Phytochemical Profiles and Antimicrobial Activities of Allium cepa Red cv. and A. sativum Subjected to Different Drying Methods: A Comparative MS-Based Metabolomics
Abstract
:1. Introduction
2. Results
2.1. Identification of Allium Species Volatiles via SPME-GC/MS
2.2. Multivariate Data Analysis of Allium Species Analysed via SPME-GC/MS
2.3. Identification of Allium Species Non-Volatile Metabolites via UPLC/PDA/qTOF-MS
2.3.1. Identification of Dipeptides and Amino Acid Conjugates
2.3.2. Identification of Flavonoids
2.3.3. Identification of Fatty Acids and Oxylipins
2.3.4. Identification of Phenolic Acids
2.4. Multivariate Data Analysis of Allium Species Analysed via UPLC-MS
2.5. Antimicrobial Activity of Allium Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Fibers
4.3. Headspace Volatiles Analysis of A. sativum and A. cepa Bulbs
4.4. GC–MS Data Processing for Multivariate Analysis
4.5. Extraction Procedure and Sample Preparation for UPLC/PDA/ESI–MS Analyses and Antimicrobial Assay
4.6. UPLC-Orbitrap HRMS Analysis
4.7. UPLC/MS Data Processing for Multivariate Analysis
4.8. Antimicrobial Effect Determined Using Minimum Inhibitory Concentration (MIC)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, H.; Chen, F.; Hua, Y.; Jiang, Y. Phytochemical analyses of Ziziphus jujuba Mill. var. spinosa seed by ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry. Analyst 2013, 138, 6881–6888. [Google Scholar] [CrossRef] [PubMed]
- Marsili, R. Flavor, Fragrance, and Odor Analysis; CRC Press: Boca Raton, FL, USA, 2001; Volume 115. [Google Scholar]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfidesulfides and garlic powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; Farag, M.A.; Holvoet, P.; Hanafi, R.S.; Gad, M.Z. A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction. Sci. Rep. 2016, 6, 36359. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Abdollahi, M. Are medicinal plants polluted with phthalates? Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2013, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Al-Hammady, M.A.; Hegazy, M.E.; Meyer, A.; Mohamed, T.A.; Westphal, H.; Wessjohann, L.A. Soft Corals Biodiversity in the Egyptian Red Sea: A Comparative MS and NMR Metabolomics Approach of Wild and Aquarium Grown Species. J. Proteome Res. 2016, 15, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Lokke, M.M.; Edelenbos, M.; Larsen, E.; Feilberg, A. Investigation of volatiles emitted from freshly cut onions (Allium cepa L.) by real time proton-transfer reaction-mass spectrometry (PTR-MS). Sensors (Basel) 2012, 12, 16060–16076. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; El-Ahmady, S.H.; Elian, F.S.; Wessjohann, L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 2013, 95, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.L.; Lee, S.; Lee, S.Y.; Ko, S.; Yoo, M. UPLC/ESI-MS/MS analysis of compositional changes for organosulphur compounds in garlic (Allium sativum L.) during fermentation. Food Chem. 2016, 211, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2017, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D. Thiamine tetrahydrofurfuryl disulfides: A little known therapeutic agent. Med. Sci. Monitor 2004, 10, RA199–RA203. [Google Scholar]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Farag, M.A.; Eldin, M.G.S.; Kassem, H.; Abou el Fetouh, M. Metabolome Classification of Brassica napus L. Organs via UPLC-QTOF-PDA-MS and Their Anti-oxidant Potential. Phytochem. Anal. 2013, 24, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Martin-Arjol, I.; Bassas-Galia, M.; Bermudo, E.; Garcia, F.; Manresa, A. Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. Chem. Phys. Lipids 2010, 163, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Dehpour, A.A.; Babakhani, B.; Khazaei, S.; Asadi, M. Chemical composition of essential oil and antibacterial activity of extracts from flower of Allium atroviolaceum. J. Med. Plants Res. 2011, 5, 3667–3672. [Google Scholar]
- Fenwick, G.R.; Hanley, A.B. The genus Allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC-MS coupled to chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martı’nez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Rodriguez Galdon, B.; Rodriguez Rodriguez, E.M.; Diaz Romero, C. Flavonoids in onion cultivars (Allium cepa L.). J. Food Sci. 2008, 73, C599–C605. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Kyle, J.A.; Collins, A.R. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.L.; Lou, X.Y.; Guo, Y.; Leung, K.S.; Zeng, E.Y. Occurrence of phthalate esters in over-the-counter medicines from China and its implications for human exposure. Environ. Int. 2017, 98, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, P.F.; Galmarini, C.R. Effect of processing and cooking conditions on onion (Allium cepa L.) induced antiplatelet activity and thiosulphinate content. J. Agric. Food Chem. 2012, 60, 8731–8737. [Google Scholar] [CrossRef] [PubMed]
- Durenkamp, M.; De Kok, L.J. Impact of pedospheric and atmospheric sulphur nutrition on sulphur metabolism of Allium cepa L., a species with a potential sink capacity for secondary sulphur compounds. J. Exp. Bot. 2004, 55, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.S.; Nyberg, N.T.; Staerk, D. Assessment of constituents in Allium by multivariate data analysis, high-resolution alpha-glucosidase inhibition assay and HPLC-SPE-NMR. Food Chem. 2014, 161, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, P.; Vericel, E.; Lelli, M.; Beguin, L.; Lagarde, M.; Guichardant, M. Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. J. Lipid Res. 2013, 54, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C.; Cottrell, S.L.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Rasheed, D.M.; Kamal, I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res. Int. 2015, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Reddy, I.R.; Duran, A.L.; Zhao, X.; Sumner, L.W. MET-IDEA: Data extraction tool for mass spectrometry-based metabolomics. Anal. Chem. 2006, 78, 4334–4341. [Google Scholar] [CrossRef] [PubMed]
- Zajmi, A.; Mohd Hashim, N.; Noordin, M.I.; Khalifa, S.A.; Ramli, F.; Mohd Ali, H.; El-Seedi, H.R. Ultrastructural Study on the Antibacterial Activity of Artonin E versus Streptomycin against Staphylococcus aureus Strains. PLoS ONE 2015, 10, e0128157. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the analysed frozen Allium species are available from the authors. |
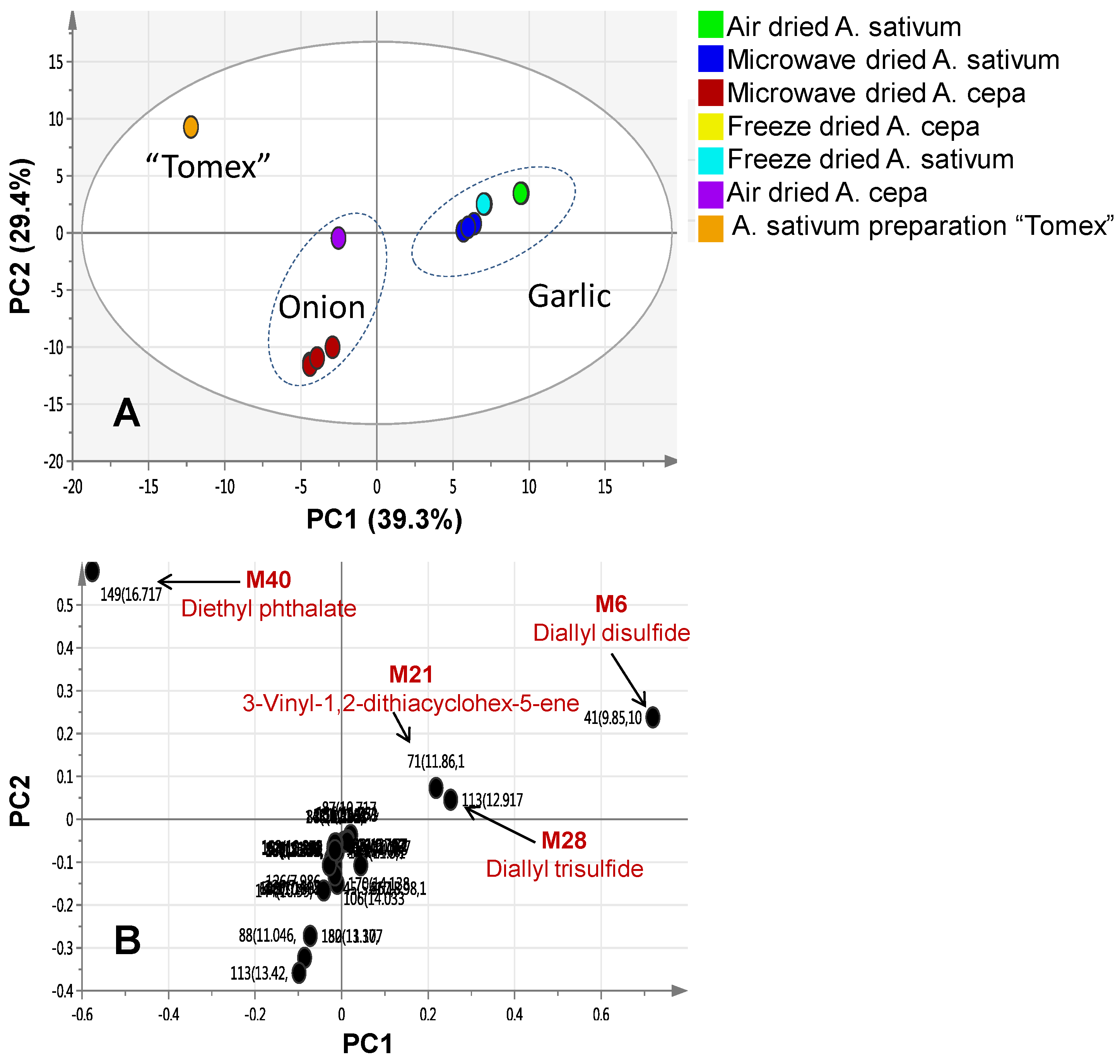

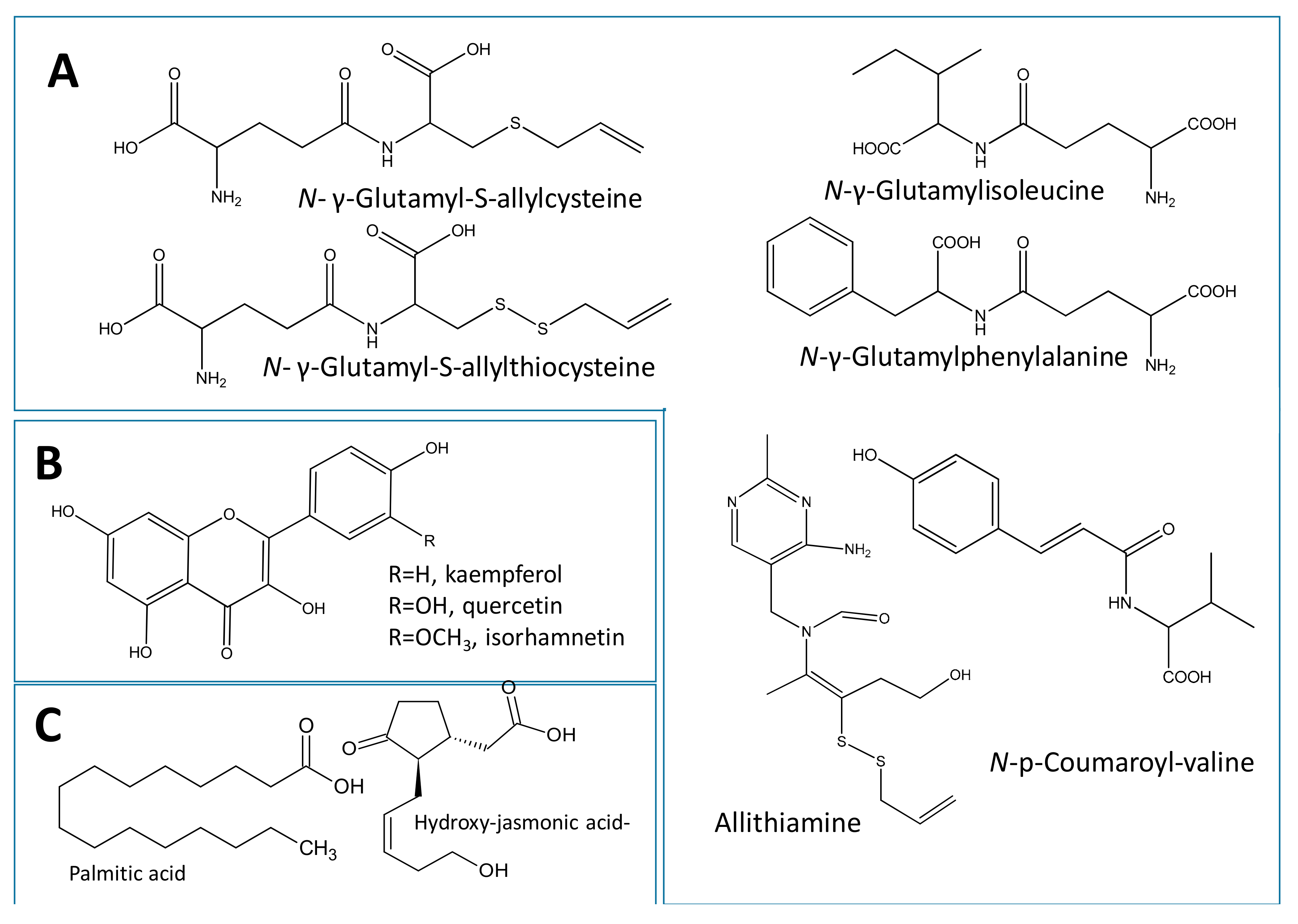
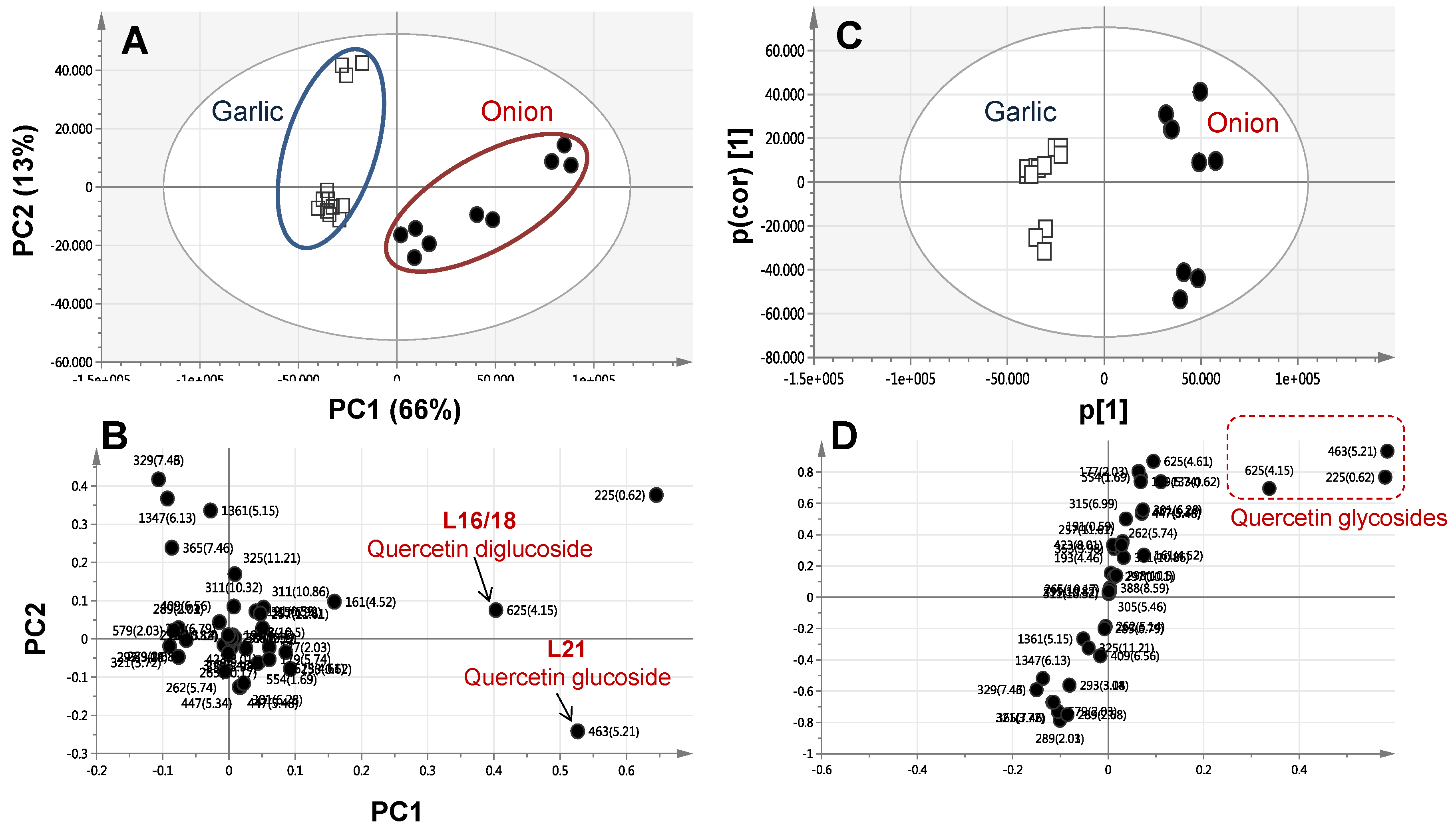

| Peak No. | r.t. (min) | RI | Volatiles | Relative Abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. sativum | A. cepa Red cv. | |||||||||
| Fresh | Sun-Dried | Microwave-Dried | Tomex | Fresh | Sun-Dried | Microwave-Dried | ||||
| M1 | 5.867 | 847 | Diallyl sulphide | tr. | 0.05 | 0.53 | tr. | - | - | - |
| M2 | 7.442 | 924 | 1-propenyl methyl disulphide | - | - | - | - | 5.27 | 5.32 | 4.41 |
| M3 | 7.986 | 958 | Dimethyl trisulphide | - | - | - | - | 6.09 | 6.46 | 4.35 |
| M4 | 8.003 | 969 | Dimethyl trisulphide isomer | tr. | tr. | 0.03 | tr. | - | - | - |
| M5 | 9.792 | 1068 | 2-Acetylpyrrole | - | - | - | - | 0.45 | 12.21 | 1.94 |
| M6 | 9.85 | 1074 | Diallyl disulphide | 45.99 | 99.11 | 46.98 | 0.08 | - | - | - |
| M7 | 10.167 | 1092 | Tetramethylpyrazine | 0.10 | tr. | 3.35 | tr. | - | - | - |
| M8 | 10.243 | 1097 | 2-Propenylthioacetonitrile | 1.27 | tr. | 0.5 | tr. | - | - | - |
| M9 | 10.307 | 1101 | Isopropyl-α-mercaptopropionate | - | - | - | - | 9.5 | 1.52 | 3.51 |
| M10 | 10.717 | 1131 | Allyl methyl trisulphide | 0.43 | tr. | 1.43 | 0.04 | - | - | - |
| M11 | 10.892 | 1143 | Diethanol disulphide | - | - | - | - | 8.93 | 5.22 | 4.29 |
| M12 | 10.99 | 1149 | Methyl pentyl disulphide | - | - | - | - | 0.48 | 18.67 | 7.32 |
| M13 | 11.046 | 1153 | cis-Methyl propenyl sulphide | 0.43 | tr. | 0.02 | 0.007 | - | - | - |
| M14 | 11.107 | 1157 | Allyl methyl trisulphide | - | - | - | - | 13.98 | 18.84 | 19.43 |
| M15 | 11.118 | 1159 | Unknown sulphur | tr. | 0.004 | 0.24 | 0.0002 | - | - | - |
| M16 | 11.157 | 1162 | Geranyl nitrile | tr. | 0.003 | 0.07 | tr. | - | - | - |
| M17 | 11.357 | 1175 | Methyl 2-methylheptanoate | tr. | tr. | 0.02 | tr. | - | - | - |
| M18 | 11.5 | 1186 | 3-Ethenyl-1,2-dithi-4-ene | 0.02 | 0.008 | 0.28 | 0.0005 | - | - | - |
| M19 | 11.517 | 1189 | Diallyl disulphide isomer | 0.04 | 0.01 | 0.47 | 0.001 | - | - | - |
| M20 | 11.751 | 1203 | Unknown sulphur | 0.01 | 0.01 | 0.906 | 0.001 | - | - | - |
| M21 | 11.86 | 1212 | 3-Vinyl-1,2-dithiacyclohex-5-ene | 31.8 | 0.19 | 4.306 | 0.007 | - | - | - |
| M22 | 11.873 | 1212 | 3-Ethenyl-1,2-dithi-5-ene isomer | 0.04 | 0.03 | 0.85 | 0.001 | - | - | - |
| M23 | 11.875 | 1212 | Dimethyl tetrasulphide | - | - | - | - | 3.51 | 2.73 | 1.98 |
| M24 | 12.158 | 1234 | Unknown | - | - | - | - | 0.43 | 2.11 | 1.64 |
| M25 | 12.161 | 1234 | p-Cuminaldehyde | tr. | 0.002 | 0.03 | tr. | - | - | - |
| M26 | 12.182 | 1236 | 3-Isopropyl benzaldehyde | tr. | 0.002 | 0.106 | tr. | - | - | - |
| M27 | 12.317 | 1246 | 4,7-Dimethylundecane | - | - | - | - | 0.59 | 1.14 | 1.91 |
| M28 | 12.917 | 1293 | Diallyl trisulphide | 19.87 | 0.32 | 28.86 | 0.06 | - | - | - |
| M29 | 12.919 | 1292 | (Allylsulfanyl)acetonitrile | - | - | - | - | 3.24 | 0.34 | 4.52 |
| M30 | 13.23 | 1316 | Dipropyl trisulphide | - | - | - | - | 3.75 | 3.51 | 5.108 |
| M31 | 13.37 | 1328 | unknown sulphur | - | - | - | - | 17.06 | 9.18 | 21.01 |
| M32 | 13.42 | 1332 | Diallyl trisulphide isomer | - | - | - | - | 3.85 | 2.69 | 11.45 |
| M33 | 14.00 | 1381 | 4-(Methylsulfinyl)butanenitrile | 0.002 | 0.02 | 2.27 | 0.003 | - | - | - |
| M34 | 14.033 | 1382 | Unknown sulphur | 0.002 | 0.012 | 1.17 | 0.002 | - | - | - |
| M35 | 15.408 | 1495 | unknown hydrocarbon | - | - | - | - | 1.56 | 2.19 | 2.37 |
| M36 | 15.98 | 1535 | Diallyl tetrasulphide | 0.03 | 0.11 | 7.32 | 0.014 | - | - | - |
| M37 | 16.18 | 1549 | 2,4-Dimethyl-5,6-dithia-2,7-nonadienal | tr. | 0.002 | 0.03 | tr. | - | - | - |
| M38 | 16.183 | 1549 | Unknown | tr. | 0.009 | 0.06 | tr. | - | - | - |
| M39 | 16.291 | 1577 | Ethyl dodecanoate | tr. | tr. | 0.027 | 0.003 | - | - | - |
| M40 | 16.717 | 1597 | Diethyl phthalate | tr. | tr. | 0.03 | 99.75 | - | - | - |
| M41 | 17.00 | 1605 | 2,4-Dimethyl-5,6-dithia-2,7-nonadienal | - | - | - | - | 21.24 | 7.8 | 4.68 |
| M42 | 18.058 | 1661 | Unknown sulphur | tr. | 0.031 | 0.04 | tr. | - | - | - |
| Peak | Rt Sec | MS | UV nm | Formula | Error ppm | MS/MS | Metabolite | Class | A. sativum | A. cepa |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 26 | 176.0954 | 265 | C6H10NO3S | −0.3 | - | Unknown | Peptide | + | - |
| L2 | 38 | 191.0196 | 267 | C6H7O7 | 0.1 | - | Citric acid/Isocitric acid | Organic acid | + | - |
| L3 | 70 | 337.1711 | - | C18H27O3NS | 1.4 | 319, 257, 175 | Unknown | - | + | - |
| L4 | 86 | 554.1658 | 295 | C28H28NO11 | −2.7 | 392 | Simmondsin-di-O-de-Me, di-O-benzoyl | Nitrile | - | + |
| L5 | 100 | 451.1401 | - | C17H27O10N2S | 0.7 | 433, 361, 289 | N-Hexosyl-γ-glutamyl-S-allylcysteine | - | ||
| L6 | 109 | 289.0873 | - | C11H17N2O5S | −3.1 | 271, 215, 128 | N-γ-Glutamyl-S-allylcysteine. | Peptide | + | - |
| L7 | 129 | 259.1298 | 281 | C11H19N2O5 | 0.6 | 203 | N-γ-Glutamylisoleucine | Peptide | - | + |
| L8 | 135 | 321.0612 | - | C11H17N2O5S2 | 1.1 | 303, 249, 128 | γ-Glutamyl-S-allylthiocysteine | Peptide | + | |
| L9 | 149 | 421.182 | 281 | C17H29N2O10 | 1.1 | 403, 331, 259 | N-Hexosyl-γ-glutamylisoleucine | Peptide | - | + |
| L10 | 153 | 289.0873 | - | C11H17N2O5S | −3.1 | 271, 215, 128 | N-γ-Glutamyl-S-allylcysteine | Peptide | + | - |
| L11 | 171 | 293.1135 | 218 | C14H17N2O5 | 2.7 | 165 | N-γ-Glutamylphenylalanine | Peptide | + | + |
| L12 | 171 | 455.1666 | 290 | C20H27N2O10 | 1.2 | 437, 365, 293 | N--Hexosyl-glutamylphenylalanine | Peptide | + | + |
| L13 | 184 | 353.0285 | 225, 279 | C15H21N4O2S2 | 3.9 | 165, 121 | Allithiamine | Thiamine deriv. | + | - |
| L14 | 184 | 165.019 | - | C8H5O4 | −0.6 | - | Phthalic acid | Phenolic acid | + | - |
| L15 | 207 | 321.0587 | - | C11H17N2O5S2 | 0.0 | 249, 171 | γ-Glutamyl-S-allylthiocysteine | Peptide | + | - |
| L16 | 231 | 625.1405 | 266, 343 | C27H29O17 | 0.2 | 361, 241 | Quercetin-O-diglucoside. | Flavonol | - | + |
| L17 | 235 | 361.1081 | 266, 344 | C22H17O5 | 0.1 | 241 | Unknown | - | - | + |
| L18 | 235 | 625.1405 | 266, 343 | C27H29O17 | 0.2 | 361, 241 | Quercetin-O-diglucoside. | Flavonol | - | + |
| L19 | 256 | 161 | 360 | C9H6O3 | −0.1 | - | Umbelliferone (IS) | Coumarin | - | - |
| L20 | 273 | 179.0346 | 279 | C9H7O4 | 2.1 | - | Caffeic acid | Phenolic acid | + | - |
| L21 | 302 | 463.0883 | 266, 365 | C21H19O12 | −0.2 | 301 | Quercetin-O-glucoside | Flavonol | - | + |
| L22 | 309 | 447.0933 | 267, 362 | C21H19O11 | −1.0 | 285 | Kaempferol-O-glucoside (Astragalin) | Flavonol | - | + |
| L23 | 311 | 447.0933 | 267, 362 | C21H19O11 | −1.0 | 301 | Quercetin-O-rhamnoside | Flavonol | - | + |
| L24 | 318 | 477.1029 | 365 | C22H21O12 | 2.1 | 315 | Isorhamnetin-O-hexoside | Flavonol | - | + |
| L25 | 324 | 228.1241 | - | C11H18NO4 | 0.3 | - | Unknown | - | - | + |
| L26 | 330 | 409.091 | 276 | C26H17O3S | −1.5 | - | Unknown | - | + | - |
| L27 | 330 | 193.0509 | 276 | C10H9O4 | −1.3 | - | Ferulic acid | Phenolic acid | + | - |
| L28 | 335 | 262.1089 | - | C14H16NO4 | −1.6 | - | N-p-Coumaroyl-valine | Acylated amino acid | - | + |
| L29 | 346 | 238.109 | 296 | C12H16NO4 | 0.6 | 164 | Unknown | - | - | + |
| L30 | 351 | 262.1088 | - | C14H16NO4 | −1.1 | - | N-p-Coumaroyl-valine isomer | Acylated amino acid | - | + |
| L31 | 352 | 273.08752 | - | C14H13O4N2 | 0.6 | 229 | Unknown | - | - | + |
| L32 | 354 | 419.0927 | - | C27H15O5 | −0.4 | - | Unknown | - | + | - |
| L33 | 365 | 301.0357 | 370 | C15H9O7 | −0.8 | 161, 179 | Quercetin | Flavonol | - | + |
| L34 | 372 | 305.0709 | - | C12H17O7S | −2.9 | 287, 225 | Jasmonic acid-hydroxy-O-sulfate | Oxylipid | + | - |
| L35 | 378 | 423.1193 | - | C22H19N2O7 | 1.1 | - | Unknown | - | + | - |
| L36 | 382 | 207.0658 | - | C11H11O4 | 2.1 | 177 | Caffeic acid dimethyl ether | Phenolic acid | + | - |
| L37 | 413 | 285.0403 | - | C15H9O6 | 0.6 | 161, 175 | Kaempferol | Flavonol | - | + |
| L38 | 423 | 315.051 | - | C16H11O7 | 0.1 | 300, 161, 176 | Isorhamnetin | Flavonol | - | + |
| L39 | 439 | 329.2337 | - | C18H33O5 | −0.4 | 311, 293, 257, 229, 211, 175 | 9,12,13-trihydroxy octadeca-7-enoic acid | Fatty acid | + | + |
| L40+ | 530 | 223.0962 | - | C12H15O4 | 0.3 | 249 | Diethylphthalate | Aromatic | + | - |
| L41 | 610 | 388.3057 | - | C21H42NO5 | 3.3 | 249, 317 | Unknown | - | - | + |
| L42 | 615 | 265.1477 | - | C12H25O4S | 0.9 | 175 | Trimethylnonanol sulphate | Oxylipid | + | + |
| L43 | 641 | 297.15283 | - | C12H25O8 | −1.5 | 183 | Unknown | - | + | - |
| L44 | 652 | 297.10323 | - | C19H21O3 | 4.3 | 183 | Unknown | - | - | + |
| L45 | 662 | 311.1686 | - | C20H23O3 | −5.9 | - | Unknown | - | - | + |
| L46 | 670 | 311.1137 | - | C13H27O8 | −2.0 | - | Unknown | - | + | + |
| L47 | 680 | 295.2276 | - | C18H31O3 | 1.1 | 249 | Oxo-octadecenoic acid | Fatty acid | + | - |
| L48 | 867 | 279.2324 | - | C18H31O2 | 2.9 | 181 | Linoleic acid | Fatty acid | + | + |
| L49 | 912 | 255.2329 | - | C16H31O2 | 0.2 | - | Palmitic acid | Fatty acid | + | - |
| L50 | 927 | 281.2485 | - | C18H33O2 | 1.5 | - | Oleic acid | Fatty acid | + | + |
| L51 | 983 | 283.2638 | - | C18H35O2 | 1.5 | - | Stearic acid | Fatty acid | + | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.F.; Wessjohann, L.A. Phytochemical Profiles and Antimicrobial Activities of Allium cepa Red cv. and A. sativum Subjected to Different Drying Methods: A Comparative MS-Based Metabolomics. Molecules 2017, 22, 761. https://doi.org/10.3390/molecules22050761
Farag MA, Ali SE, Hodaya RH, El-Seedi HR, Sultani HN, Laub A, Eissa TF, Abou-Zaid FOF, Wessjohann LA. Phytochemical Profiles and Antimicrobial Activities of Allium cepa Red cv. and A. sativum Subjected to Different Drying Methods: A Comparative MS-Based Metabolomics. Molecules. 2017; 22(5):761. https://doi.org/10.3390/molecules22050761
Chicago/Turabian StyleFarag, Mohamed A., Sara E. Ali, Rashad H. Hodaya, Hesham R. El-Seedi, Haider N. Sultani, Annegret Laub, Tarek F. Eissa, Fouad O. F. Abou-Zaid, and Ludger A. Wessjohann. 2017. "Phytochemical Profiles and Antimicrobial Activities of Allium cepa Red cv. and A. sativum Subjected to Different Drying Methods: A Comparative MS-Based Metabolomics" Molecules 22, no. 5: 761. https://doi.org/10.3390/molecules22050761






