve
2.8. Long-term Antimicrobial Stability Analyze
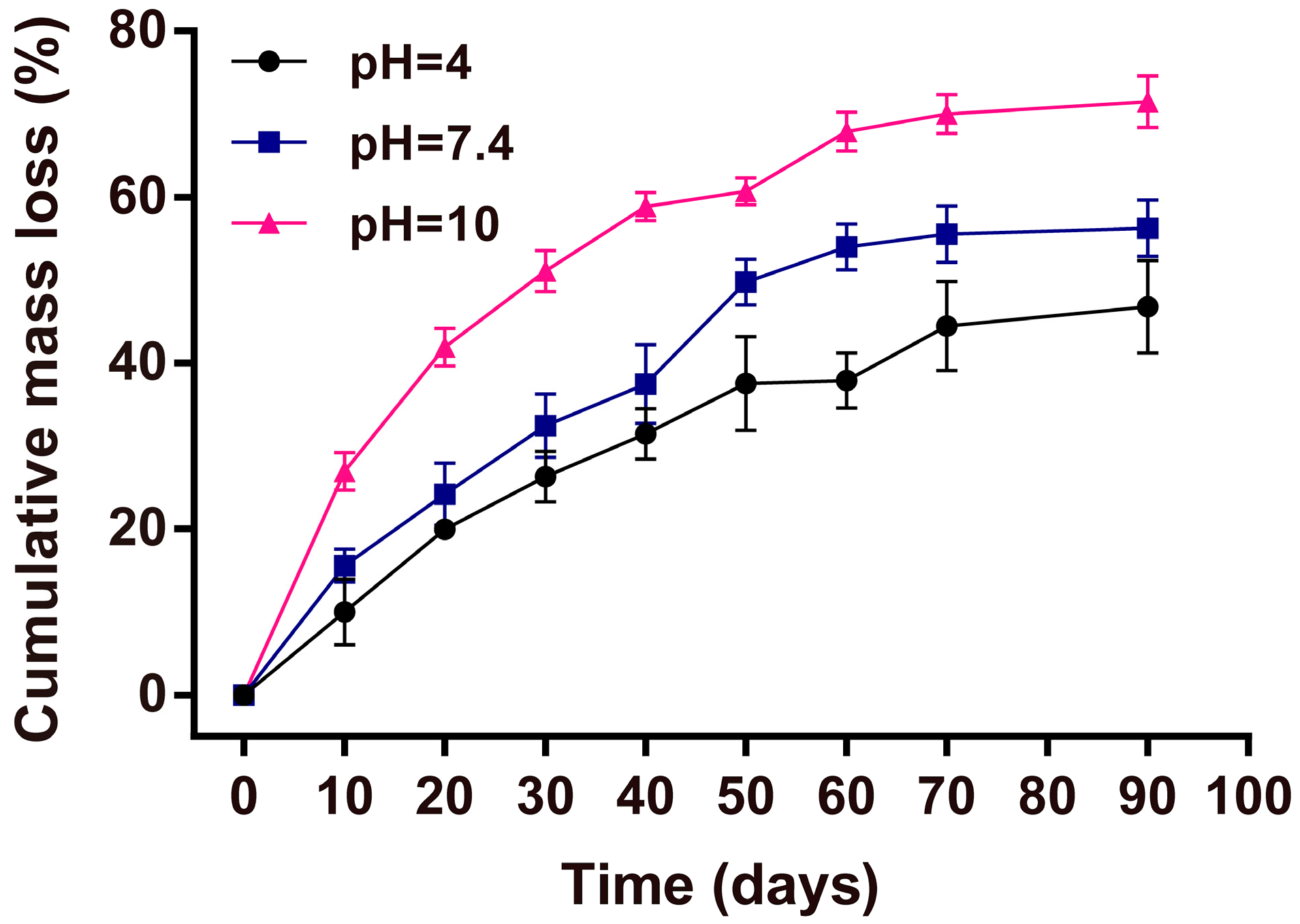
2.9. Mass Loss Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of AgNPs Modified PDA-SS/PVA Composite Film
3.3. Materials Characterization
3.4. Wettability Measurement
3.5. Water Uptake Ability
3.6. Mechanical Analysis
3.7. Inhibition Zone Assay
3.8. Growth Curve Assay
3.9. Long-Term Antimicrobial Stability Test
3.10. Mass Loss Test
3.11. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.Q.; Tao, M.L.; Shen, W.D.; Zhou, Y.Z.; Ding, Y.; Ma, Y.; Zhou, W.L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of three main sericin components from the cocoon of the silkworm, Bombyx mori. Biosci. Biotech. Bioch. 2002, 66, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- Padamwar, M.N.; Pawar, A.P. Silk sericin and its applications: A review. J. Sci. Ind. Res. India 2004, 63, 323–329. [Google Scholar]
- Zhaorigetu, S.; Yanaka, N.; Sasaki, M.; Watanabe, H.; Kato, N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J. Photochem. Photobiol. B 2003, 71, 11–17. [Google Scholar] [CrossRef]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Cho, K.Y.; Moon, J.Y.; Lee, Y.W.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Kim, K.H.; Cho, C.S. Preparation of self-assembled silk sericin nanoparticles. Int. J. Biol. Macromol. 2003, 32, 36–42. [Google Scholar] [CrossRef]
- Dash, B.C.; Mandal, B.B.; Kundu, S.C. Silk gland sericin protein membranes: Fabrication and characterization for potential biotechnological applications. J. Biotechnol. 2009, 144, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3-d scaffolds and 2-d films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Srichana, T.; Aramwit, P. The effect of sterilization methods on the physical properties of silk sericin scaffolds. Aaps PharmSciTech 2011, 12, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mora, C.; Mrowiec, A.; Garcia-Vizcaino, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolas, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Ghosh, S.K.; Kaplan, D.L.; Kundu, S.C. Purification and biochemical characterization of a 70 kDa sericin from tropical tasar silkworm, Antheraea mylitta. Comp. Biochem. Phys. B 2007, 147, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.Y.; Kim, M.K.; Um, I.C.; Lee, K.H. Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 2011, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.Y.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiu, G.; Zhou, Z.; Li, J.; Amy, G.L.; Xie, J.; Lee, J.Y. An effective design of electrically conducting thin-film composite (TFC) membranes for bio and organic fouling control in forward osmosis (FO). Environ. Sci. Technol. 2016, 50, 10596–10605. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microb. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wang, Y.J.; Liu, L.N.; Chang, H.P.; Zhao, P.; He, H.W. Preparation and characterization of silver nanoparticles composited on polyelectrolyte film coated sericin gel for enhanced antibacterial application. Sci. Adv. Mater. 2016, 8, 1547–1552. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Trevino, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Murugadoss, A.; Prasad, P.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, O.; Hejna, O.; Solovyev, A.; Kalachyova, Y.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]
- Skladanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immun. 2016, 205, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Smekalova, M.; Kilianova, M.; Prucek, R.; Bogdanova, K.; Vecerova, R.; Kolar, M.; Havrdova, M.; Plaza, G.A.; Chojniak, J.; et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 2016, 21, 26. [Google Scholar]
- Elliott, C. The effects of silver dressings on chronic and burns wound healing. Br. J. Nurs. 2010, 19, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Lim, T.-P.; Leong, D.T.; Xie, J. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of modified biodegradable PHB non-woven fabric. Mater. Sci. Eng. C 2016, 65, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.F.; Dhandapani, P.; Maruthamuthu, S.; Kulandainathan, M.A. One pot synthesis of polypyrrole silver nanocomposite on cotton fabrics for multifunctional property. Carbohyd. Polym. 2012, 90, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Swope, K.L.; Flicklinger, M.C. The use of confocal scanning laser microscopy and other tools to characterize Escherichia coli in a high-cell-density synthetic biofilm. Biotechnol. Bioeng. 1996, 52, 340–356. [Google Scholar] [CrossRef]
- Lee, J.M.; Yu, J.E.; Koh, Y.S. Experimental study on the effect of wavelength in the laser cleaning of silver threads. J. Cult. Herit. 2003, 4, 157–161. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Jia, X.; Hu, J.M.; Liu, Z.Y.; Wang, H.Y.; Zhou, F. Mussel-inspired thermosensitive polydopamine-graft-poly(N-isopropylacrylamide) coating for controlled-release fertilizer. J. Agric. Food Chem. 2013, 61, 12232–12237. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Lin, F.; Liu, Y.; Leong, D.T.; Xie, J. Highly luminescent thiolated gold nanoclusters impregnated in nanogel. Chem. Mater. 2016, 28, 4009–4016. [Google Scholar] [CrossRef]
- Chang, S.Q.; Kang, B.; Dai, Y.D.; Chen, D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J. Appl. Polym. Sci. 2009, 112, 2511–2515. [Google Scholar] [CrossRef]
- Zhang, D.; Toh, G.W.; Lin, H.; Chen, Y. In situ synthesis of silver nanoparticles on silk fabric with PNP for antibacterial finishing. J. Mater. Sci. 2012, 47, 5721–5728. [Google Scholar] [CrossRef]
- Zhang, A.; Neumeyer, J.L.; Baldessarini, R.J. Recent progress in development of dopamine receptor subtype-selective agents: Potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2007, 107, 274–302. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(dopa) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Postma, A.; Yan, Y.; Wang, Y.J.; Zelikin, A.N.; Tjipto, E.; Caruso, F. Self-Polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W.M. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.W.; Granville, A.M. Surface property modification of silver nanoparticles with dopamine-functionalized poly(pentafluorostyrene) via raft polymerization. Polymers 2016, 8, 81. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhang, B.L.; Tang, J.L. Mussel-Inspired functionalization of graphene for synthesizing Ag-polydopamine-graphene nanosheets as antibacterial materials. Nanoscale 2013, 5, 118–123. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Aguilar, L.E.; Ambade, R.B.; Lee, S.-H.; Park, C.H.; Kim, C.S. Immobilization of silver nanoparticles on electropolymerized polydopamine films for metal implant applications. Colloids Interface Sci. Commun. 2015, 6, 5–8. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, Y.; Lyu, B.; Zhang, R.R.; Zhang, H.; Ma, H.W.; Lyu, Y.L.; Wei, S.C. Rapidly-deposited polydopamine coating via high temperature and vigorous stirring: formation, characterization and biofunctional evaluation. PLoS ONE 2014, 9, e113087. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Toniazzo, V.; Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Liu, L.N.; Wang, Y.J.; Chang, H.P.; Zhao, P.; Zuo, H.; He, H.W. Characterization of silver nanoparticle in situ synthesis on porous sericin gel for antibacterial application. J. Nanomater. 2016, 2016, 1–8. [Google Scholar]
- Abbasi, A.R.; Morsali, A. Synthesis and properties of silk yarn containing Ag nanoparticles under ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.J.; Zhang, P.P.; Wang, A.J.; Liao, Q.C.; Xi, J.L.; Chen, J.R. One-Step synthesis of monodisperse polydopamine-coated silver core-shell nanostructures for enhanced photocatalysis. New J. Chem. 2012, 36, 148–154. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wyeth, P. Using FTIR spectroscopy to detect sericin on historic silk. Sci. China Chem. 2010, 53, 626–631. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.S.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.X.; Deng, D.Y.; Cheng, Y.R.; Kong, L.Q.; Xiao, F. Annealing-Free and strongly adhesive silver nanowire networks with long-term reliability by introduction of a nonconductive and biocompatible polymer binder. Nanoscale 2014, 6, 4812–4818. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.B.; Kordestani, S.S.; Mirzadeh, H.; Mansoori, P. Poly(vinyl alcohol)-chitosan blends: preparation, mechanical and physical properties. Iran Polym. J. 2003, 12, 139–146. [Google Scholar]
- Cervera, M.F.; Heinamaki, J.; Krogars, K.; Jorgensen, A.C.; Karjalainen, M.; Colarte, A.I.; Yliruusi, J. Solid-State and mechanical properties of aqueous chitosan-amylose starch films plasticized with polyols. Aaps PharmSciTech 2004, 5, 109. [Google Scholar]
- Xu, F.; Wen, T.J.; Lu, T.J.; Seffen, K.A. Skin biothermomechanics for medical treatments. J. Mech. Behav. Biomed. 2008, 1, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Abhilash, S.; Manzoor, K.; Nair, S.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohyd. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Tao, W.; Li, M.Z.; Xie, R.J. Preparation and structure of porous silk sericin materials. Macromol. Mater. Eng. 2005, 290, 188–194. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.S.; Zhang, J.X.; Huang, L.; Liu, J.; Li, Y.K.; Zhang, G.Z.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: an injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Vazquez, B.; Roman, J.S.; Peniche, C.; Cohen, M.E. Polymeric hydrophilic hydrogels with flexible hydrophobic chains. Control of the hydration and interactions with water molecules. Macromolecules 1997, 30, 8440–8446. [Google Scholar] [CrossRef]
- Guan, J.; Porter, D.; Vollrath, F. Thermally induced changes in dynamic mechanical properties of native silks. Biomacromolecules 2013, 14, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Lucke, F.K. Antibacterial activity of lactobacillus-sake isolated from meat. Appl. Environ. Microb. 1989, 55, 1901–1906. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
Samples of the silk cocoons are available from the authors. |


| Bacteria | Control (cm) | PDA-SS/PVA (cm) | AgNPs-PDA-SS/PVA (cm) |
|---|---|---|---|
| E. coil | 1.55 ± 0.10 | 1.65 ± 0.11 | 2.03 ± 0.08 |
| S. aureus | 1.55 ± 0.15 | 1.62 ± 0.06 | 1.86 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Tao, G.; He, H.; Song, K.; Zuo, H.; Jiang, W.; Wang, Y. One-Step Synthesis of Silver Nanoparticles on Polydopamine-Coated Sericin/Polyvinyl Alcohol Composite Films for Potential Antimicrobial Applications. Molecules 2017, 22, 721. https://doi.org/10.3390/molecules22050721
Cai R, Tao G, He H, Song K, Zuo H, Jiang W, Wang Y. One-Step Synthesis of Silver Nanoparticles on Polydopamine-Coated Sericin/Polyvinyl Alcohol Composite Films for Potential Antimicrobial Applications. Molecules. 2017; 22(5):721. https://doi.org/10.3390/molecules22050721
Chicago/Turabian StyleCai, Rui, Gang Tao, Huawei He, Kai Song, Hua Zuo, Wenchao Jiang, and Yejing Wang. 2017. "One-Step Synthesis of Silver Nanoparticles on Polydopamine-Coated Sericin/Polyvinyl Alcohol Composite Films for Potential Antimicrobial Applications" Molecules 22, no. 5: 721. https://doi.org/10.3390/molecules22050721