Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proline Profile of the Different Unifloral Honeys
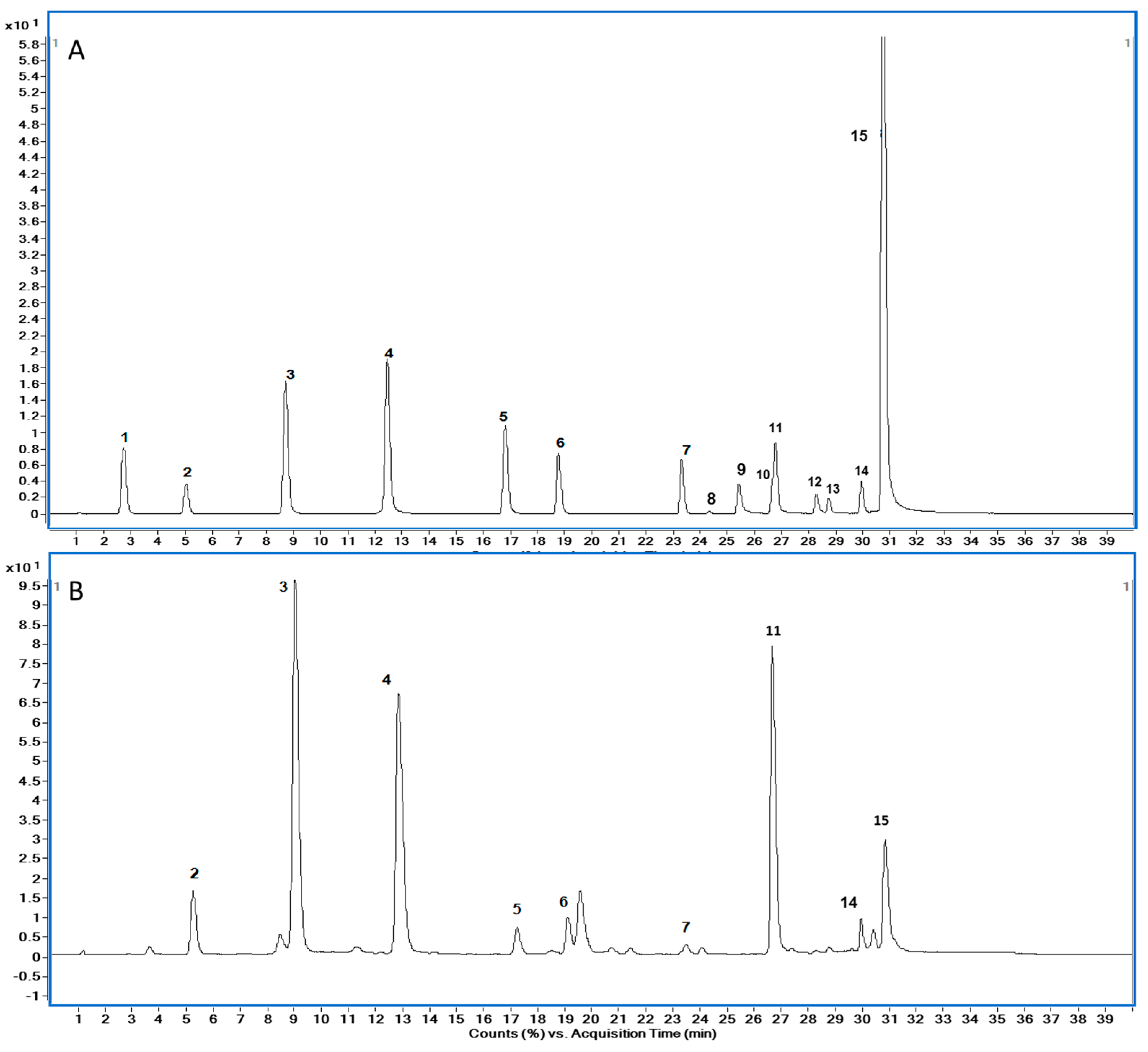
2.2. Phenolic Compounds in Four Different Honey Types
2.3. Multivariate Statistical Analysis
2.3.1. Principal Component Analysis (PCA)
2.3.2. Hierarchical Clustering (HCA)
2.4. Discrimination of Honey Samples Based on Linear Discriminate Analysis (LDA)
3. Materials and Methods
3.1. Honey Samples and Pollen Analysis
3.2. Chemicals
3.3. Solid-Phase Extraction and HPLC–ESI-MS/MS Analysis
3.4. HPLC analysis of Proline
3.5. Data Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagai, T.; Inoue, R.; Kanamori, N.; Suzuki, N.; Nagashima, T. Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem. 2006, 97, 256–262. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Asokan, M.K.; Subramanian, A.P.; John, A.A.; Supriyanto, E.; Razak, S.I.A.; Marvibaigi, M. A review on antiproliferative and apoptotic activities of natural honey. Anticancer Agents Med. Chem. 2015, 15, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Oliveira Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Yaoa, L.; Jiang, Y.M.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in australian melaleuca, guioa, lophostemon, banksia and helianthus honeys and their potential for floral authentication. Food Res. Int. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of markers of botanical origin and other compounds extracted from unifloral honeys. J. Agric. Food Chem. 2013, 61, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Kadar, M.; Juan-Borras, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and classification of greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Zhao, J.; Du, X.; Cheng, N.; Chen, L.; Xue, X.; Zhao, J.; Wu, L.; Cao, W. Identification of monofloral honeys using hplc–ecd and chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. Hplc flavonoid profiles as markers for the botanical origin of european unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Oelschlaegel, S.; Gruner, M.; Wang, P.N.; Boettcher, A.; Koelling-Speer, I.; Speer, K. Classification and characterization of manuka honeys based on phenolic compounds and methylglyoxal. J. Agric. Food Chem. 2012, 60, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Xue, X.F.; Du, X.J.; Cheng, N.; Chen, L.Z.; Zhao, J.; Zheng, J.B.; Cao, W. Identification of acacia honey adulteration with rape honey using liquid chromatography-electrochemical detection and chemometrics. Food Anal. Method 2014, 7, 2003–2012. [Google Scholar] [CrossRef]
- Zhou, J.H.; Yao, L.H.; Li, Y.; Chen, L.Z.; Wu, L.M.; Zhao, J. Floral classification of honey using liquid chromatography-diode array detection-tandem mass spectrometry and chemometric analysis. Food Chem. 2014, 145, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, 4–17. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Giroud, B.; Albert, M.; Lheritier, J.; Grenier-Loustalot, M.F. Characterization of honey amino acid profiles using high-pressure liquid chromatography to control authenticity. Anal. Bioanal. Chem. 2004, 378, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Keckes, J.; Trifkovic, J.; Andric, F.; Jovetic, M.; Tesic, Z.; Milojkovic-Opsenica, D. Amino acids profile of serbian unifloral honeys. J. Sci. Food Agric. 2013, 93, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Oddo, L.; Piro, R.; Ivanov, T.; Piškulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; Von Der OHE, W.; Von Der Ohe, K. Main european unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Chen, H.; Fan, C.L.; Chang, Q.Y.; Pang, G.F.; Hu, X.Y.; Lu, M.L.; Wang, W.W. Chemometric determination of the botanical origin for chinese honeys on the basis of mineral elements determined by ICP-MS. J. Agric. Food Chem. 2014, 62, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zheng, Y.; Xu, B. Floral origin identification and amino acid profiles of chinese unifloral honeys. Int. J. Food Prop. 2013, 16, 1860–1870. [Google Scholar] [CrossRef]
- Wu, Z.B.; Chen, L.Z.; Wu, L.M.; Xue, X.F.; Zhao, J.; Li, Y.; Ye, Z.H.; Lin, G.H. Classification of chinese honeys according to their floral origins using elemental and stable isotopic compositions. J. Agric. Food Chem. 2015, 63, 5388–5394. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, L.; Chang, Q.; Peng, T.; Hu, X.; Fan, C.; Pang, G.; Lu, M.; Wang, W. Discrimination of botanical origins for chinese honey according to free amino acids content by high-performance liquid chromatography with fluorescence detection with chemometric approaches. J. Sci. Food Agric. 2017, 97, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Senyuva, H.Z.; Gilbert, J.; Silici, S.; Charlton, A.; Dal, C.; Gurel, N.; Cimen, D. Profiling turkish honeys to determine authenticity using physical and chemical characteristics. J. Agric. Food Chem. 2009, 57, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Gardini, S.; Marcazzan, G.L.; Caboni, M.F. Buckwheat honeys: Screening of composition and properties. Food Chem. 2013, 141, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin c content and antioxidant activity of italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Galkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The determination of phenolic profiles of serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Cheng, N.; Gao, H.; Wang, B.; Wei, Y.; Cao, W. Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem. Toxicol. 2012, 50, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Guyot, C.; Scheirman, V.; Collin, S. Floral origin markers of heather honeys: Calluna vulgaris and erica arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and antioxidative activities in buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruška, A.; Kornyšova, O.; Charczun, N.; Ligor, M.; Buszewski, B. Quantitative and qualitative determination of phenolic compounds in honey. Chem. Technol. 2009, 52, 74–80. [Google Scholar]
- Lachman, J.; Hejtmánková, A.; Sýkora, J.; Karban, J.; Orsák, M.; Rygerová, B. Contents of major phenolic and flavonoid antioxidants in selected czech honey. Czech J. Food Sci. 2010, 28, 412–426. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Song, X.Y.; Yao, Y.F.; Yang, W.D. Pollen analysis of natural honeys from the central region of Shanxi, North China. PLoS ONE 2012, 7, e49545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Xue, X.; Wu, L.; Chen, L.; Zhang, J.; Yang, S. Fluorometric determination of proline in honey by high-performance liquid chromatography after pre-column derivatization with 7-fluoro-4-nitrobenzo-2-oxa-1, 3-diazole (NBD-F). Anal. Methods 2015, 7, 7625–7630. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucl. Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The honey samples are available from the authors for limited time. |
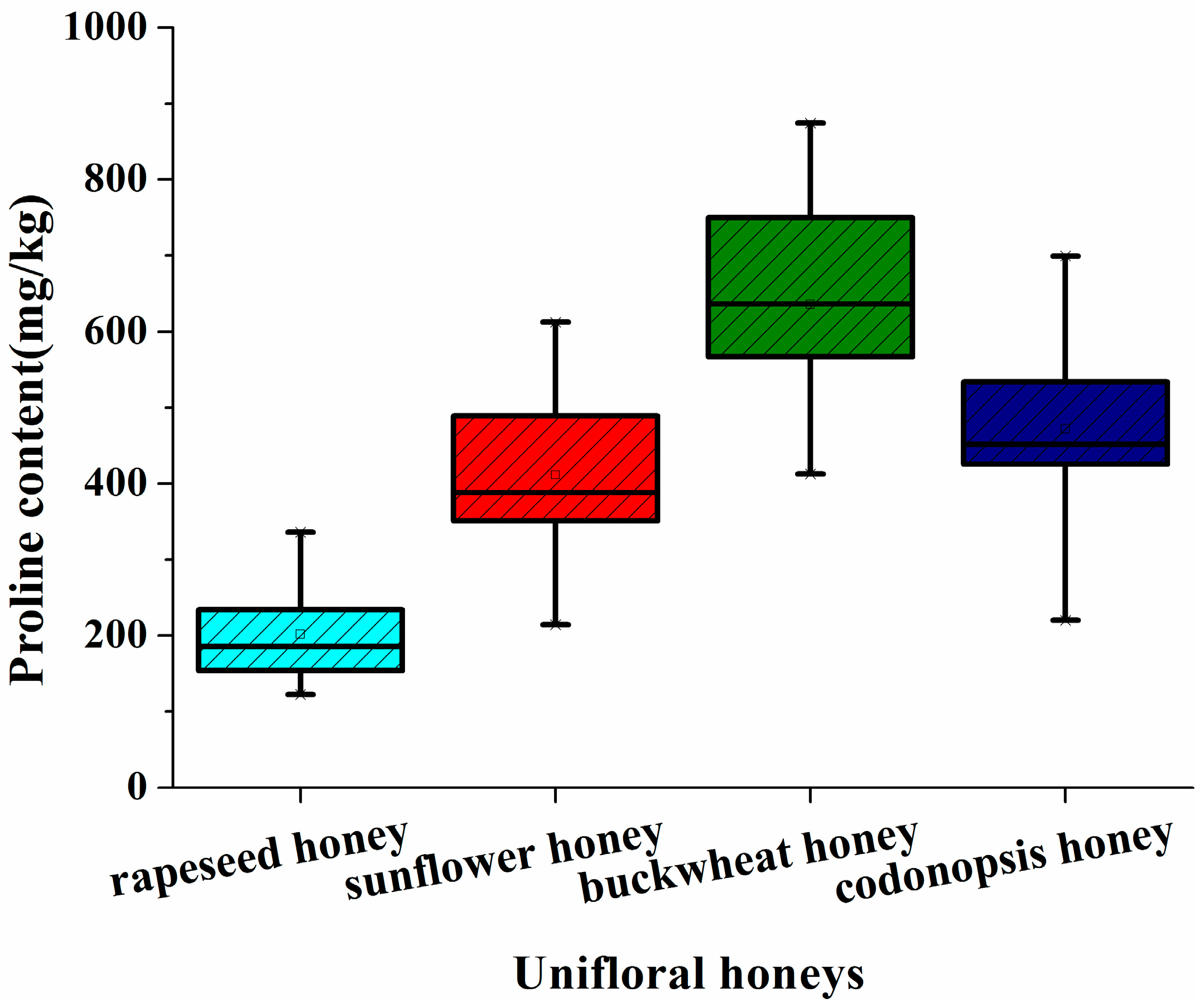
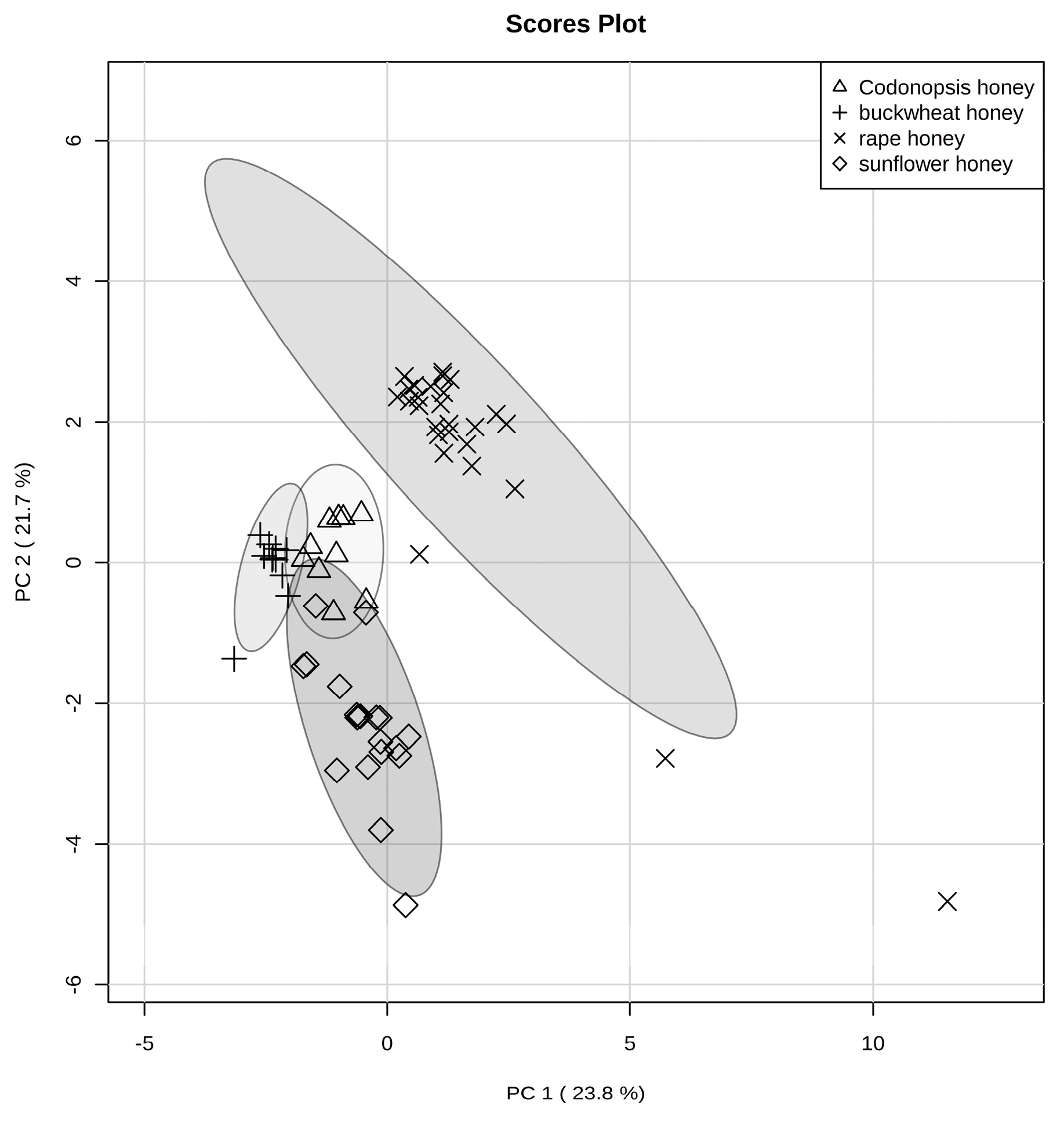
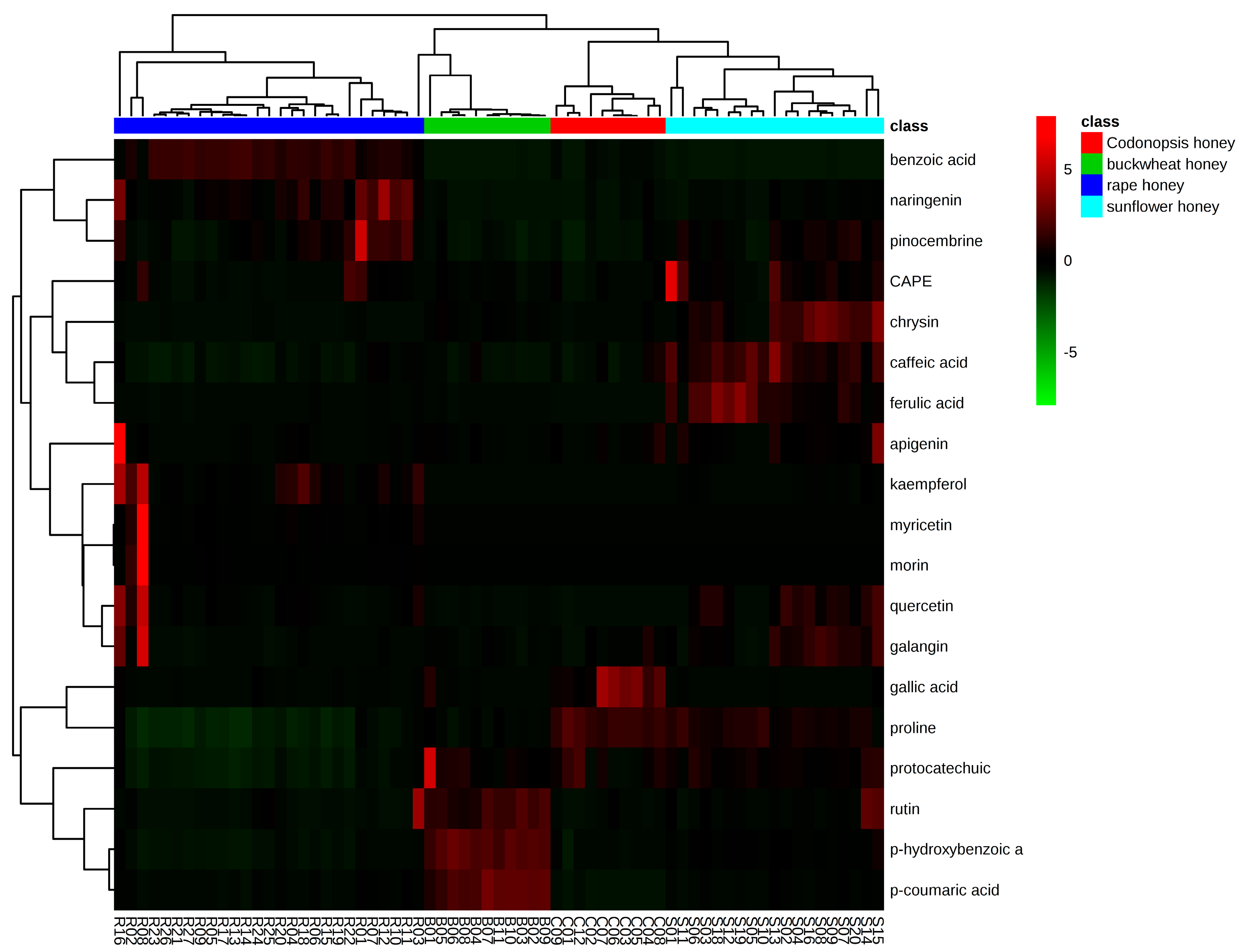
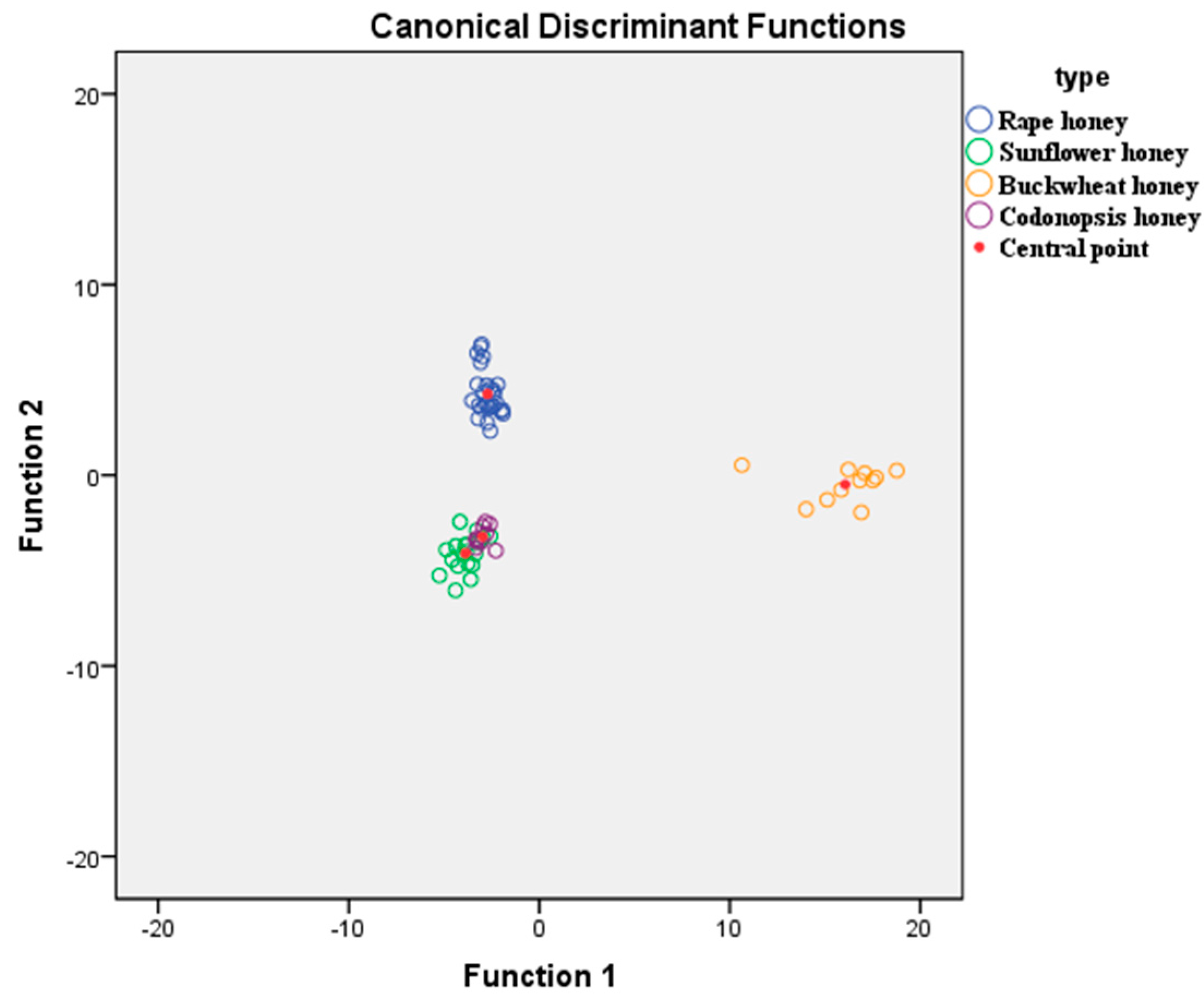

| Compounds | Rapeseed Honey (n = 27) | Sunflower Honey (n = 19) | Buckwheat Honey (n = 11) | Codonopsis Honey (n = 10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | |
| Gallic acid | 0.080 | 0.003 | 0.015 ± 0.018 b | 0.088 | 0.002 | 0.011 ± 0.020 b | 0.324 | 0.001 | 0.039 ± 0.086 b | 0.3 | 0.012 | 0.157 ± 0.103 a |
| Protocatechuic acid | 0.118 | 0.033 | 0.066 ± 0.021 b | 0.262 | 0.082 | 0.133 ± 0.050 b | 1.314 | 0.017 | 0.361 ± 0.324 a | 0.198 | 0.042 | 0.096 ± 0.051 b |
| p-Hydroxybenzoic acid | 1.220 | 0.431 | 0.726 ± 0.169 b | 1.912 | 0.105 | 0.939 ± 0.369 b | 11.174 | 0.067 | 7.967 ± 3.764 a | 0.715 | 0.376 | 0.513 ± 0.111 b |
| Caffeic acid | 0.465 | 0.015 | 0.122 ± 0.095 b | 1.023 | 0.017 | 0.460 ± 0.195 a | 0.872 | 0.004 | 0.202 ± 0.223 b | 0.348 | 0.015 | 0.145 ± 0.110 b |
| p-Coumaric acid | 0.323 | 0.101 | 0.188 ± 0.054 b | 0.272 | ND | 0.138 ± 0.067 b | 3.188 | 0.022 | 2.101 ± 1.061 a | 0.051 | 0.002 | 0.017 ± 0.019 b |
| Ferulic acid | 0.066 | 0.013 | 0.032 ± 0.015 b | 0.449 | 0.067 | 0.217 ± 0.114 a | 0.100 | 0.016 | 0.038 ± 0.026 b | 0.01 | ND | 0.005 ± 0.003 b |
| Benzoic acid | 18.108 | 1.800 | 9.475 ± 4.479 a | 0.176 | 0.006 | 0.097 ± 0.031 b | 0.478 | 0.018 | 0.228 ± 0.121 b | 1.369 | 0.078 | 0.611 ± 0.364 b |
| Rutin | 0.248 | ND | 0.077 ± 0.049 b | 0.187 | ND | 0.049 ± 0.048 bc | 0.304 | 0.159 | 0.225 ± 0.095 a | 0.018 | ND | 0.018 ± 0.005 c |
| Myricetin | 0.507 | ND | 0.116 ± 0.097 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Morin | 11.115 | ND | 0.607 ± 2.133 a | 0.007 | ND | 0.004 ± 0.002 a | 0.015 | ND | 0.015 ± 0.004 a | ND | ND | ND |
| Quercetin | 5.184 | 0.022 | 0.402 ± 0.989 a | 0.659 | 0.002 | 0.280 ± 0.215 a | 0.154 | 0.001 | 0.046 ± 0.043 a | 0.016 | 0.004 | 0.092 ± 0.039 a |
| Naringenin | 0.032 | 0.001 | 0.013 ± 0.008 a | ND | ND | ND | ND | ND | ND | 0.004 | 0.002 | 0.003 ± 0.001 b |
| Kaempferol | 1.897 | 0.012 | 0.214 ± 0.367 a | 0.077 | ND | 0.026 ± 0.019 a | 0.026 | 0.011 | 0.019 ± 0.008 a | 0.019 | 0.008 | 0.014 ± 0.006 a |
| Apigenin | 0.036 | ND | 0.009 ± 0.007 a | 0.015 | ND | 0.004 ± 0.003 a | 0.006 | ND | 0.003 ± 0.002 a | 0.005 | ND | 0.003 ± 0.002 a |
| Pinocembrin | 0.119 | ND | 0.046 ± 0.030 a | 0.029 | ND | 0.024 ± 0.013 b | 0.035 | ND | 0.035 ± 0.010 c | 0.024 | ND | 0.024 ± 0.007 c |
| CAPE | 0.087 | ND | 0.013 ± 0.021 a | 0.059 | ND | 0.016 ± 0.014 a | 0.026 | ND | 0.014 ± 0.008 a | 0.027 | ND | 0.008 ± 0.008 a |
| Chrysin | 0.096 | ND | 0.035 ± 0.021 b | 1.384 | ND | 0.700 ± 0.461 a | 0.762 | 0.016 | 0.277 ± 0.208 b | 0.175 | ND | 0.059 ± 0.057 b |
| Galangin | 0.329 | ND | 0.194 ± 0.064 a | 0.042 | ND | 0.034 ± 0.014 b | ND | ND | ND | ND | ND | ND |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Kaempferol | 0.856 | 0.089 | 0.258 |
| Myricetin | 0.722 | 0.247 | 0.435 |
| Quercetin | 0.713 | 0.589 | 0.182 |
| Morin | 0.702 | 0.266 | 0.433 |
| Protocatechuic acid | −0.509 | 0.509 | 0.109 |
| Benzoic acid | 0.457 | −0.802 | −0.143 |
| Caffeic acid | −0.147 | 0.729 | −0.469 |
| Galangin | 0.607 | 0.715 | 0.146 |
| Chrysin | −0.084 | 0.7 | −0.194 |
| Ferulic acid | −0.145 | 0.56 | −0.396 |
| Proline | −0.435 | 0.519 | −0.392 |
| CAPE | 0.136 | 0.462 | −0.225 |
| p-Coumaric acid | −0.454 | 0.051 | 0.752 |
| p-Hydroxybenzoic acid | −0.546 | 0.214 | 0.692 |
| Rutin | −0.372 | 0.216 | 0.652 |
| Gallic acid | −0.19 | 0 | −0.164 |
| Pinocembrin | 0.355 | 0.004 | −0.403 |
| Naringenin | 0.535 | −0.32 | −0.244 |
| Apigenin | 0.294 | 0.447 | −0.052 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.-Q.; Zhang, J.; Li, Y.; Chen, L.; Zhao, W.; Zhou, J.; Jin, Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules 2017, 22, 735. https://doi.org/10.3390/molecules22050735
Wen Y-Q, Zhang J, Li Y, Chen L, Zhao W, Zhou J, Jin Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules. 2017; 22(5):735. https://doi.org/10.3390/molecules22050735
Chicago/Turabian StyleWen, Ya-Qin, Jinzhen Zhang, Yi Li, Lanzhen Chen, Wen Zhao, Jinhui Zhou, and Yue Jin. 2017. "Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis" Molecules 22, no. 5: 735. https://doi.org/10.3390/molecules22050735





