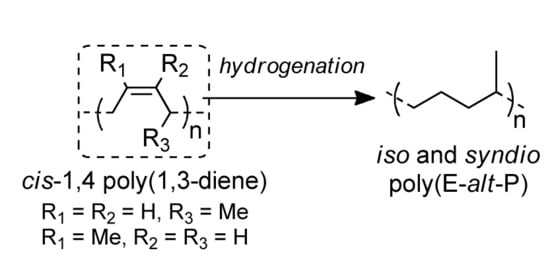
Isotactic and Syndiotactic Alternating Ethylene/Propylene Copolymers Obtained Through Non-Catalytic Hydrogenation of Highly Stereoregular cis-1,4 Poly(1,3-diene)s
Abstract
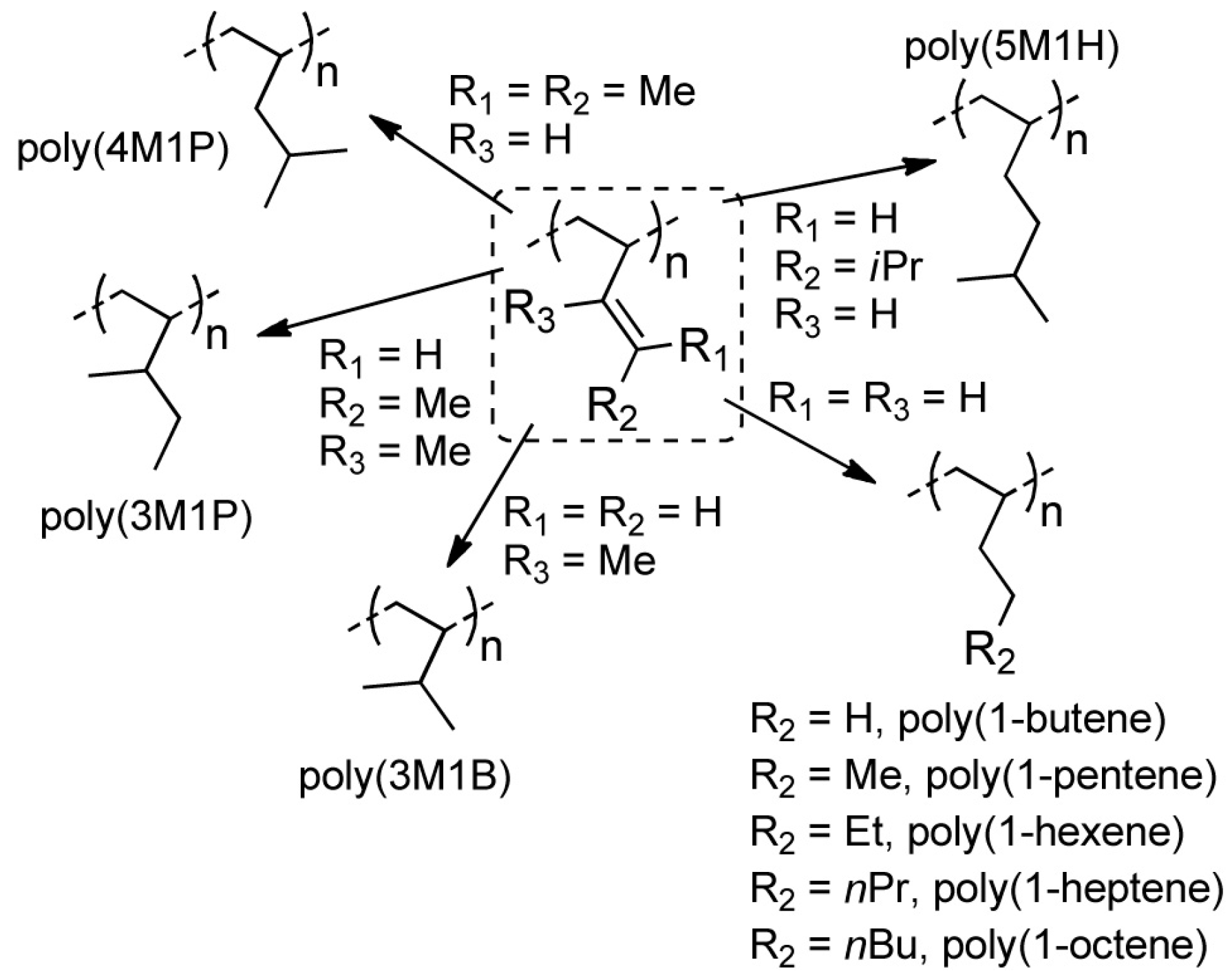
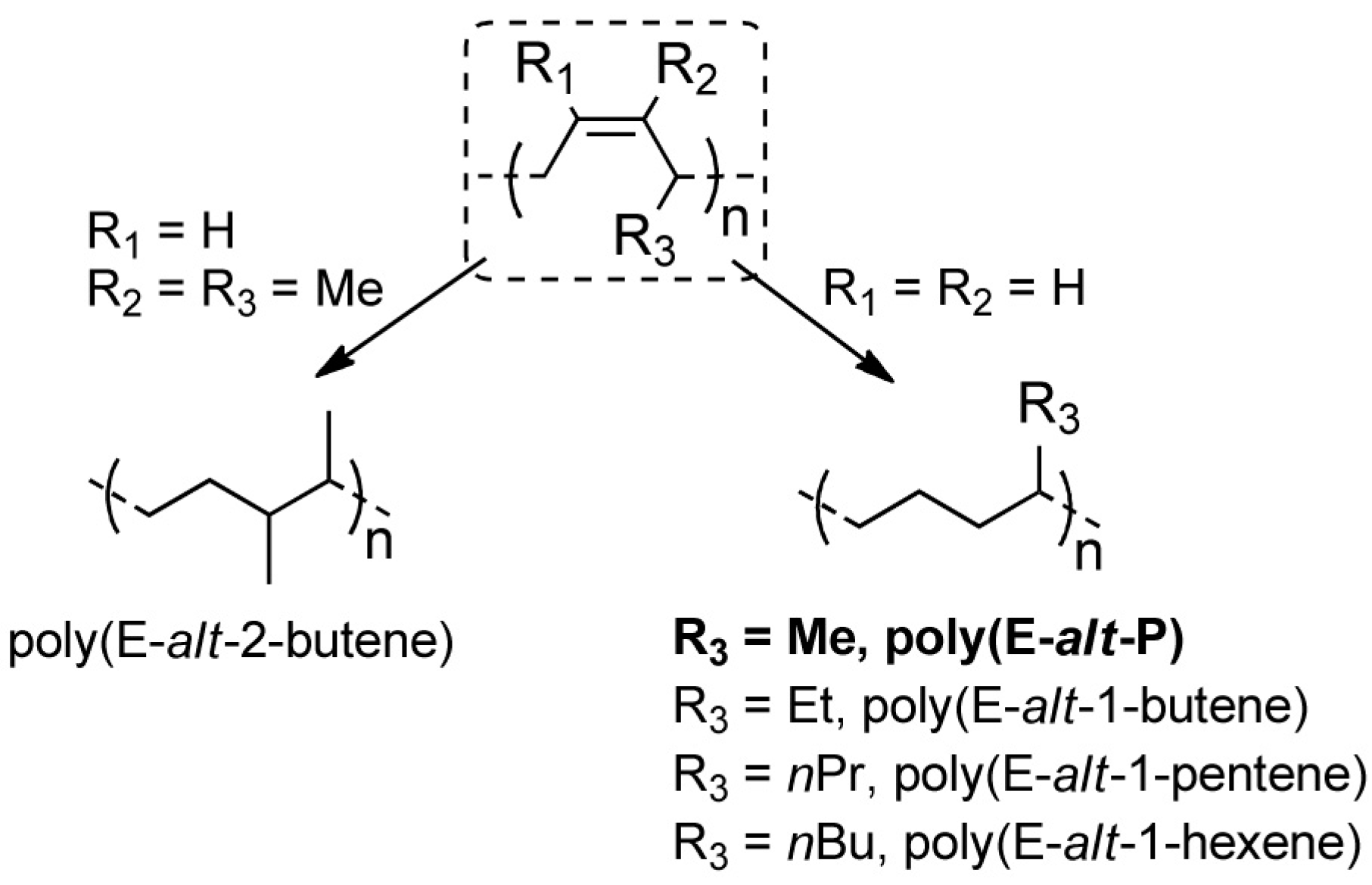
:1. Introduction
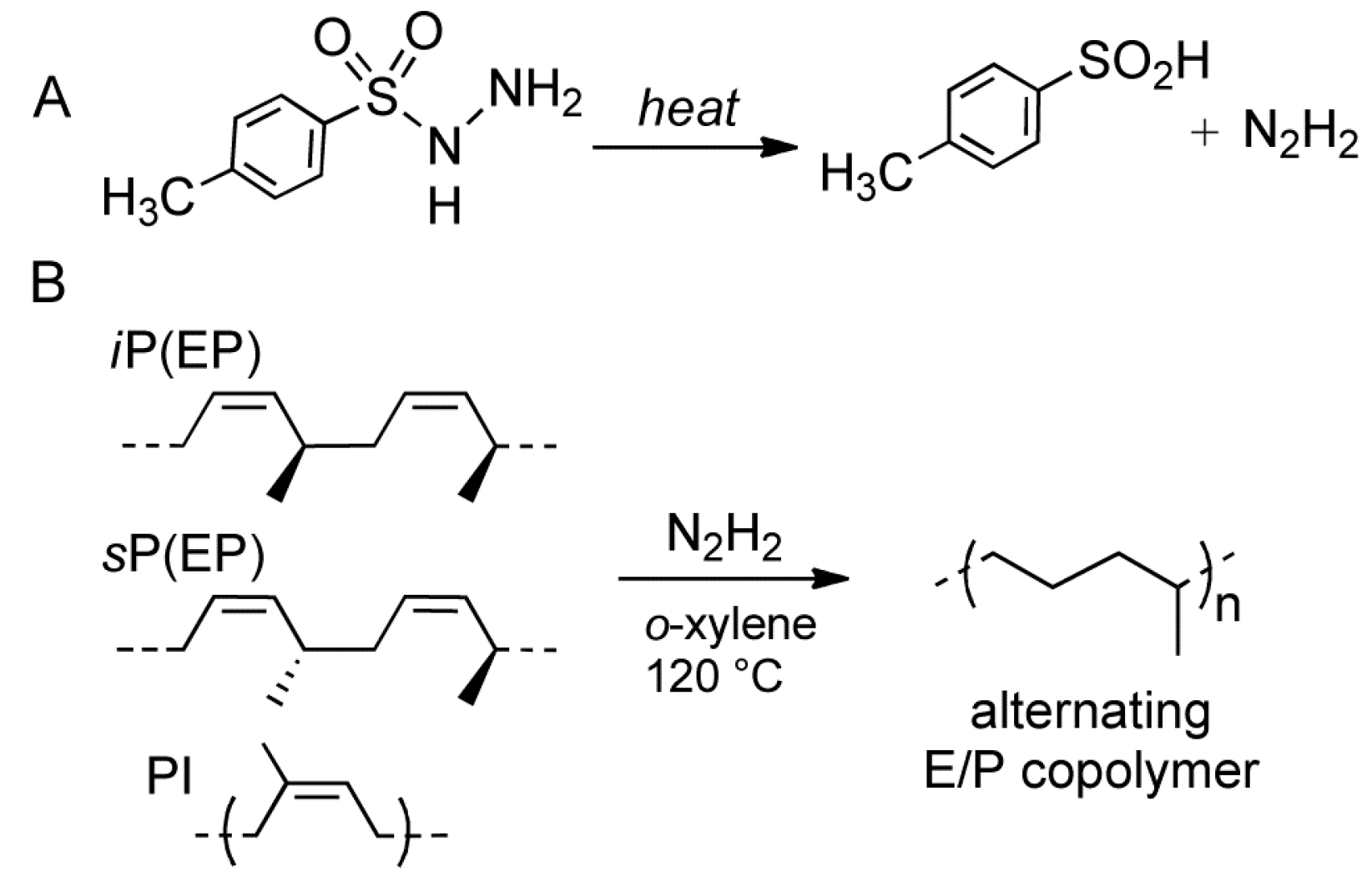
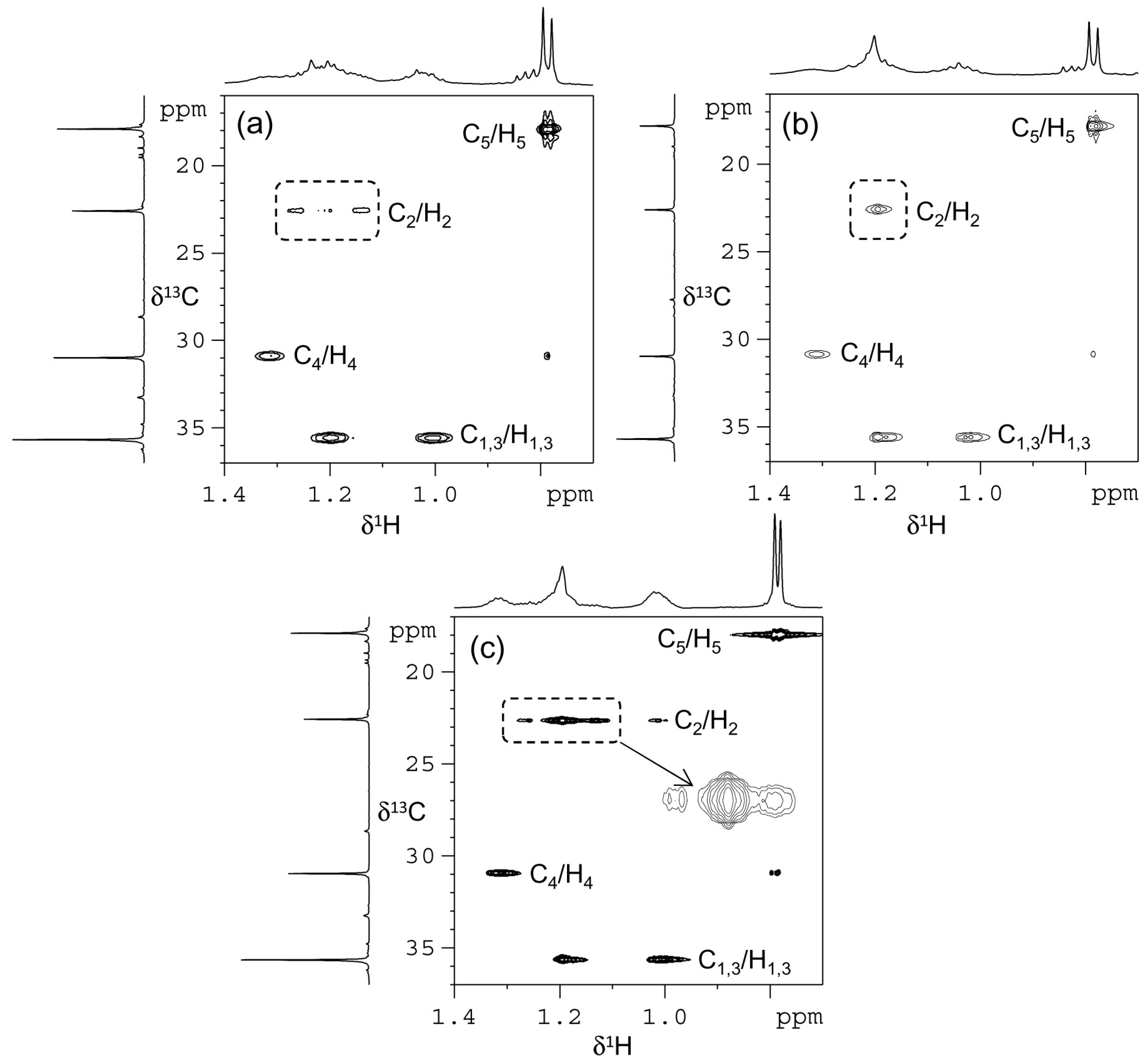
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Hydrogenation Procedure
3.2.1. Hydrogenation of Isotactic cis-1,4 poly(1,3-pentadiene)
3.2.2. Hydrogenation of Syndiotactic cis-1,4 poly(1,3-pentadiene)
3.2.3. Hydrogenation of cis-1,4 poly(isoprene)
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kravchenko, R.; Waymouth, R.M. Ethylene-propylene copolymerization with 2-arylindene zirconocenes. Macromolecules 1998, 31, 1–6. [Google Scholar] [CrossRef]
- D’Orazio, L.; Mancarella, C.; Martuscelli, E.; Sticotti, G.; Massari, P. Melt rheology, phase structure and impact properties of injection-moulded samples of isotactic polypropylene/ethylene-propylene copolymer (iPP/EPR) blends: Influence of molecular structure of EPR copolymers. Polymer 1993, 34, 3671–3676. [Google Scholar] [CrossRef]
- Kaminsky, W. Trends in Polyolefin Chemistry. Macromol. Chem. Phys. 2008, 209, 459–466. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1345. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Waymouth, R.M. Alternating copolymerization of ethylene and propylene: Evidence for selective chain transfer to ethylene. Macromolecules 2001, 34, 8619–8625. [Google Scholar] [CrossRef]
- Leclerc, M.K.; Waymouth, R.M. Alternating ethene/propene copolymerization with a metallocene catalyst. Angew. Chem. Int. Ed. 1998, 37, 922–925. [Google Scholar] [CrossRef]
- Jin, J.; Uozumi, T.; Sano, T.; Teranishi, T.; Soga, K.; Shiono, T. Alternating copolymerization of ethylene and propene with the [ethylene(1-indenyl)(9-fluorenyl)]zirconium dichloride-methylaluminoxane catalyst system. Macromol. Rapid. Commun. 1998, 19, 337–339. [Google Scholar] [CrossRef]
- Rose, J.M.; Cherian, A.E.; Coates, G.W. Living polymerization of α-olefins with an α-Diimine Ni(II) catalyst: Formation of well-defined ethylene−propylene copolymers through controlled chain-walking. J. Am. Chem. Soc. 2006, 128, 4186–4187. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1,3-dienes with iron complexes based catalysts. Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Cat A: Chem. 2003, 204/205, 287–293. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and stereospecificity of chromium (II) catalysts for 1,3-diene polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Giarrusso, A.; Porri, L. Polymerization of 1,3-dienes with the soluble catalyst system methylaluminoxanes-[CpTiCl3]. Influence of monomer structure on polymerization stereospecificity. J. Organomet. Chem. 1993, 451, 67–72. [Google Scholar] [CrossRef]
- Leone, G.; Boccia, A.C.; Ricci, G.; Giarrusso, A.; Porri, L. Polymerization of (E)-1,3-pentadiene and (E)-2-methyl-1,3-pentadiene with neodymium catalysts: Examination of the factors that affect the stereoselectivity. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 3227–3232. [Google Scholar] [CrossRef]
- Boccia, A.C.; Leone, G.; Boglia, A.; Ricci, G. Novel stereoregular cis-1,4 and trans-1,2 poly(diene)s: Synthesis, characterization, and mechanistic considerations. Polymer 2013, 54, 3492–3503. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and characterization of isotactic 1,2-Poly(E-3-methyl-1,3-pentadiene). Some remarks about the influence of monomer structure on polymerization stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Boccia, A.C.; Zetta, L. cis-1,4-alt-3,4 Polyisoprene: Synthesis and characterization. Macromolecules 2009, 42, 9263–9267. [Google Scholar] [CrossRef]
- Pirozzi, B.; Napolitano, R.; Giusto, G.; Ricci, G. Determination of the crystal structure of isotactic cis-1,4 Poly(1,3-hexadiene) by X-Ray Diffraction and molecular mechanics. Macromol. Chem. Phys. 2008, 209, 1012–1020. [Google Scholar] [CrossRef]
- Pirozzi, B.; Napolitano, R.; Giusto, G.; Esposito, S.; Ricci, G. Determination of the crystal structure of syndiotactic 1,2-poly(E-3-methyl-1,3-pentadiene) by X-ray diffraction and molecular mechanics. Macromolecules 2007, 40, 8962–8968. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Famulari, A.; Arosio, P.; Meille, S.V. Synthesis and structural characterization of syndiotactic trans-1,2 and cis-1,2 polyhexadienes. J. Polym. Sci Part A: Polym. Chem. 2007, 45, 5339–5353. [Google Scholar] [CrossRef]
- Ricci, G.; Motta, T.; Boglia, A.; Alberti, E.; Zetta, L.; Bertini, F.; Arosio, P.; Famulari, A.; Meille, S.V. Synthesis, characterization, and crystalline structure of syndiotactic 1,2-polypentadiene: The trans polymer. Macromolecules 2005, 38, 8345–8352. [Google Scholar] [CrossRef]
- Ricci, G.; Alberti, E.; Zetta, L.; Motta, T.; Bertini, F.; Mendichi, R.; Arosio, P.; Famulari, A.; Meille, S.V. Synthesis, characterization and molecular conformation of syndiotactic 1,2 polypentadiene: The cis polymer. Macromolecules 2005, 38, 8353–8365. [Google Scholar] [CrossRef]
- Purevsuren, B.; Allegra, G.; Meille, S.V.; Farina, A.; Porri, L.; Ricci, G. cis-Isotactic 1,4-polypentadiene. NMR solution characterization and crystal structure of polymers prepared with neodymium-catalytic systems. Polymer J. 1998, 30, 431–434. [Google Scholar] [CrossRef]
- Meille, S.V.; Capelli, S.; Ricci, G. Structural characterization of syndiotactic 1,2-poly(4-methyl-1,3-pentadiene). Macromol. Rapid Commun. 1995, 16, 891–897. [Google Scholar] [CrossRef]
- Meille, S.V.; Capelli, S.; Allegra, G.; Ricci, G. Isotactic cis-1,4-poly(3-methyl-1,3-pentadiene): A new conformation for isotactic cis-1,4-polydienes. Macromol. Rapid Commun. 1995, 16, 329–335. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Porri, L. Polymerization of (Z)-1,3-pentadiene with CpTiCl3/MAO. Effect of temperature on polymer structure and mechanistic implications. Macromolecules 1994, 27, 868–869. [Google Scholar] [CrossRef]
- Cabassi, F.; Porzio, W.; Ricci, G.; Bruckner, S.; Meille, S.V.; Porri, L. Structural studies on 1,4-cis-poly(2-methyl-1,3-pentadiene) synthesized with Nd catalysts. Makromol. Chem. 1988, 189, 2135–2143. [Google Scholar] [CrossRef]
- Bruckner, S.; Meille, S.V.; Porzio, W.; Ricci, G. The structure of one crystalline form of isotactic 1,4-cis-poly(2-methyl-1,3-pentadiene). An application of the rietveld method. Makromol. Chem. 1988, 189, 2145–2152. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Santillo, C.; Di Girolamo, R.; Leone, G.; Ricci, G. Chirality, entropy and crystallization in polymers: Isotactic poly(3-methyl-1-pentene) as an example of influence of chirality and entropy on the crystal structure. CrystEngComm. 2015, 17, 6006–6013. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Santillo, C.; Di Girolamo, R.; Leone, G.; Boccia, A.C.; Ricci, G. Crystal structure of isotactic poly((R,S)-3-methyl-1-pentene). Macromolecules 2015, 48, 5251–5266. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boccia, A.C.; Pierro, I.; Zanchin, G.; Mauri, M.; Scoti, M.; Malafronte, A.; Auriemma, F.; De Rosa, C. Perfectly alternating ethylene/2-butene copolymers by hydrogenation of highly stereoregular 1,4-Poly(1,3-diene)s: Synthesis and characterization. Macromolecules 2017, 50, 754–761. [Google Scholar] [CrossRef]
- Ricci, G.; Boffa, G.; Porri, L. Polymerization of 1,3-dialkenes with neodymium catalysts. Some remarks on the influence of the solvent. Makromol. Chem. Rapid Commun. 1986, 7, 355–359. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Cabassi, F.; Porri, L. Neodymium catalysts for 1,3-diene polymerization: Influence of the preparation conditions on activity. Polymer Commun. 1987, 28, 223–226. [Google Scholar]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T. Synthesis, structure, and butadiene polymerization behavior of alkylphosphine cobalt(II) complexes. J. Mol. Catal. A: Chem. 2005, 226, 235–241. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Rempel, G.L. Homogeneous hydrogenation art of nitrile butadiene rubber: A review. Polym. Rev. 2013, 53, 192–239. [Google Scholar] [CrossRef]
- Santin, C.K.; Jacobi, M.M.; Schuster, R.H. Influence of 1,2 units content on the hydrogenation of polydienes by TSH. J. Appl. Polym. Sci. 2011, 119, 1195–1203. [Google Scholar] [CrossRef]
- Samran, J.; Phinyocheep, P.; Daniel, P.; Kittipoom, S. Hydrogenation of unsaturated rubbers using diimide as a reducing agent. J. Appl. Polym. Sci. 2005, 95, 16–27. [Google Scholar] [CrossRef]
- Mango, L.A.; Lenz, R.W. Hydrogenation of unsaturated polymers with diimide. Makromol. Chem. 1973, 163, 13–36. [Google Scholar] [CrossRef]
- Zetta, L.; Gatti, G.; Audisio, G. Long-range steric effects on C-13 chemical-shift of hydrocarbon polymers. Macromolecules 1978, 11, 763–766. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Markova, N.; Ivanova, G.; Enchev, V.; Simeonova, M. Tacticity of poly(butyl-α-cyanoacrylate) chains in nanoparticles: NMR spectroscopy and DFT calculations. Struct. Chem. 2012, 23, 815–824. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Alternating Ethylene/Propylene Copolymers are available from the authors. |

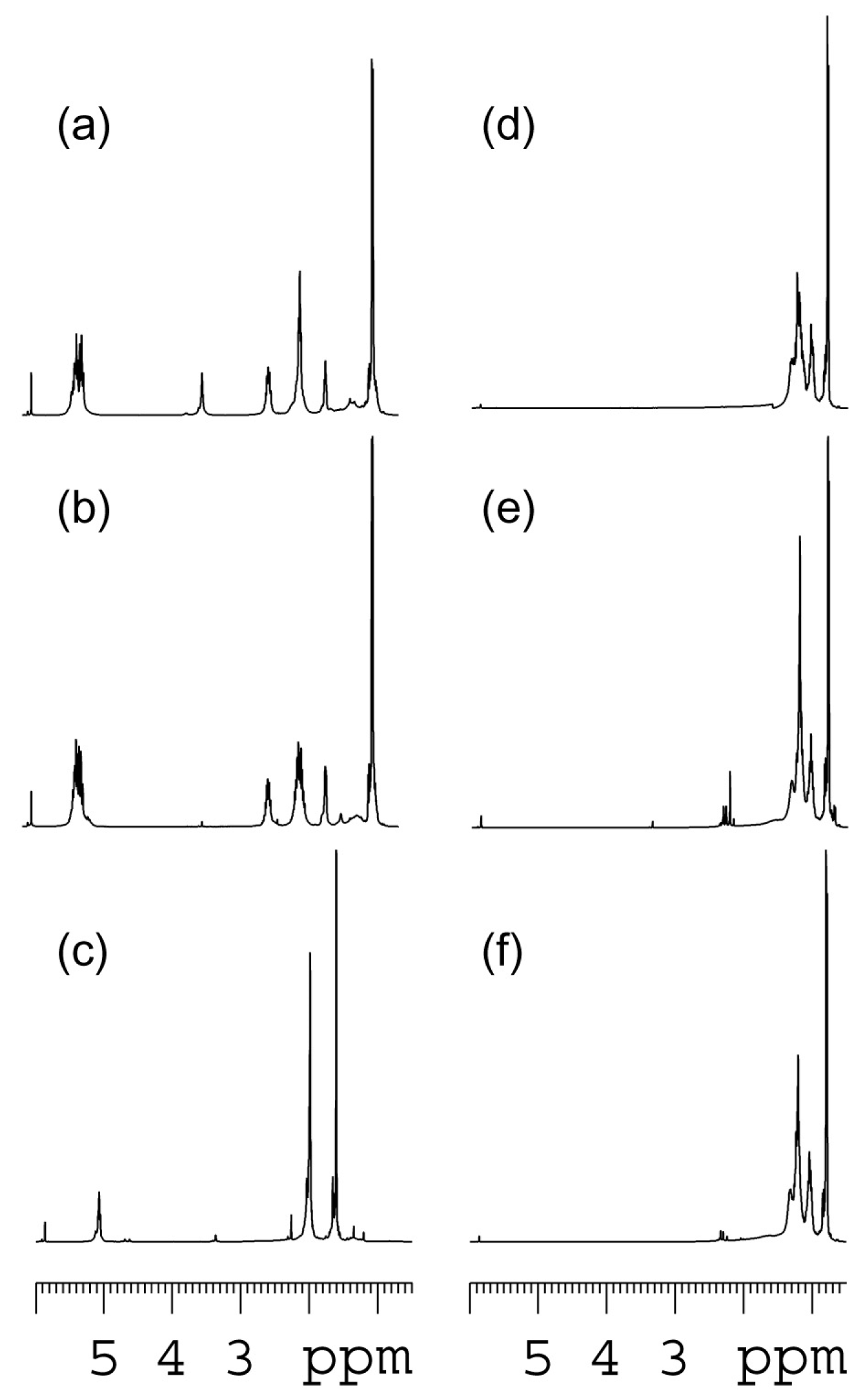
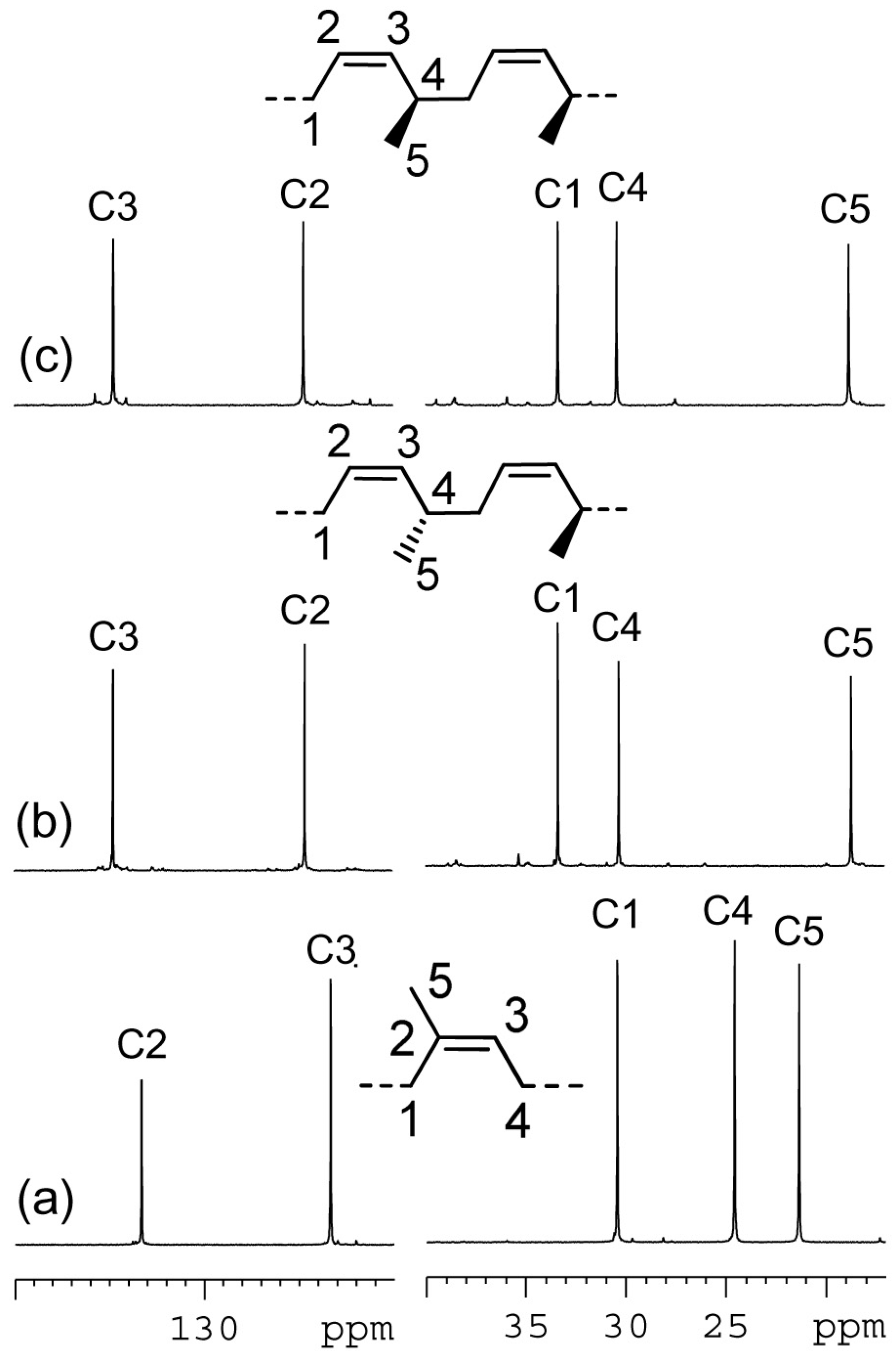
| polymer/ppm | C1 | C2 | C3 | C4 | C5 |
| PI | 30.44 | 123.35 | 133.30 | 24.60 | 21.38 |
| sP(EP) | 33.37 | 124.62 | 134.75 | 30.31 | 18.73 |
| iP(EP) | 33.38 | 124.73 | 134.72 | 30.42 | 18.84 |
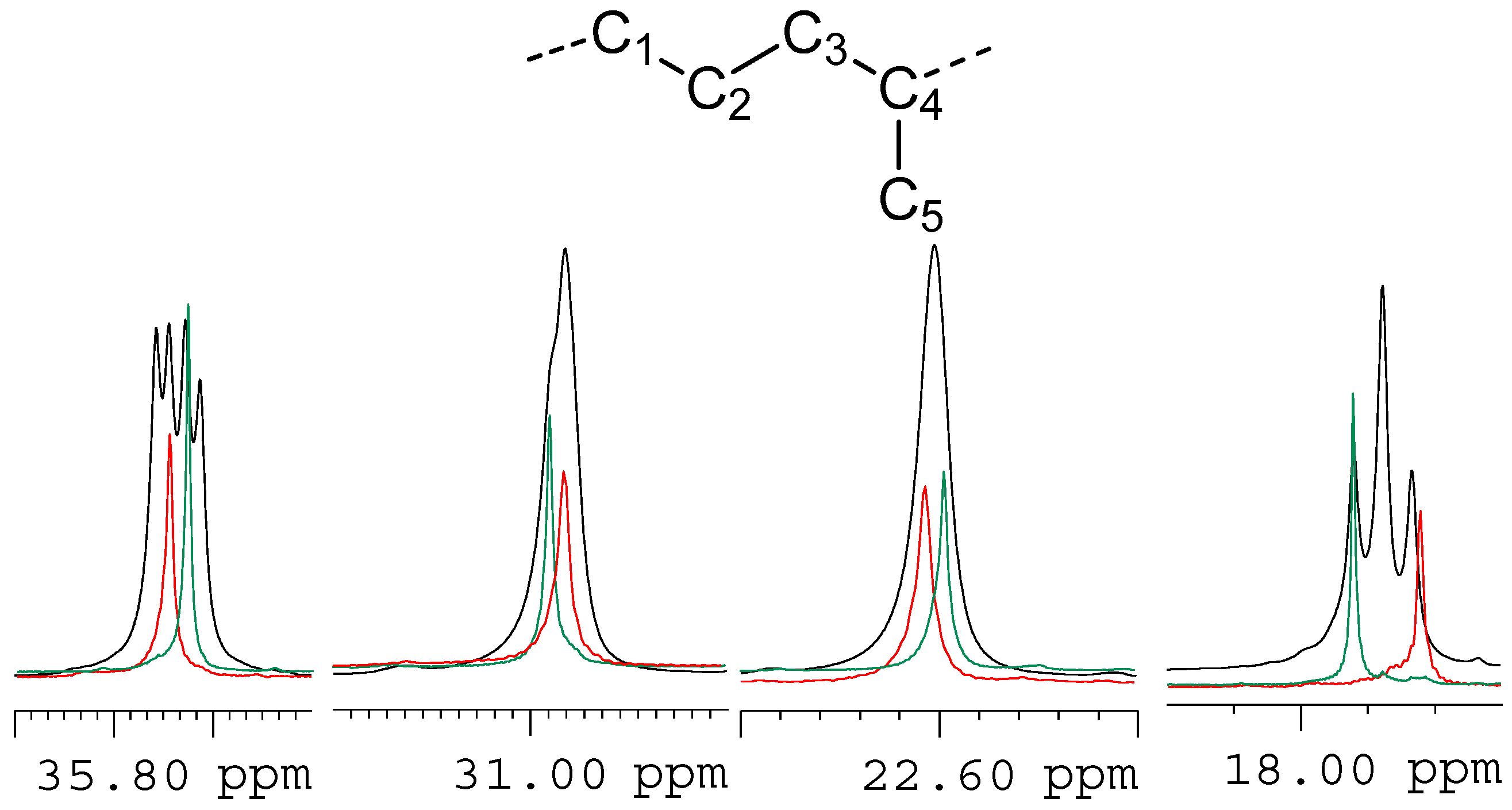
| Polymer/ppm | C1/C3 | C2 | C4 | C5 |
| H-PI | 35.72 | 22.60 | 30.96 | 17.92 |
| 35.69 | 17.88 | |||
| 35.66 | 17.84 | |||
| 35.63 | ||||
| H-sP(EP) | 35.69 | 22.61 | 30.96 | 17.82 |
| H-iP(EP) | 35.66 | 22.59 | 30.98 | 17.92 |
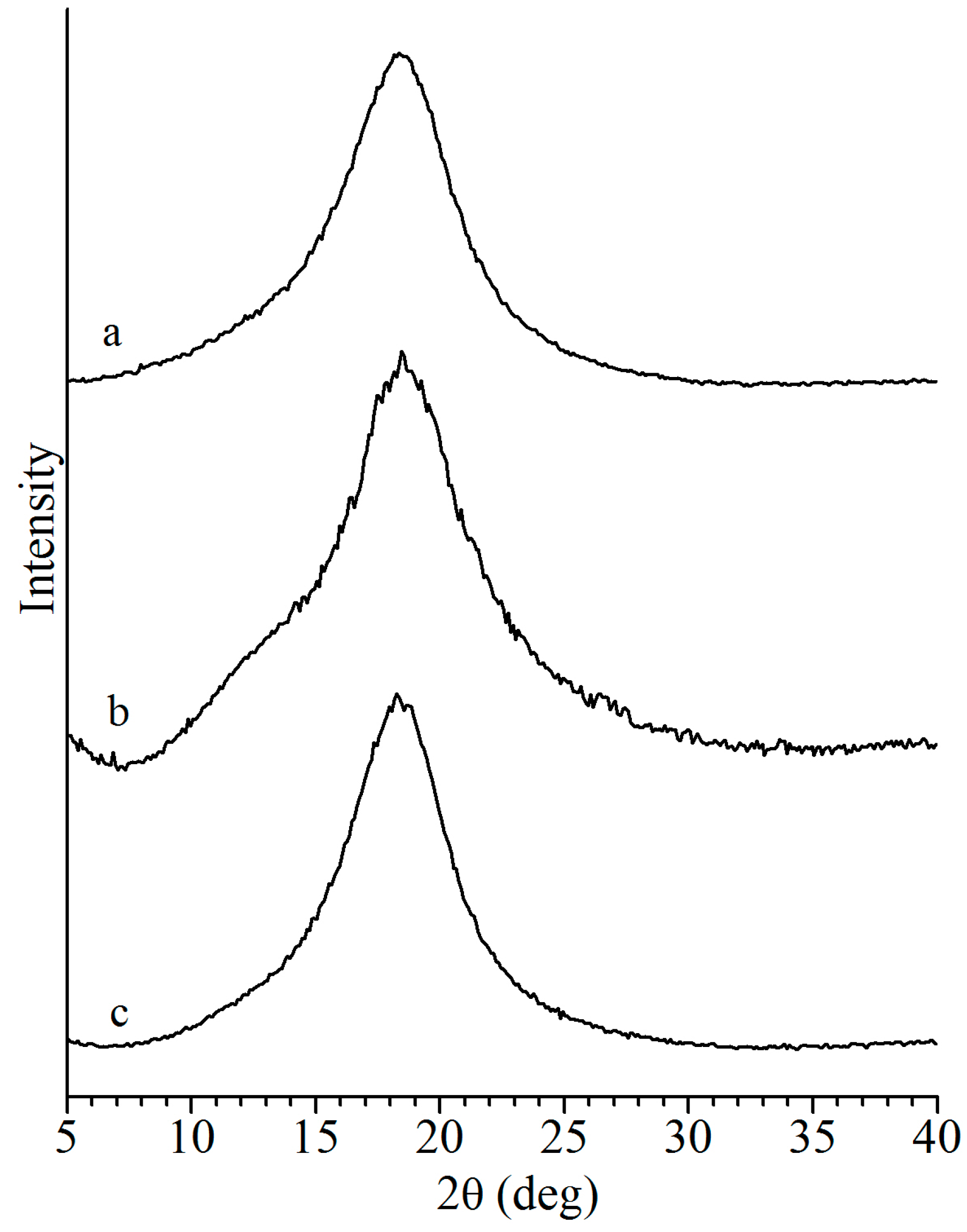

| Sample | Monomer | Catalyst | Time | Yield | cis-1,4 2 | Tg 3 |
|---|---|---|---|---|---|---|
| (min) | (%) | (%) | (°C) | |||
| iP(EP) | (E)-1,3-pentadiene | AlEt2Cl/Nd(OCOC7H15)3/AliBu3 | 60 | 87 | ≥90 | −66 |
| sP(EP) | (E)-1,3-pentadiene | CoCl2(PtBu2Me)2/MAO | 144 | 69 | ≥99 | −66 |
| PI | isoprene | AlEt2Cl/Nd(OCOC7H15)3/AliBu3 | 30 | 100 | ≥97 | −65 |
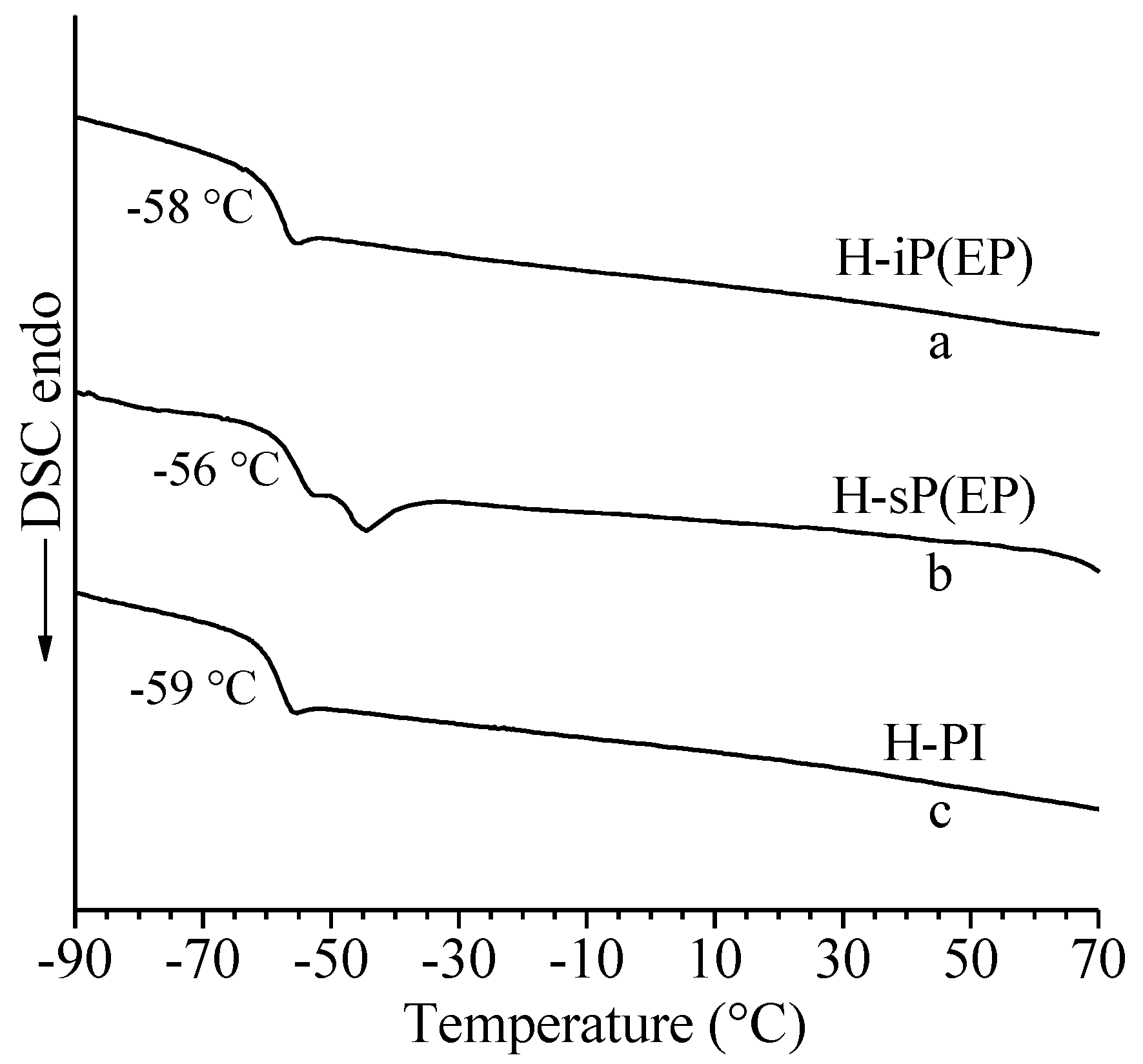
| E/P Copolymers | Corresponding Poly(1,3-diene) | Mw 1 (×10−3) | Mw/Mn1 | Tg 2 (°C) |
|---|---|---|---|---|
| H-iP(EP) | isotactic cis-1,4 poly(1,3-pentadiene) | 194.8 | 2.8 | −58 |
| H-sP(EP) | syndiotactic cis-1,4-poly(1,3-pentadiene) | 60.5 | 3.1 | –56 |
| H-PI | cis-1,4 poly(isoprene) | 249.3 | 2.8 | –59 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, G.; Boccia, A.C.; Leone, G.; Pierro, I.; Zanchin, G.; Scoti, M.; Auriemma, F.; De Rosa, C. Isotactic and Syndiotactic Alternating Ethylene/Propylene Copolymers Obtained Through Non-Catalytic Hydrogenation of Highly Stereoregular cis-1,4 Poly(1,3-diene)s. Molecules 2017, 22, 755. https://doi.org/10.3390/molecules22050755
Ricci G, Boccia AC, Leone G, Pierro I, Zanchin G, Scoti M, Auriemma F, De Rosa C. Isotactic and Syndiotactic Alternating Ethylene/Propylene Copolymers Obtained Through Non-Catalytic Hydrogenation of Highly Stereoregular cis-1,4 Poly(1,3-diene)s. Molecules. 2017; 22(5):755. https://doi.org/10.3390/molecules22050755
Chicago/Turabian StyleRicci, Giovanni, Antonella Caterina Boccia, Giuseppe Leone, Ivana Pierro, Giorgia Zanchin, Miriam Scoti, Finizia Auriemma, and Claudio De Rosa. 2017. "Isotactic and Syndiotactic Alternating Ethylene/Propylene Copolymers Obtained Through Non-Catalytic Hydrogenation of Highly Stereoregular cis-1,4 Poly(1,3-diene)s" Molecules 22, no. 5: 755. https://doi.org/10.3390/molecules22050755