Acetylcholinesterase Inhibitory Meroterpenoid from a Mangrove Endophytic Fungus Aspergillus sp. 16-5c
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. X-Ray Crystallographic Analysis of 1, 3, 4, 6, 7, 8, 9 and 10
3.5. Assays for Enzyme Inhibiting Activities and Cytotoxic Activities
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornforth, J.W. Terpenoid biosynthesis. Chem. Br. 1968, 4, 102–106. [Google Scholar] [PubMed]
- Geris, R.; Simpson, T. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Chexal, K.; Springer, J.; Clardy, J.; Cole, R.; Kirksey, J.; Dorner, J.; Cutler, H.; Strawter, B. Austin, a novel polyisoprenoid mycotoxin from Aspergillus ustus. J. Am. Chem. Soc. 1976, 98, 6748–6750. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Scott, F.E.; Stenzel, D.J.; Simpson, T.J.; Moore, R.W.; Trimble, L.A.; Arai, K.; Vederas, J.C. Studies on the biosynthesis of the mycotoxin austin, a meroterpenoid metabolite of Aspergillus ustus. J. Chem. Soc. Perkin Trans. I. 1989, 807–816. [Google Scholar]
- Chen, J.-W.; Luo, Y.-L.; Hwang, M.-J.; Peng, F.-C.; Ling, K.-H. Territrem B, a tremorgenic mycotoxin that inhibits acetylcholinesterase with a noncovalent yet irreversible binding mechanism. J. Biol. Chem. 1999, 274, 34916–34923. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.; Entwistle, R.; Guo, C.; Ahuja, M.; Szewczyk, E.; Hung, J.; Chiang, Y.; Oakley, B.; Wang, C. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 4709–4720. [Google Scholar] [CrossRef] [PubMed]
- Fill, T.P.; Pereira, G.K.; Geris dos Santos, R.M.; Rodrigues-Fo, E. Four additional meroterpenes produced by Penicillium sp. found in association with Melia azedarach. Possible biosynthetic intermediates to austin. Z. Naturforsch. 2007, 62b, 1035–1044. [Google Scholar]
- Geris dos Santos, R.M.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef]
- Dos Santos, R.M.G.; Rodrigues-Fo, E. Structures of meroterpenes produced by Penicillium sp., an endophytic fungus found associated with Melia azedarach. J. Braz. Chem. Soc. 2003, 14, 722–727. [Google Scholar] [CrossRef]
- Geris dos Santos, R.M.; Rodrigues-Fo, E. Further meroterpenes produced by Penicillium sp., an endophyte obtained from Melia azedarach. Z. Naturforsch. 2003, 58c, 663–669. [Google Scholar]
- Fukuyama, K.; Katsube, Y.; Ishido, H.; Yamazaki, M.; Maebayashi, Y. The absolute configuration of desacetylaustin isolated from Emericella nidulans var. dentate. Chem. Pharm. Bull. 1980, 28, 2270–2271. [Google Scholar] [CrossRef]
- Hayashi, H.; Mukaihara, M.; Murao, S.; Arai, M.; Lee, A.; Clardy, J. Acetoxydehydroaustin, a new bioactive compound, and related compound neoaustin from Penicillium sp. MG-11. J. Biosci. Biotechnol. Biochem. 1994, 58, 334–338. [Google Scholar] [CrossRef]
- Scott, F.; Simpson, T.; Trimble, L.; Vederas, J. Biosynthesis of the meroterpenoid austin, by Aspergillus ustus: synthesis and incorporation of carbon-13, oxygen-18-labeled ethyl 3,5-dimethylorsellinate. J. Chem. Soc. Chem. Commun. 1986, 3, 214–215. [Google Scholar] [CrossRef]
- Scudiero, D.; Shoemaker, R.; Paull, K.; Monks, A.; Tierney, S.; Nofziger, T.; Currens, M.; Seniff, D.; Boyd, M. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Simpson, T.; Ahmed, S.; McIntyre, C.; Scott, F.; Sadler, I. Biosynthesis of polyketide-terpenoid (meroterpenoid) metabolites andibenin B and andilesin A in Aspergillus variecolor. Tetrahedron 1997, 53, 4013–4034. [Google Scholar] [CrossRef]
- Song, Y.-X.; Qiao, L.-T.; Wang, J.-J.; Zeng, H.-M.; She, Z.-G.; Miao, C.-D.; Hong, K.; Gu, Y.-C.; Liu, L.; Lin, Y.-C. Two new meroterpenes from the mangrove endophytic fungus Aspergillus sp. 085241B. Helv. Chim. Acta. 2011, 94, 1875–1879. [Google Scholar] [CrossRef]
- Huang, H.; Feng, X.; Xiao, Z.; Liu, L.; Li, H.; Ma, L.; Lu, Y.; Ju, J.; She, Z.; Lin, Y. Azaphilones and p-terphenyls from the mangrove endophytic fungus Penicillium chermesinum (ZH4-E2) isolated from the South China Sea. J. Nat. Prod. 2011, 74, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.; Vrijmoed, L.L.P.; Jones, E.B.G.; Lin, Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 2009, 74, 1093–1098. [Google Scholar]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A. 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Meroterpenoid, isocoumarin, and phenol derivatives from these agrass-derived fungus Pestalotiopsis sp. PSU-ES194. Tetrahedron 2015, 71, 882–888. [Google Scholar] [CrossRef]
- Tang, H.; Wei, Y.B.; Zhang, C.; Ning, F.X.; Qiao, W.; Huang, S.L.; Ma, L.; Huang, Z.S.; Gu, L.Q. Synthesis, biological evaluation and molecular modeling of oxoisoaporphine and oxoaporphine derivatives as new dual inhibitors of acetylcholinesterase/ butyrylcholinesterase. Eur. J. Med. Chem. 2009, 44, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Compounds 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 are available from the authors. |
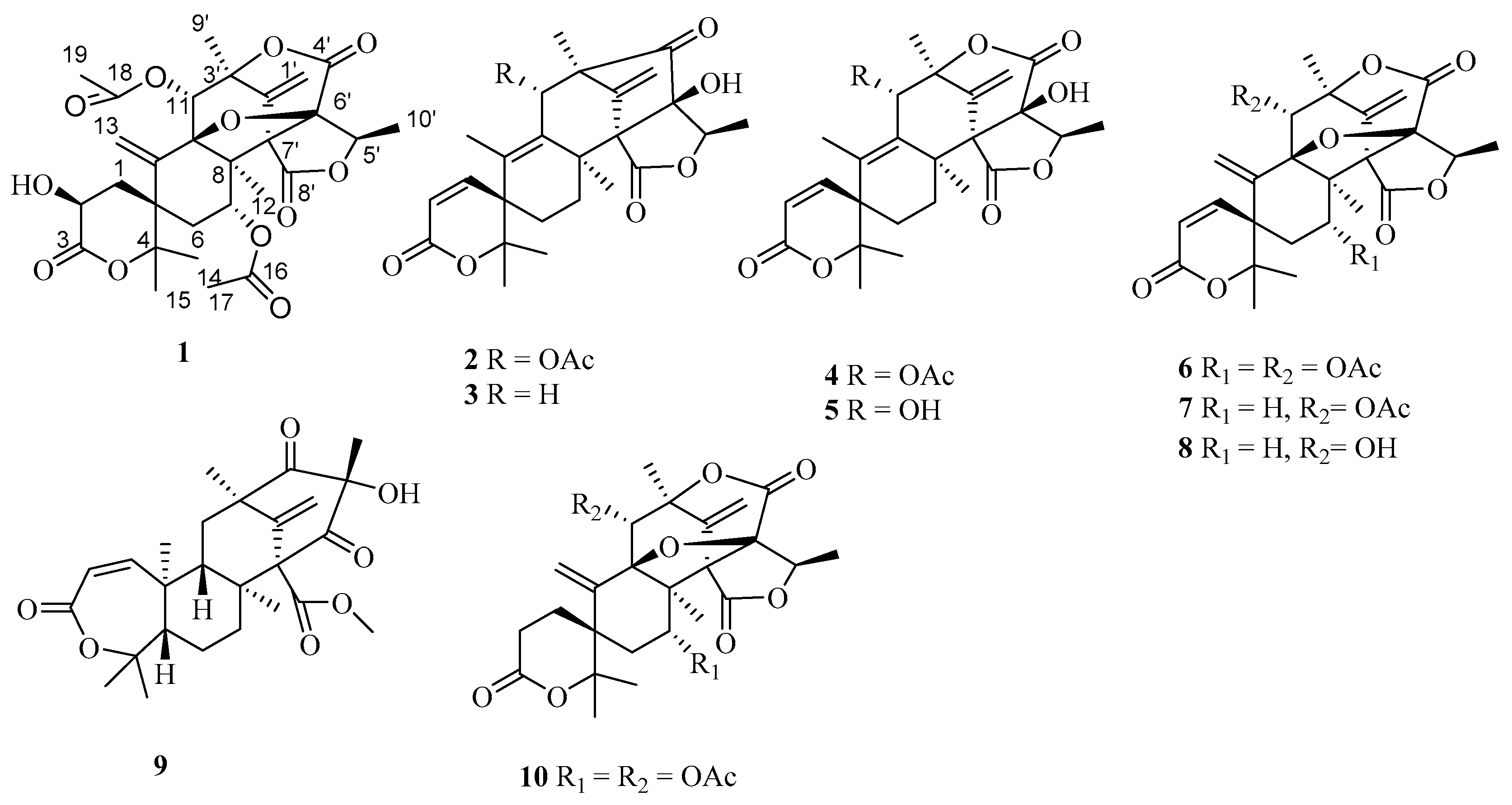
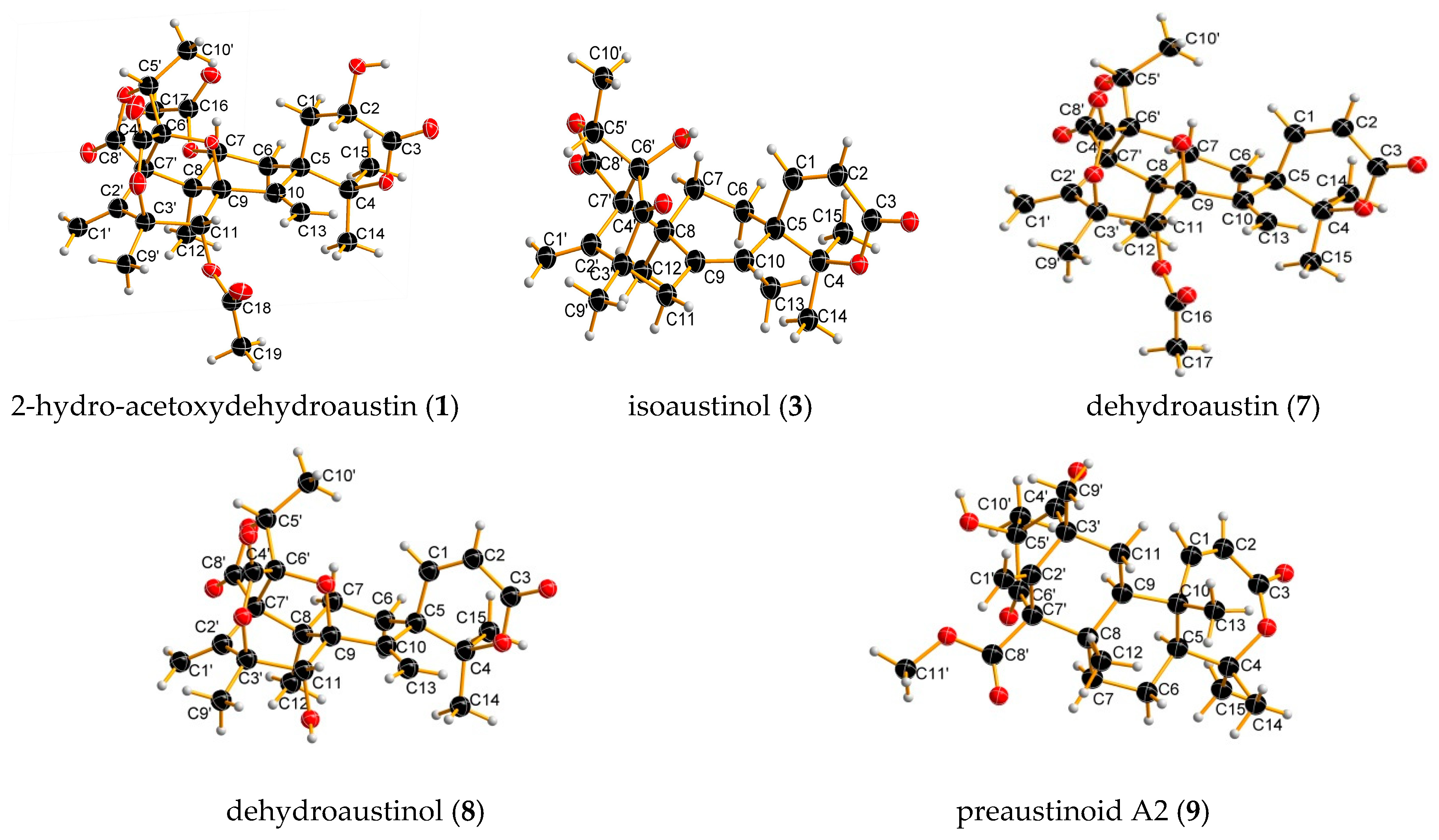
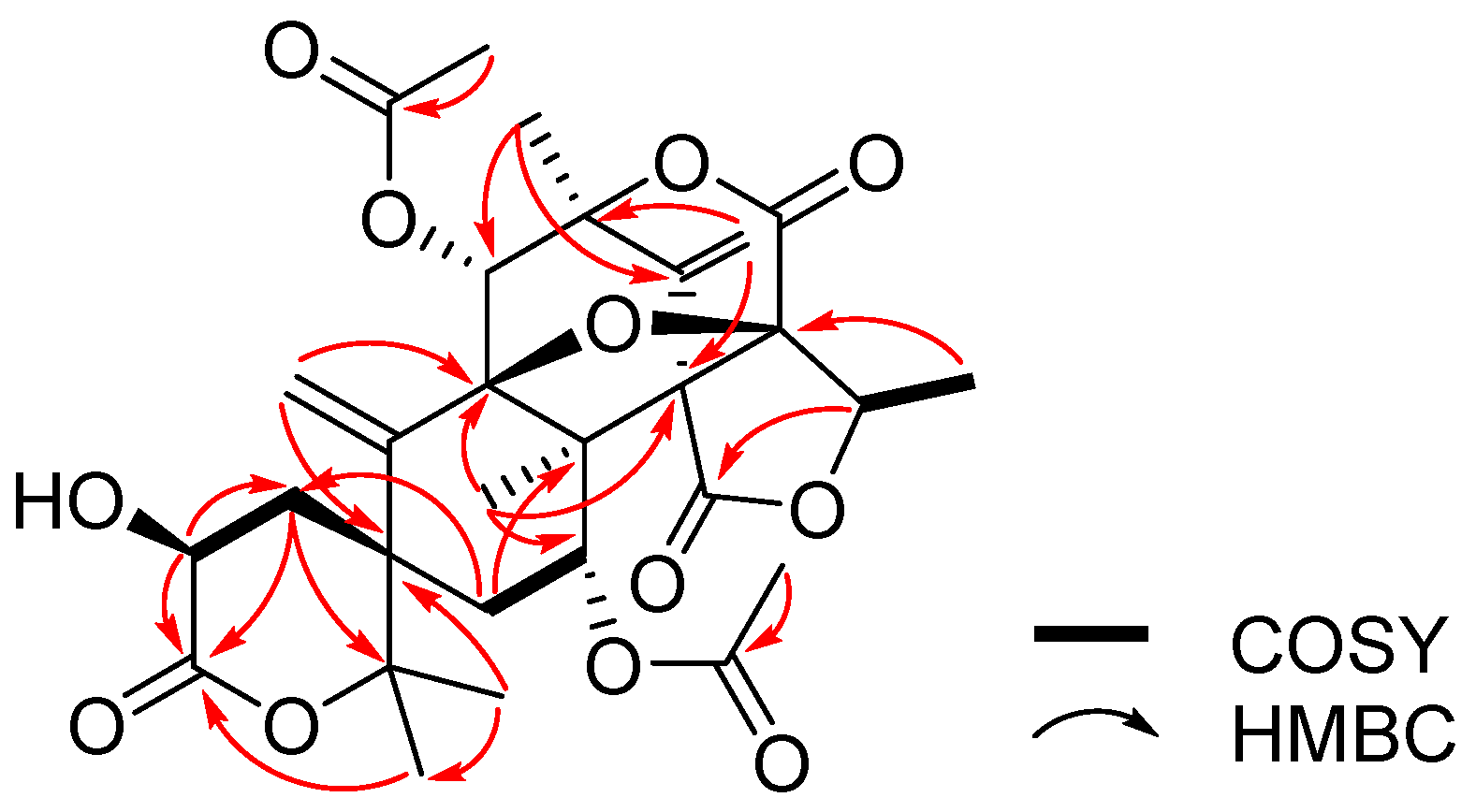
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 2.23, dd (11.5, 13.4) | 37.1, CH2 | 6.52, d (9.9) | 146.7, CH |
| 2.98, dd (7.4, 13.4) | ||||
| 2 | 4.28, dd (7.4, 11.5) | 64.8, CH | 6.03, d (9.9) | 120.0, CH |
| 3 | 174.9, C | 163.7, C | ||
| 4 | 90.0, C | 85.6, C | ||
| 5 | 46.1, C | 46.3, C | ||
| 6 | 1.76, dd (11.9, 13.0) | 36.1, CH2 | 2.66, td (13.5, 3.6) | 27.1, CH2 |
| 1.90, dd (3.9, 13.0) | 1.79, dt (13.5, 3.6) | |||
| 7 | 5.35, dd (3.9, 11.9) | 67.8, CH | 1.67,dd (12.2, 3.6) | 27.0, CH2 |
| 1.61,dd (12.2, 3.6) | ||||
| 8 | 56.6, C | 41.3, C | ||
| 9 | 93.2, C | 134.8, C | ||
| 10 | 138.1, C | 138.1, C | ||
| 11 | 5.70, s | 74.3, CH | 5.74, s | 74.1, CH |
| 12 | 1.37, s | 12.0, CH3 | 1.57, s | 23.0, CH3 |
| 13 | 5.81, d (1.5) | 124.4, CH2 | 1.75, s | 15.2, CH3 |
| 5.85, d (1.5) | ||||
| 14 | 1.43, s | 23.5, CH3 | 1.37, s | 25.9, CH3 |
| 15 | 1.47, s | 27.4, CH3 | 1.20, s | 22.8, CH3 |
| 16 | 170.6, C | 2.04, s | 20.7, CH3 | |
| 17 | 2.07, s | 20.8, CH3 | 171.5, C | |
| 18 | 168.9, C | |||
| 19 | 2.05, s | 21.1, CH3 | ||
| 1′ | 5.73, s | 116.9, CH2 | 5.41, d (0.9) | 111.7, CH |
| 6.14, s | 5.33, d (0.9) | |||
| 2′ | 136.9, C | 142.6, C | ||
| 3′ | 82.5, C | 59.2, C | ||
| 4′ | 168.9, C | 209.5, C | ||
| 5′ | 5.26, q (6.9) | 76.6 d | 4.35, q (6.4) | 76.2, CH |
| 6′ | 85.5, C | 91.2, C | ||
| 7′ | 61.6, C | 66.4, C | ||
| 8′ | 167.3, C | 169.1, C | ||
| 9′ | 1.58, s | 19.6, CH3 | 1.25, s | 12.9, CH3 |
| 10′ | 1.70, d (6.9) | 13.8, CH3 | 1.30, d (6.4) | 12.7, CH3 |
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | HUP b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| >50 | >50 | 2.50 | >50 | >50 | >50 | 0.40 | 3.00 | >50 | >50 | 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Cui, H.; Liu, X.; Xiao, Z.; Wen, S.; She, Z.; Huang, X. Acetylcholinesterase Inhibitory Meroterpenoid from a Mangrove Endophytic Fungus Aspergillus sp. 16-5c. Molecules 2017, 22, 727. https://doi.org/10.3390/molecules22050727
Long Y, Cui H, Liu X, Xiao Z, Wen S, She Z, Huang X. Acetylcholinesterase Inhibitory Meroterpenoid from a Mangrove Endophytic Fungus Aspergillus sp. 16-5c. Molecules. 2017; 22(5):727. https://doi.org/10.3390/molecules22050727
Chicago/Turabian StyleLong, Yuhua, Hui Cui, Xinglie Liu, Ze’en Xiao, Shitong Wen, Zhigang She, and Xishan Huang. 2017. "Acetylcholinesterase Inhibitory Meroterpenoid from a Mangrove Endophytic Fungus Aspergillus sp. 16-5c" Molecules 22, no. 5: 727. https://doi.org/10.3390/molecules22050727






