Design, Synthesis and Fungicidal Activity of 2-Substituted Phenyl-2-oxo-, 2-Hydroxy- and 2-Acyloxyethylsulfonamides
Abstract
:1. Introduction
2. Results
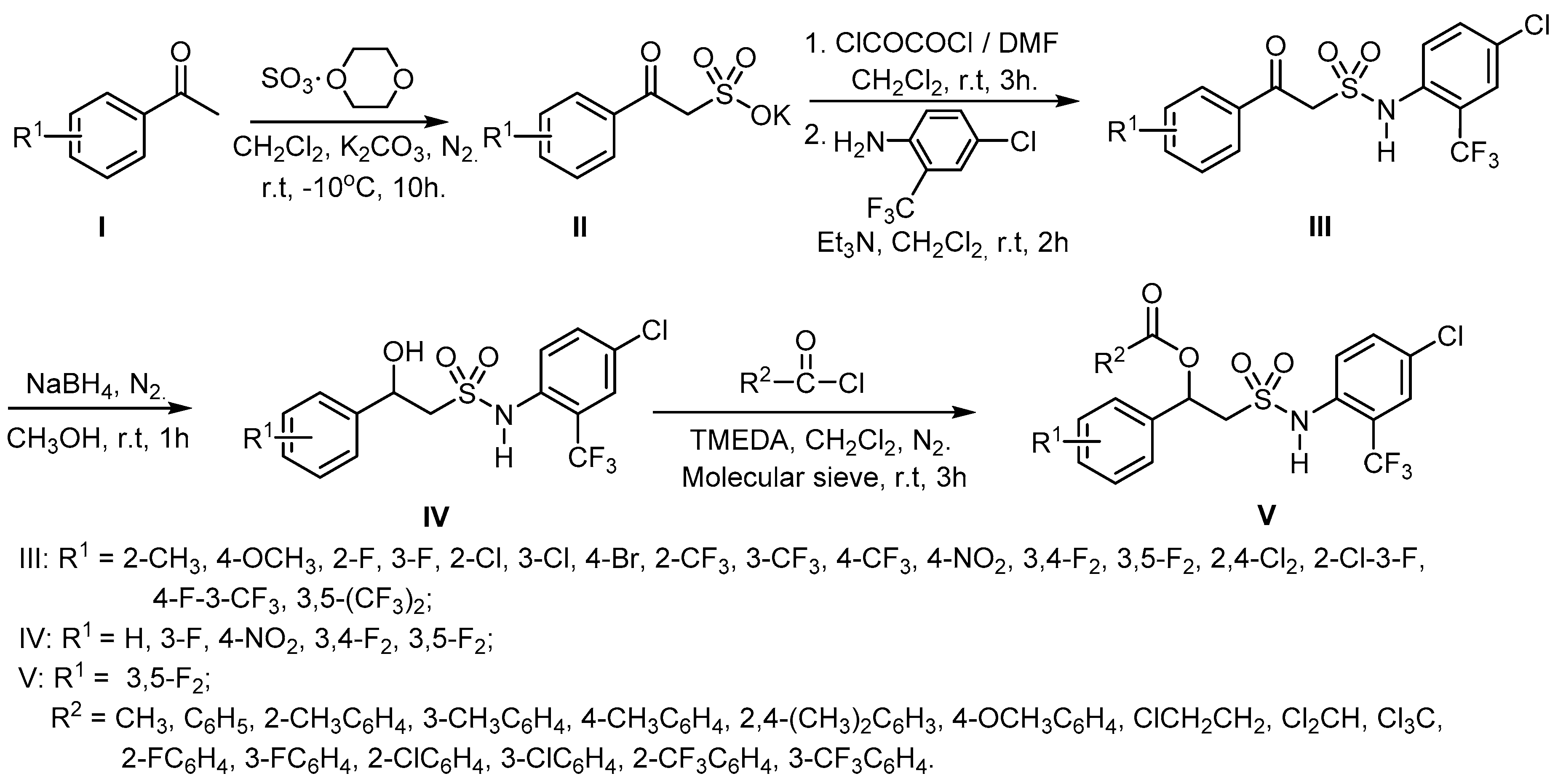
2.1. Chemistry
2.2. Biological Assay
2.2.1. In Vitro Fungicidal Activity against Botrytis cinerea
2.2.2. In Vivo Fungicidal Activity against Botrytis cinerea
2.2.3. Fungicidal Activity against Different Phytopathogenic Fungi
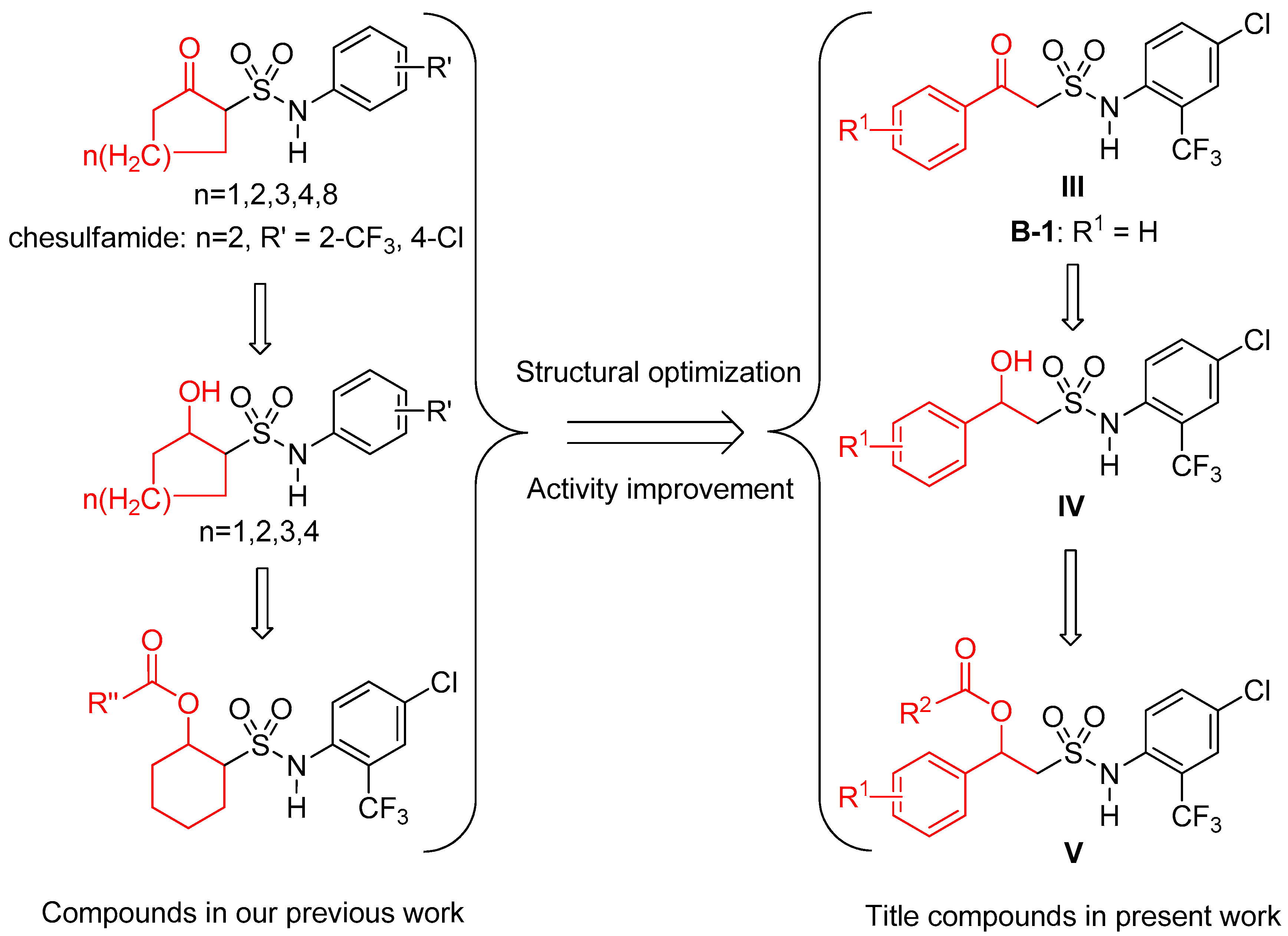
3. Discussion
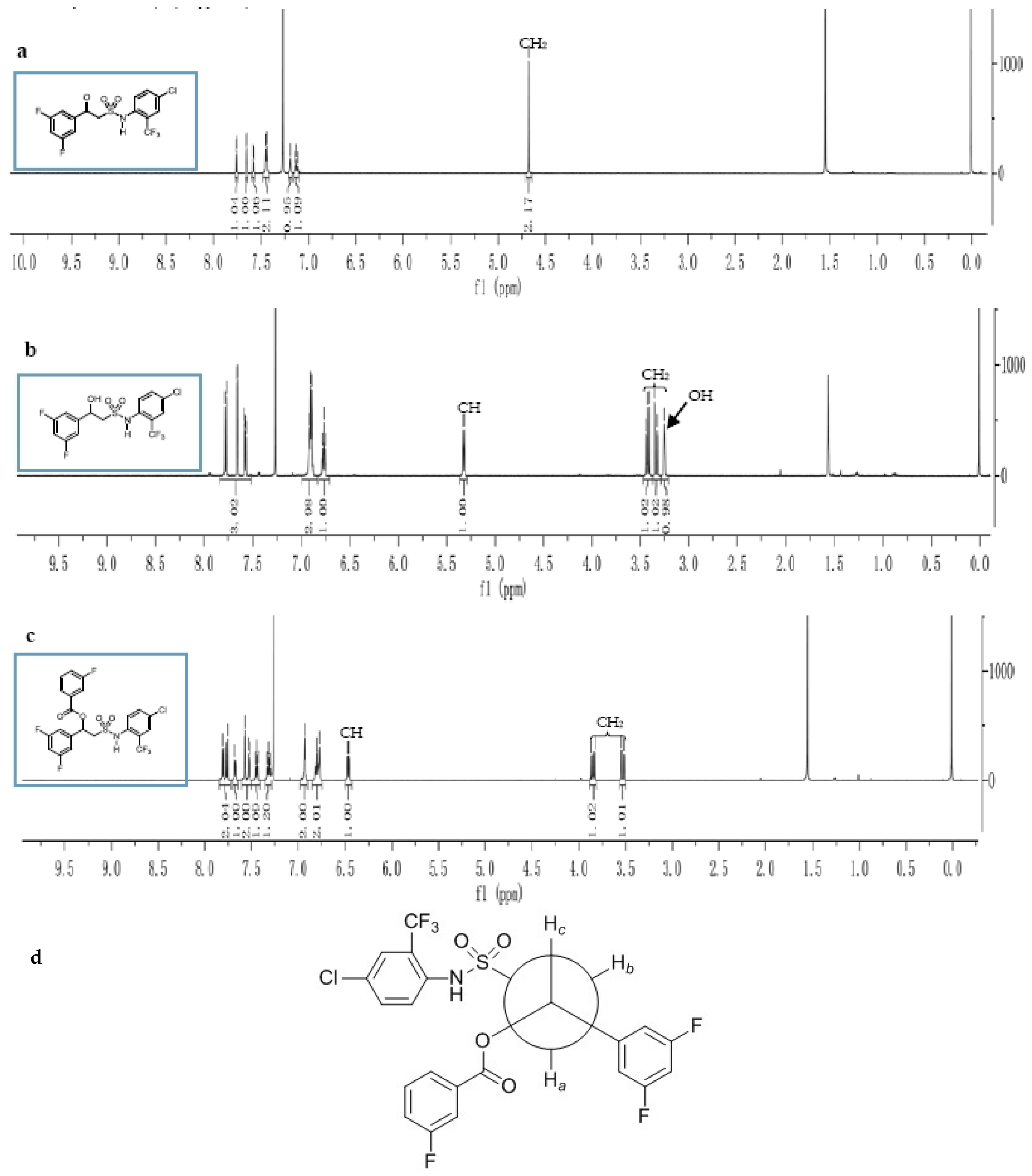
3.1. Synthesis and Structure Elucidation
3.2. Screening of Fungicidal Activity and Structure-Activity Relationships
4. Materials and Methods
4.1. General Information
4.2. Synthetic Procedures
4.2.1. General Synthetic Procedure for the Target Compounds III
4.2.2. General Synthetic Procedure for the Target Compounds IV
4.2.3. General Synthetic Procedure for the Target Compounds V
4.3. Fungicidal Activity Bioassays
4.3.1. Evaluation of Compounds III, IV, V on the Mycelia Growth of Botrytis cinerea in Solid Media
4.3.2. In Vivo Fungicidal Activity against Botrytis cinerea by Greenhouse Pot Experiments
4.3.3. In vitro Fungicidal Activity of Compounds III, IV, V against Different Phytopathogenic Fungi
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alsughayer, A.; Elassar, A.-Z.A.; Mustafa, S.; Sagheer, F.A. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J. Biomater. Nanobiotechnol. 2011, 2, 144–149. [Google Scholar]
- Wang, X.L.; Wang, X.L.; Geng, R.X.; Zhou, C.H. Advance in research of antimicrobial drugs with sulfamide group. Chin. J. New Drug 2010, 19, 2050–2059. [Google Scholar]
- Wilkinson, B.L.; Bornaghi, L.F.; Wright, A.D.; Houston, T.A.; Poulsen, S.-A. Anti-mycobacterial activity of a bis-sulfonamide. Bioorg. Med. Chem. Lett. 2007, 17, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Hen, N.; Bialer, M.; Wlodarczyk, B.; Finnell, R.H.; Yagen, B. Syntheses and evaluation of anticonvulsant profile and teratogenicity of novel amide derivatives of branched aliphatic carboxylic acids with 4-aminobenzensulfonamide. J. Med. Chem. 2010, 53, 4177–4186. [Google Scholar] [PubMed]
- Namba, K.; Zheng, X.X.; Motoshima, K.; Kobayashi, H.; Tai, A.; Takahashi, E. Design and synthesis of benzenesulfonanilides active against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Bioorg. Med. Chem. 2008, 16, 6131–6144. [Google Scholar] [PubMed]
- Zhao, F.; Wang, J.; Ding, X.; Shu, S.J.; Liu, H. Application of sulfonyl in drug design. Chin. J. Org. Chem. 2016, 36, 490–501. [Google Scholar] [CrossRef]
- Vandyck, K.; Cummings, M.D.; Nyanguile, O.; Boutton, C.W.; Vendeville, S.; McGowan, D.; Devogelaere, B.; Amssoms, K.; Last, S.; Rombauts, K.; et al. Structure-based design of a benzodiazepine scaffold yields a potent allosteric inhibitor of hepatitis C NS5B RNA polymerase. J. Med. Chem. 2009, 52, 4099–4102. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, P.; Demopoulos, V.J. A diverse series of substituted benzenesulfonamides as aldose reductase inhibitors with antioxidant activity: Design, synthesis, and in vitro activity. J. Med. Chem. 2010, 53, 7756–7766. [Google Scholar] [CrossRef] [PubMed]
- Ala, P.J.; Gonneville, L.; Hillman, M.; Becker-Pasha, M.; Yue, E.W.; Douty, B.; Wayland, B.; Polam, P.; Crawley, M.L.; McLaughlin, E.; et al. Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2006, 281, 38013–38021. [Google Scholar] [CrossRef] [PubMed]
- Terrett, N.K.; Bell, A.S.; Brown, D.; Ellis, P. Sildenafil (Viagra TM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg. Med. Chem. Lett. 1996, 6, 1819–1824. [Google Scholar]
- Roda, A.; Cerrè, C.; Manetta, A.C.; Cainelli, G.; Umani-Ronchi, A.; Panunzio, M. Synthesis and physicochemical, biological, and pharmacological properties of new bile acids amidated with cyclic amino acids. J. Med. Chem. 1996, 39, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Breslin, H.J.; Lane, B.M.; Ott, G.R.; Ghose, A.K.; Angeles, T.S.; Albom, M.S.; Cheng, M.; Wan, W.; Haltiwanger, R.C.; Wells-Knecht, K.J.; et al. Design, synthesis, and Anaplastic Lymphoma Kinase (ALK) inhibitory activity for a Novel Series of 2,4,8,22-tetraazatetracyclo [14.3.1.13,7.19,13]docosa-1(20),3(22),4,6,9(21),10,12,16,18-nonaene macrocycles. J. Med. Chem. 2012, 55, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Kamisuki, S.; Shirakawa, T.; Kugimiya, A.; Abu-Elheiga, L.; Choo, H.-Y.P.; Yamada, K.; Shimogawa, H.; Wakil, S.J.; Uesugi, M. Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. J. Med. Chem. 2011, 54, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Pinard, E.; Alberati, D.; Borroni, E.; Fischer, H.; Hainzl, D.; Jolidon, S.; Moreau, J.L.; Narquizian, R.; Nettekoven, M.; Norcross, R.D. Discovery of benzoylpiperazines as a novel class of potent and selective GlyT1 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5134–5139. [Google Scholar] [CrossRef] [PubMed]
- Sturino, C.F.; O’Neill, G.; Lachance, N.; Boyd, M.; Berthelette, C.; Labelle, M.; Li, L.; Roy, B.; Scheigetz, J.; Tsou, N.; et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J. Med. Chem. 2007, 50, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.K.; Naylor, E.M.; Chen, A.; Chang, R.S.L.; Chen, T.-B.; Faust, K.A.; Lotti, V.J.; Kivlighn, S.D.; Gable, R.A. A highly potent, orally active imidazo[4,5-b]pyridine biphenyl acylsulfonamide (MK-996; L-159,282): A new AT1-selective angiotensin II receptor antagonist. J. Med. Chem. 1994, 37, 4068–4072. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Update 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Page, D.; Wei, Z.Y.; Liu, Z.P.; Tremblay, M.; Desfosses, H.; Milburn, C.; Srivastava, S.; Yang, H.; Brown, W.; Walpole, C.; et al. 5-Sulfonamide benzimidazoles: A class of cannabinoid receptors agonists with potent in vivo antinociception activity. Lett. Drug Des. Discov. 2010, 7, 208–213. [Google Scholar] [CrossRef]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pest. Biochem. Physiol. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium, 15th ed.; British Crop. Production Council: Alton, UK, 2009. [Google Scholar]
- Li, X.H.; Pan, Q.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo- and 2-hydroxycycloalkylsulfonamides. Lett. Drug Des. Discov. 2013, 10, 353–359. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, Z.M. Synthesis and bioactivities of novel 2-methoxycarbonyl-5-arylazomethine benzenesulfonamide. Chin. J. Synth. Chem. 2012, 20, 65–68. [Google Scholar]
- Chen, Z.; Xu, W.M.; Liu, K.M.; Yang, S.; Fan, H.T.; Bhadury, P.S.; Huang, D.Y.; Zhang, Y. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Shivashankar, K.; Kulkarni, M.V.; Rasal, V.P.; Patel, H.; Mutha, S.S.; Mohite, A.A. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem. 2010, 45, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.M.; Chen, R.; Xing, R.G.; Chen, X.; Liu, S.; Guo, Z.Y.; Ji, X.; Wang, L.; Li, P. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydr. Res. 2007, 342, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yang, X.L.; Liang, X.M.; Kai, Z.P.; Yuan, H.Z.; Yuan, D.K.; Zhang, J.J.; Wang, R.Q.; Ran, F.X.; Qi, S.H.; et al. Synthesis and biological activities of 2-oxocycloalkylsulfonamides. Bioorg. Med. Chem. 2008, 16, 4538–4544. [Google Scholar]
- Liang, X.M.; Wang, D.Q.; Yang, H.Y.; Wu, J.P.; Zhang, J.J.; Yan, X.J. Preparation Method of 1-Oxotetralyl-2-sulfonamide and Its Usage as a Fungicide. Patent CN 101,503,381, 12 August 2009. [Google Scholar]
- Li, X.H.; Yang, X.L.; Ling, Y.; Fan, Z.J.; Liang, X.M.; Wang, D.Q.; Chen, F.H.; Li, Z.M. Synthesis and fungicidal activity of novel 2-oxocycloalkylsulfonylureas. J. Agric. Food Chem. 2005, 53, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Wu, D.C.; Qi, Z.Q.; Li, X.W.; Gu, Z.M.; Wei, S.S.; Zhang, Y.; Wang, Y.Z.; Ji, M.S. Synthesis, fungicidal activity, and structure–activity relationship of 2-oxo-and 2-hydroxycycloalkylsulfonamides. J. Agric. Food Chem. 2010, 58, 11384–11389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.S.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenyl-sulfonamides and their activity against Botrytis cinerea. Pest Manag. Sci. 2011, 67, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Cui, Z.N.; Chen, X.Y.; Wu, D.C.; Qi, Z.Q.; Ji, M.S. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int. J. Mol. Sci. 2013, 14, 22544–22557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Qi, Z.Q.; Zhong, C.J.; Zhang, Y.; Ji, M.S.; Wang, Y.Z.; Wang, D.Q. The synthesis and fungicidal activity of benzoyl methanesulfonamides. Chin. J. Pestic. Sci. 2008, 10, 136–140. [Google Scholar]
- Li, X.H.; Rui, P.; Pan, Q.; Chen, X.Y.; Qi, Z.Q.; Ji, M.S. Combined synthesis and fungicidal activity evolution of the 2-oxo-2-phenyl ethyl sulfonamide derivatives. Chin. J. Pestic. Sci. 2016, 18, 28–36. [Google Scholar]
- Hou, Z.; Yang, R.; Zhang, C.; Zhu, L.F.; Miao, F.; Yang, X.J.; Zhou, L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as novel antifungal lead compounds: biological evaluation and structure-activity relationships. Molecules 2013, 18, 10413–10424. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.S.; He, R.H.; Li, X.H.; Xiao, S.Q.; Niu, M.Y.; Feng, H. Inhibition effect of cycloakylsulfonamide on Phytophthora capsici. Plant Prot. 2016, 42, 214–218. [Google Scholar]
- Qi, Z.Q.; Sun, Q.B.; Li, X.H.; Gu, Z.M.; Li, X.W.; Ji, M.S. Inhibitory effect of N-(2,4,5-trichlorophenyl)-2-oxocyclohexylsulfonamide against Botrytis cinerea. Chin. J. Pestic. Sci. 2014, 16, 523–528. [Google Scholar]
Sample Availability: Samples of the compounds III, IV and V are available from the authors. |
| Compd. | DL-11 | HLD-15 | ||
|---|---|---|---|---|
| EC50 (mg L−1) | 95% Confidence Limit (mg L−1) | EC50 (mg L−1) | 95% Confidence Limit (mg L−1) | |
| III-1 | 8.67 | 6.41–11.73 | 16.25 | 11.82–22.33 |
| III-2 | 10.81 | 6.81–17.16 | 17.70 | 10.99–28.51 |
| III-3 | 7.51 | 5.14–10.99 | 8.12 | 6.22–10.61 |
| III-4 | 8.54 | 6.38–11.44 | 8.61 | 6.01–12.33 |
| III-5 | 5.44 | 3.18–9.30 | 7.94 | 5.83–10.82 |
| III-6 | 8.60 | 6.71–11.00 | 7.91 | 5.89–10.62 |
| III-7 | 3.98 | 2.86–5.52 | 8.00 | 6.05–10.58 |
| III-8 | 2.68 | 1.96–3.67 | 4.16 | 2.82–6.15 |
| III-9 | 5.15 | 3.79–7.01 | 7.22 | 5.01–10.40 |
| III-10 | 3.82 | 2.58–5.65 | 5.40 | 3.66–7.97 |
| III-11 | 4.07 | 2.95–5.59 | 6.83 | 4.83–9.67 |
| III-12 | 4.69 | 3.05–7.23 | 6.89 | 5.10–9.31 |
| III-13 | 1.58 | 1.03–2.43 | 3.48 | 2.37–5.12 |
| III-14 | 2.02 | 1.68–2.36 | 3.97 | 2.76–5.71 |
| III-15 | 1.64 | 1.15–2.34 | 8.53 | 5.16–14.12 |
| III-16 | 5.67 | 4.19–7.67 | 6.48 | 4.69–8.95 |
| III-17 | 7.15 | 5.29–9.67 | 7.74 | 5.45–10.99 |
| B-1 | 14.25 | 10.08–19.02 | 22.79 | 19.22–27.74 |
| IV-1 | 13.33 | 10.29–17.27 | 33.16 | 23.80–46.20 |
| IV-2 | 3.01 | 2.25–4.03 | 7.17 | 5.50–9.34 |
| IV-3 | 3.50 | 2.59–4.72 | 4.07 | 2.92–5.68 |
| IV-4 | 0.74 | 0.38–1.44 | 2.49 | 1.72–3.59 |
| IV-5 | 0.70 | 0.38–1.30 | 0.61 | 0.28–1.35 |
| V-1 | 0.10 | 0.02–0.45 | 3.32 | 2.26–4.88 |
| V-2 | 1.96 | 1.18–3.25 | 4.69 | 3.05–7.23 |
| V-3 | 6.10 | 4.01–9.27 | 132.52 | 54.23–323.82 |
| V-4 | 14.27 | 9.43–21.58 | 3648.50 | 197.38–67442.80 |
| V-5 | 1383.25 | 116.31–16450.90 | 1443.05 | 184.66–11277.00 |
| V-6 | 131.12 | 39.92–430.68 | / | / |
| V-7 | 11.72 | 7.33–18.73 | 9.80 | 5.63–17.07 |
| V-8 | 1.32 | 0.80–2.17 | 13.80 | 9.20–20.71 |
| V-9 | 0.01 | 0.00–0.22 | 7.72 | 5.81–10.25 |
| V-10 | 1.18 | 0.69–2.00 | 4.73 | 3.36–6.67 |
| V-11 | 5.82 | 3.84–8.82 | 119.32 | 44.64–318.93 |
| V-12 | 6.99 | 5.14–9.51 | 21.09 | 14.97–29.72 |
| V-13 | 35.18 | 14.78–83.75 | 825.50 | 111.18–6129.14 |
| V-14 | 23.63 | 17.45–32.00 | 338.33 | 130.28–780.13 |
| V-15 | 20.33 | 10.69–38.66 | 553.23 | 144.46–2118.63 |
| V-16 | 1279.16 | 117.52–13923.30 | 139.55 | 71.51–272.32 |
| procymidone | 2.59 | 1.78–3.76 | 15.95 | 11.78–21.60 |
| chlorothalonil | 1.66 | 0.97–2.84 | 17.52 | 8.04–38.19 |
| Compd. | Control Efficiency (%) against Strain DL-11 ± SEM | Compd. | Control Efficiency (%) against Strain DL-11 ± SEM |
|---|---|---|---|
| III-1 | 54.9 ± 9.1 | IV-3 | 58.2 ± 7.3 |
| III-2 | 50.5 ± 9.5 | IV-4 | 67.1 ± 8.2 |
| III-3 | 65.9 ± 8.1 | IV-5 | 62.9 ± 7.6 |
| III-4 | 60.4 ± 8.6 | V-1 | 70.8 ± 15.4 |
| III-5 | 52.7 ± 9.3 | V-2 | 69.2 ± 13.9 |
| III-6 | 40.7 ± 10.4 | V-3 | 67.1 ± 4.2 |
| III-7 | 25.3 ± 11.8 | V-4 | 68.3 ± 9.7 |
| III-8 | 57.1 ± 8.9 | V-5 | 61.45 ± 6.0 |
| III-9 | 44.0 ± 10.1 | V-6 | 71.3 ± 5.2 |
| III-10 | 47.3 ± 9.8 | V-7 | 68.3 ± 9.7 |
| III-11 | 58.2 ± 8.8 | V-8 | 70.8 ± 10.4 |
| III-12 | 58.2 ± 8.8 | V-9 | 65.4 ± 9.0 |
| III-13 | 62.6 ± 8.4 | V-10 | 71.3 ± 14.6 |
| III-14 | 33.0 ± 11.1 | V-11 | 66.7 ± 4.8 |
| III-15 | 53.8 ± 9.3 | V-12 | 68.3 ± 5.5 |
| III-16 | 57.1 ± 8.9 | V-13 | 74.6 ± 11.5 |
| III-17 | 42.9 ± 10.2 | V-14 | 73.3 ± 11.8 |
| IV-1 | 67.1 ± 8.7 | V-15 | 67.1 ± 9.4 |
| IV-2 | 52.9 ± 7.4 | V-16 | 67.9 ± 8.0 |
| procymidone | 72.5 ± 6.1 | pyrimethanil | 61.7 ± 8.1 |
| Compd. | EC50 (mg L−1) | |||
|---|---|---|---|---|
| Fg a | Tc a | Pa a | Pc a | |
| III-1 | 8.27 | 1.39 | 10.49 | 3.7 |
| III-2 | 5.12 | 4.02 | 9.36 | 4.17 |
| III-3 | 4.72 | 1.65 | 14.53 | 3.06 |
| III-4 | 3.55 | 3.07 | 13.41 | 2.77 |
| III-6 | 1.41 | 0.99 | 7.25 | 2.25 |
| III-10 | 1.05 | 0.60 | 8.65 | 4.47 |
| III-12 | 2.84 | 2.46 | 11.42 | 6.02 |
| III-13 | 0.79 | 1.86 | 8.62 | 2.74 |
| III-16 | 3.26 | 3.67 | 11.17 | 4.26 |
| IV-5 | 3.17 | 1.88 | 6.52 | 4.44 |
| V-1 | 7.48 | 15.2 | 27.33 | 28.4 |
| V-8 | 4.59 | 1.52 | 10.72 | 4.79 |
| V-9 | 9.8 | 1.57 | 19.94 | 5.6 |
| V-10 | 2.41 | 1.07 | 6.96 | 3.22 |
| B-1 | 4.13 | 2.33 | 16.92 | 3.18 |
| chlorothalonil | 0.35 | 0.02 | 5.93 | 17.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Rui, P.; Liu, C.; Du, Y.; Qin, P.; Qi, Z.; Ji, M.; Li, X.; Cui, Z. Design, Synthesis and Fungicidal Activity of 2-Substituted Phenyl-2-oxo-, 2-Hydroxy- and 2-Acyloxyethylsulfonamides. Molecules 2017, 22, 738. https://doi.org/10.3390/molecules22050738
Wang M, Rui P, Liu C, Du Y, Qin P, Qi Z, Ji M, Li X, Cui Z. Design, Synthesis and Fungicidal Activity of 2-Substituted Phenyl-2-oxo-, 2-Hydroxy- and 2-Acyloxyethylsulfonamides. Molecules. 2017; 22(5):738. https://doi.org/10.3390/molecules22050738
Chicago/Turabian StyleWang, Minlong, Peng Rui, Caixiu Liu, Ying Du, Peiwen Qin, Zhiqiu Qi, Mingshan Ji, Xinghai Li, and Zining Cui. 2017. "Design, Synthesis and Fungicidal Activity of 2-Substituted Phenyl-2-oxo-, 2-Hydroxy- and 2-Acyloxyethylsulfonamides" Molecules 22, no. 5: 738. https://doi.org/10.3390/molecules22050738






