Template Effect of the Graphene Moiré Lattice on Phthalocyanine Assembly
Abstract
:1. Introduction
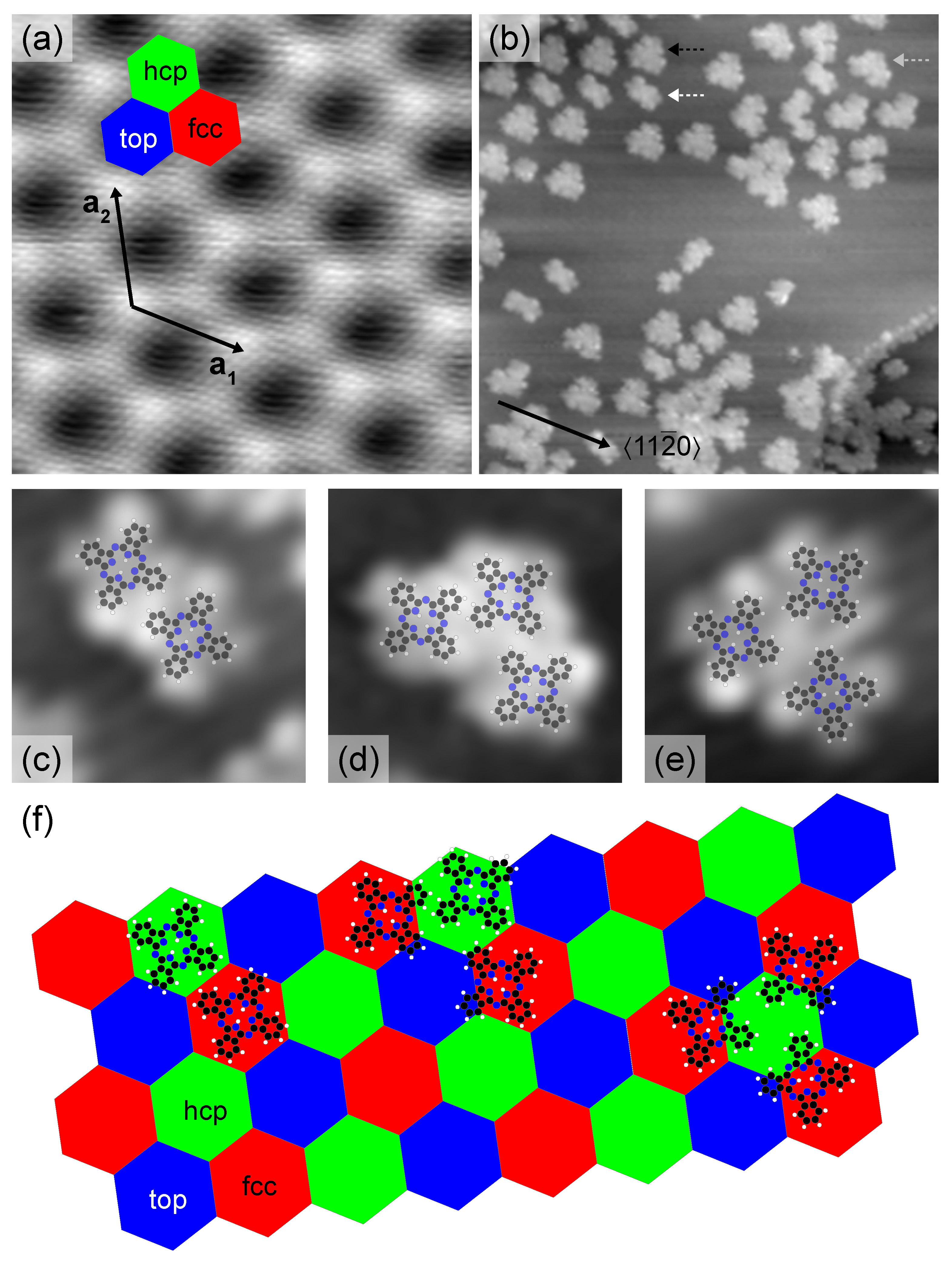
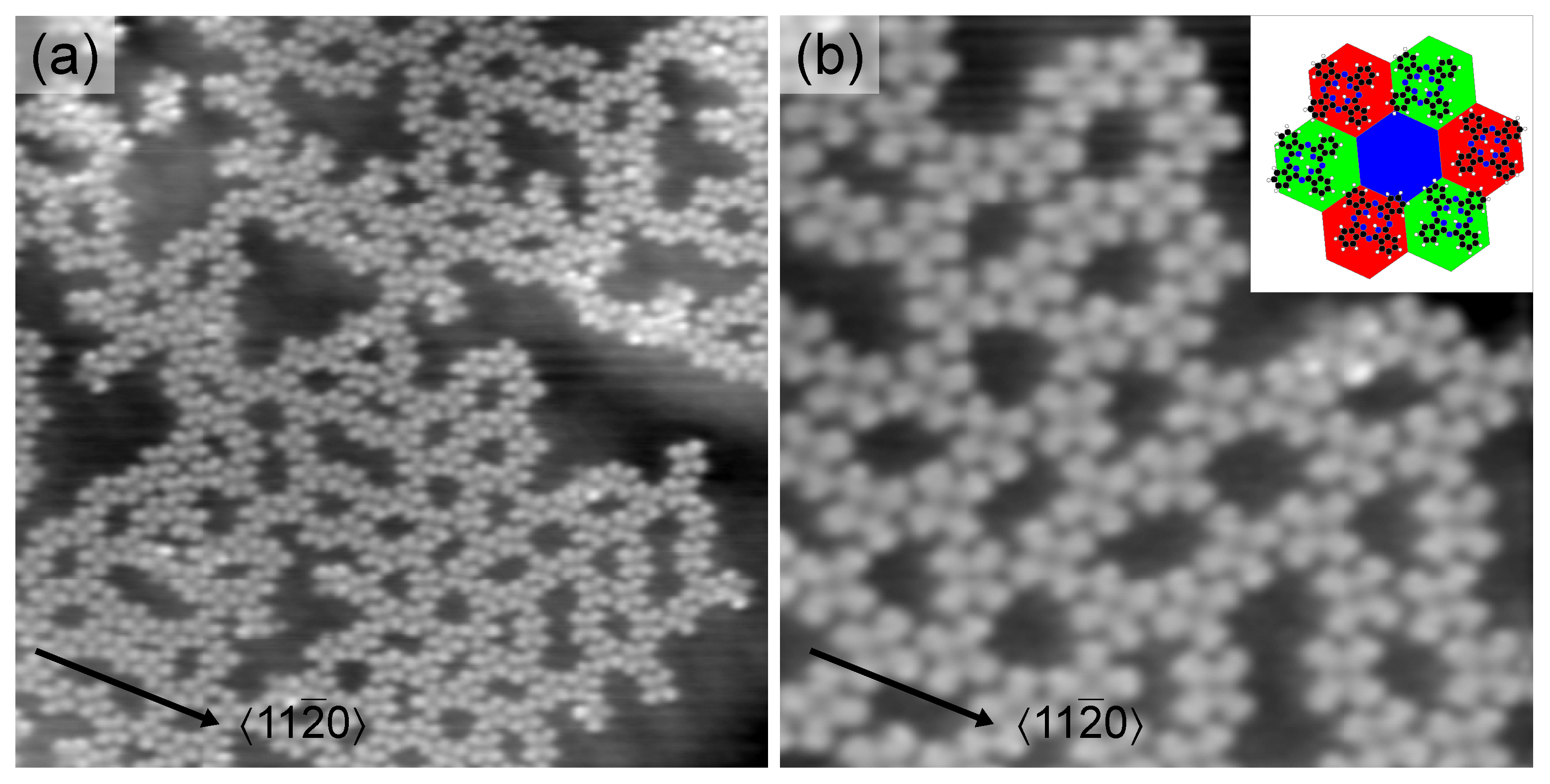
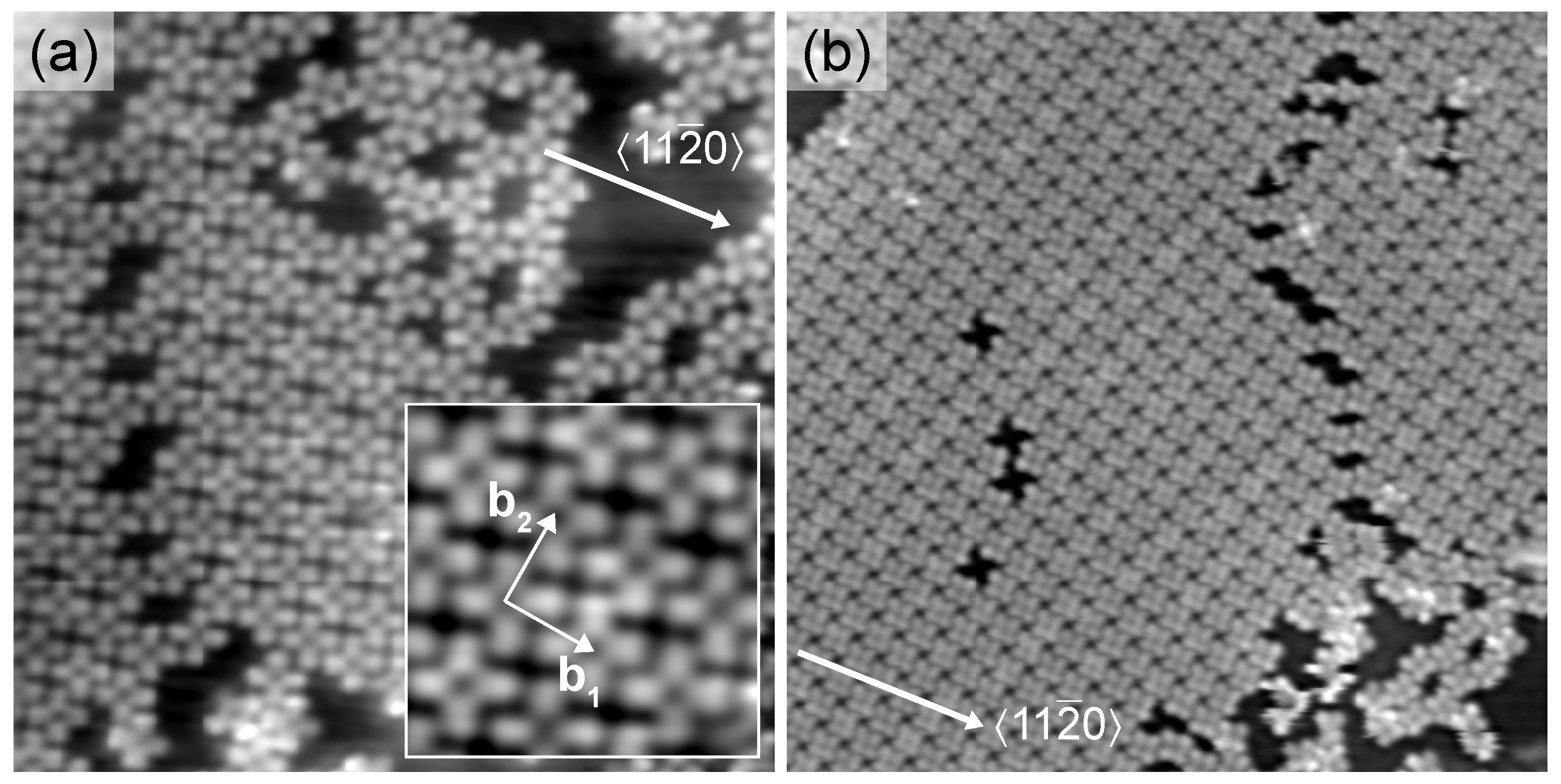
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Natterer, F.D.; Yang, K.; Paul, W.; Willke, P.; Choi, T.; Greber, T.; Heinrich, A.J.; Lutz, C.P. Reading and writing single-atom magnets. Nature 2017, 543, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Kröger, J.; Berndt, R.; Hofer, W.A. Pushing and pulling a Sn ion through an adsorbed phthalocyanine molecule. J. Am. Chem. Soc. 2009, 131, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Somorjai, G.A. Nanoscale advances in catalysis and energy applications. Nano Lett. 2010, 10, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Valden, M.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T. The active site in nanoparticle gold catalysis. Science 2004, 306, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Pellin, M.J.; Greeley, J.P.; Marshall, C.L.; Curtiss, L.A.; Ballentine, G.A.; Elam, J.W.; Catillon-Mucherie, S.; Redfern, P.C.; Mehmood, F.; Zapol, P. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Chambliss, D.D.; Wilson, R.J.; Chiang, S. Nucleation of ordered Ni island arrays on Au(111) by surface-lattice dislocations. Phys. Rev. Lett. 1991, 66, 1721–1724. [Google Scholar] [CrossRef] [PubMed]
- Brune, H. Microscopic view of epitaxial growth: Nucleation and aggregation. Surf. Sci. Rep. 1998, 31, 121–229. [Google Scholar] [CrossRef]
- Shen, J.; Skomski, R.; Klaua, M.; Jenniches, H.; Manoharan, S.S.; Kirschner, J. Magnetism in one dimension: Fe on Cu(111). Phys. Rev. B 1997, 56, 2340–2343. [Google Scholar] [CrossRef]
- Gambardella, P.; Blanc, M.; Brune, H.; Kuhnke, K.; Kern, K. One-dimensional metal chains on Pt vicinal surfaces. Phys. Rev. B 2000, 61, 2254–2262. [Google Scholar] [CrossRef]
- Lin, J.L.; Petrovykh, D.Y.; Kirakosian, A.; Rauscher, H.; Himpsel, F.J.; Dowben, P.A. Self-assembled Fe nanowires using organometallic chemical vapor deposition and CaF2 masks on stepped Si(111). Appl. Phys. Lett. 2001, 78, 829–831. [Google Scholar] [CrossRef]
- Repain, V.; Baudot, G.; Ellmer, H.; Rousset, S. Two-dimensional long-range-ordered growth of uniform cobalt nanostructures on a Au(111) vicinal template. Europhys. Lett. 2002, 58, 730–736. [Google Scholar] [CrossRef]
- Ya Ohno, S.; Yagyuu, K.; Nakatsuji, K.; Komori, F. One-dimensional self-organized patterns on vicinal Cu(001)- c (2×2)N surfaces. Jpn. J. Appl. Phys. 2002, 41, L1243–L1246. [Google Scholar] [CrossRef]
- Néel, N.; Kröger, J.; Berndt, R. Highly periodic fullerene nanomesh. Adv. Mater. 2006, 18, 174–177. [Google Scholar] [CrossRef]
- Néel, N.; Kröger, J.; Berndt, R. Fullerene nanowires on a vicinal gold surface. Appl. Phys. Lett. 2006, 88, 163101. [Google Scholar] [CrossRef]
- Kröger, J.; Jensen, H.; Berndt, R.; Rurali, R.; Lorente, N. Molecular orbital shift of perylenetetracarboxylic-dianhydride on gold. Chem. Phys. Lett. 2007, 438, 249–253. [Google Scholar] [CrossRef]
- Kröger, J.; Jensen, H.; Néel, N.; Berndt, R. Self-organization of cobalt-phthalocyanine on a vicinal gold surface revealed by scanning tunnelling microscopy. Surf. Sci. 2007, 601, 4180–4184. [Google Scholar] [CrossRef]
- Böhringer, M.; Morgenstern, K.; Schneider, W.D.; Berndt, R.; Mauri, F.; De Vita, A.; Car, R. Two-dimensional self-assembly of supramolecular clusters and chains. Phys. Rev. Lett. 1999, 83, 324–327. [Google Scholar] [CrossRef]
- Barth, J.V.; Weckesser, J.; Cai, C.; Günter, P.; Bürgi, L.; Jeandupeux, O.; Kern, K. Building supramolecular nanostructures at surfaces by hydrogen bonding. Angew. Chem. Int. Ed. 2000, 39, 1230–1234. [Google Scholar] [CrossRef]
- Theobald, J.A.; Oxtoby, N.S.; Phillips, M.A.; Champness, N.R.; Beton, P.H. Controlling molecular deposition and layer structure with supramolecular surface assemblies. Nature 2003, 424, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Makoudi, Y.; Duverger, E.; Arab, M.; Chérioux, F.; Ample, F.; Rapenne, G.; Bouju, X.; Palmino, F. Room-temperature electronic template effect of the SmSi(111)-8×2 interface for self-alignment of organic molecules. ChemPhysChem 2008, 9, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, T.G.; Néel, N.; Kröger, J.; Berndt, R. Spatial modulation of d states in a nanoscale Co island. Chem. Phys. Lett. 2009, 484, 59–63. [Google Scholar] [CrossRef]
- Lin, X.; Nilius, N. Self-assembly of MgPc molecules on polar FeO thin films. J. Phys. Chem. C 2008, 112, 15325–15328. [Google Scholar] [CrossRef]
- Nilius, N.; Rienks, E.D.L.; Rust, H.P.; Freund, H.J. Self-organization of gold atoms on a polar FeO(111) surface. Phys. Rev. Lett. 2005, 95, 066101. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.T.; Bleikamp, S.; Feibelman, P.J.; Michely, T. Two-dimensional Ir cluster lattice on a graphene moiré on Ir(111). Phys. Rev. Lett. 2006, 97, 215501. [Google Scholar] [CrossRef] [PubMed]
- Preobrajenski, A.B.; Ng, M.L.; Vinogradov, A.S.; Mårtensson, N. Controlling graphene corrugation on lattice-mismatched substrates. Phys. Rev. B 2008, 78, 073401. [Google Scholar] [CrossRef]
- Marchini, S.; Günther, S.; Wintterlin, J. Scanning tunneling microscopy of graphene on Ru(0001). Phys. Rev. B 2007, 76, 075429. [Google Scholar] [CrossRef]
- Wintterlin, J.; Bocquet, M.L. Graphene on metal surfaces. Surf. Sci. 2009, 603, 1841–1852. [Google Scholar] [CrossRef]
- Corso, M.; Auwärter, W.; Muntwiler, M.; Tamai, A.; Greber, T.; Osterwalder, J. Boron nitride nanomesh. Science 2004, 303, 217–220. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.T.; Gerber, T.; Busse, C.; Myslivecek, J.; Coraux, J.; Michely, T. A versatile fabrication method for cluster superlattices. New J. Phys. 2009, 11, 103045. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, M.; Huang, L.; Liu, F.; Gao, H.J. Directed self-assembly of monodispersed platinum nanoclusters on graphene moiré template. Appl. Phys. Lett. 2009, 95, 093106. [Google Scholar] [CrossRef]
- Donner, K.; Jakob, P. Structural properties and site specific interactions of Pt with the graphene/Ru(0001) moiré overlayer. J. Chem. Phys. 2009, 131, 164701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, F.; Goodman, D.W. Deposition of metal clusters on single-layer graphene/Ru(0001): Factors that govern cluster growth. Surf. Sci. 2010, 604, L31–L38. [Google Scholar] [CrossRef]
- Sutter, E.; Albrecht, P.; Wang, B.; Bocquet, M.L.; Wu, L.; Sutter, E. Arrays of Ru nanoclusters with narrow size distribution templated by monolayer graphene on Ru. Surf. Sci. 2011, 605, 1676–1684. [Google Scholar] [CrossRef]
- Sicot, M.; Bouvron, S.; Zander, O.; Rüdiger, U.; Dedkov, Y.S.; Fonin, M. Nucleation and growth of nickel nanoclusters on graphene moiré on Rh(111). Appl. Phys. Lett. 2010, 96, 093115. [Google Scholar] [CrossRef]
- Cavallin, A.; Pozzo, M.; Africh, C.; Baraldi, A.; Vesselli, E.; Dri, C.; Comelli, G.; Larciprete, R.; Lacovig, P.; Lizzit, S.; et al. Local electronic structure and density of edge and facet atoms at Rh nanoclusters self-assembled on a graphene template. ACS Nano 2012, 6, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.; Coletti, C.; Iwasaki, T.; Zakharov, A.A.; Starke, U. Quasi-free-standing epitaxial graphene on SiC obtained by hydrogen intercalation. Phys. Rev. Lett. 2009, 103, 246804. [Google Scholar] [CrossRef] [PubMed]
- Pacilé, D.; Leicht, P.; Papagno, M.; Sheverdyaeva, P.M.; Moras, P.; Carbone, C.; Krausert, K.; Zielke, L.; Fonin, M.; Dedkov, Y.S.; et al. Artificially lattice-mismatched graphene/metal interface: Graphene/Ni/Ir(111). Phys. Rev. B 2013, 87, 035420. [Google Scholar] [CrossRef]
- Halle, J.; Néel, N.; Kröger, J. Filling the gap: Li-intercalated graphene on Ir(111). J. Phys. Chem. C 2016, 120, 5067–5073. [Google Scholar] [CrossRef]
- Goriachko, A.; He, Y.; Knapp, M.; Over, H.; Corso, M.; Brugger, T.; Berner, S.; Osterwalder, J.; Greber, T. Self-assembly of a hexagonal boron nitride nanomesh on Ru(0001). Langmuir 2007, 23, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Goriachko, A.; He, Y.B.; Over, H. Complex growth of nanoAu on BN nanomeshes supported by Ru(0001). J. Phys. Chem. C 2008, 112, 8147–8152. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, H.; Jiang, Y.; Pan, Y.; Gao, M.; Xiao, W.; Gao, H.J. Tunability of supramolecular kagome lattices of magnetic phthalocyanines using graphene-based moiré patterns as templates. J. Am. Chem. Soc. 2009, 131, 14136–14137. [Google Scholar] [CrossRef] [PubMed]
- Roos, M.; Künzel, D.; Uhl, B.; Huang, H.H.; Brandao Alves, O.; Hoster, H.E.; Gross, A.; Behm, R.J. Hierarchical interactions and their influence upon the adsorption of organic molecules on a graphene film. J. Am. Chem. Soc. 2011, 133, 9208–9211. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Perkins, E.W.; Smith, N.A.; Saywell, A.; Goretzki, G.; Phillips, A.G.; Argent, S.P.; Sachdev, H.; Müller, F.; Hüfner, S.; et al. Supramolecular assemblies formed on an epitaxial graphene superstructure. Angew. Chem. Int. Ed. 2010, 49, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yeo, P.S.E.; Zheng, Y.; Yang, Z.; Bao, Q.; Gan, C.K.; Loh, K.P. Using the graphene moiré pattern for the trapping of C60 and homoepitaxy of graphene. ACS Nano 2012, 6, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Bazarnik, M.; Brede, J.; Decker, R.; Wiesendanger, R. Tailoring molecular self-assembly of magnetic phthalocyanine molecules on Fe- and Co-intercalated graphene. ACS Nano 2013, 7, 11341–11349. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, P.; Hämäläinen, S.K.; Banerjee, K.; Häkkinen, P.; Ijäs, M.; Harju, A.; Liljeroth, P. Molecular self-assembly on graphene on SiO2 and h-BN substrates. Nano Lett. 2013, 13, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, P.; Hämäläinen, S.K.; Ijäs, M.; Harju, A.; Liljeroth, P. Self-assembly and orbital imaging of metal phthalocyanines on a graphene model surface. J. Phys. Chem. C 2014, 118, 13320–13325. [Google Scholar] [CrossRef]
- Banerjee, K.; Kumar, A.; Canova, F.F.; Kezilebieke, S.; Foster, A.S.; Liljeroth, P. Flexible self-assembled molecular templates on graphene. J. Phys. Chem. C 2016, 120, 8772–8780. [Google Scholar] [CrossRef]
- Li, J.; Gottardi, S.; Solianyk, L.; Moreno-López, J.C.; Stöhr, M. 1,3,5-benzenetribenzoic acid on Cu(111) and graphene/Cu(111): A comparative STM study. J. Phys. Chem. C 2016, 120, 18093–18098. [Google Scholar] [CrossRef] [PubMed]
- Dil, H.; Lobo-Checa, J.; Laskowski, R.; Blaha, P.; Berner, S.; Osterwalder, J.; Greber, T. Surface trapping of atoms and molecules with dipole rings. Science 2008, 319, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Berner, S.; Corso, M.; Widmer, R.; Groening, O.; Laskowski, R.; Blaha, P.; Schwarz, K.; Goriachko, A.; Over, H.; Gsell, S.; et al. Boron nitride nanomesh: Functionality from a corrugated monolayer. Angew. Chem. Int. Ed. 2007, 46, 5115–5119. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bocquet, M.L. Monolayer graphene and h-BN on metal substrates as versatile templates for metallic nanoclusters. J. Phys. Chem. Lett. 2011, 2, 2341–2345. [Google Scholar] [CrossRef]
- Yang, K.; Xiao, W.D.; Jiang, Y.H.; Zhang, H.G.; Liu, L.W.; Mao, J.H.; Zhou, H.T.; Du, S.X.; Gao, H.J. Molecule-substrate coupling between metal phthalocyanines and epitaxial graphene grown on Ru(0001) and Pt(111). J. Phys. Chem. C 2012, 116, 14052–14056. [Google Scholar] [CrossRef]
- Hämäläinen, S.K.; Stepanova, M.; Drost, R.; Liljeroth, P.; Lahtinen, J.; Sainio, J. Self-assembly of cobalt-phthalocyanine molecules on epitaxial graphene on Ir(111). J. Phys. Chem. C 2012, 116, 20433–20437. [Google Scholar] [CrossRef]
- N’Diaye, A.T.; Coraux, J.; Plasa, T.N.; Busse, C.; Michely, T. Structure of epitaxial graphene on Ir(111). New J. Phys. 2008, 10, 043033. [Google Scholar] [CrossRef]
- Voloshina, E.N.; Fertitta, E.; Garhofer, A.; Mittendorfer, F.; Fonin, M.; Thissen, A.; Dedkov, Y.S. Electronic structure and imaging contrast of graphene moiré on metals. Sci. Rep. 2013, 3, 1072. [Google Scholar] [CrossRef] [PubMed]
- Endlich, M.; Gozdzik, S.; Néel, N.; da Rosa, A.L.; Frauenheim, T.; Wehling, T.O.; Kröger, J. Phthalocyanine adsorption to graphene on Ir(111): Evidence for decoupling from vibrational spectroscopy. J. Chem. Phys. 2014, 141, 184308. [Google Scholar] [CrossRef] [PubMed]
- Néel, N.; Lattelais, M.; Bocquet, M.L.; Kröger, J. Depopulation of single-phthalocyanine molecular orbitals upon pyrrolic-hydrogen abstraction on graphene. ACS Nano 2016, 10, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bocquet, M.L.; Marchini, S.; Günther, S.; Wintterlin, J. Chemical origin of a graphene moiré overlayer on Ru(0001). Phys. Chem. Chem. Phys. 2008, 10, 3530–3534. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, S.J.; Kröger, J.; Wang, B.; Bocquet, M.L.; Lorente, N.; Berndt, R. Graphene on Ru(0001): Contact formation and chemical reactivity on the atomic scale. Phys. Rev. Lett. 2010, 105, 236101. [Google Scholar] [CrossRef] [PubMed]
- Wamg, Y.; Ge, X.; Manzano, C.; Kröger, J.; Berndt, R.; Hofer, W.A.; Tang, H.; Cerda, J. Supramolecular patterns controlled by electron interference and direct intermolecular interactions. J. Am. Chem. Soc. 2009, 131, 10400–10402. [Google Scholar] [CrossRef] [PubMed]
- Hattab, H.; N’Diaye, A.T.; Wall, D.; Jnawali, G.; Coraux, J.; Busse, C.; van Gastel, R.; Poelsema, B.; Michely, T.; zu Heringdorf, F.J.M.; von Hoegen, M.H. Growth temperature dependent graphene alignment on Ir(111). Appl. Phys. Lett. 2011, 98, 141903. [Google Scholar] [CrossRef]
- Endlich, M.; Molina-Sánchez, A.; Wirtz, L.; Kröger, J. Screening of electron-phonon coupling in graphene on Ir(111). Phys. Rev. B 2013, 88, 204403. [Google Scholar] [CrossRef]
- Endlich, M.; Miranda, H.P.C.; Molina-Sánchez, A.; Wirtz, L.; Kröger, J. Moiré-induced replica of graphene phonons on Ir(111). Ann. Phys. 2014, 526, 372–380. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Néel, N.; Kröger, J. Template Effect of the Graphene Moiré Lattice on Phthalocyanine Assembly. Molecules 2017, 22, 731. https://doi.org/10.3390/molecules22050731
Néel N, Kröger J. Template Effect of the Graphene Moiré Lattice on Phthalocyanine Assembly. Molecules. 2017; 22(5):731. https://doi.org/10.3390/molecules22050731
Chicago/Turabian StyleNéel, Nicolas, and Jörg Kröger. 2017. "Template Effect of the Graphene Moiré Lattice on Phthalocyanine Assembly" Molecules 22, no. 5: 731. https://doi.org/10.3390/molecules22050731




