Inferring the Genetic Determinants of Fruit Colors in Tomato by Carotenoid Profiling
Abstract
:1. Introduction
2. Results and Discussion
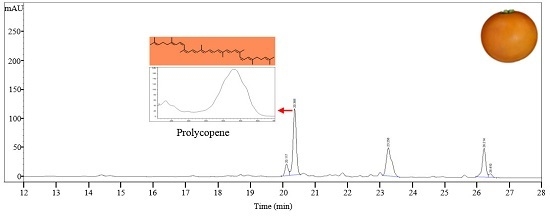
2.1. Separation and Identification of Carotenoid Compounds in Red Tomato Fruits Using HPLC
2.2. Analysis of Carotenoid Profiles in Tomatoes Showing Various Colors of Ripe Fruits
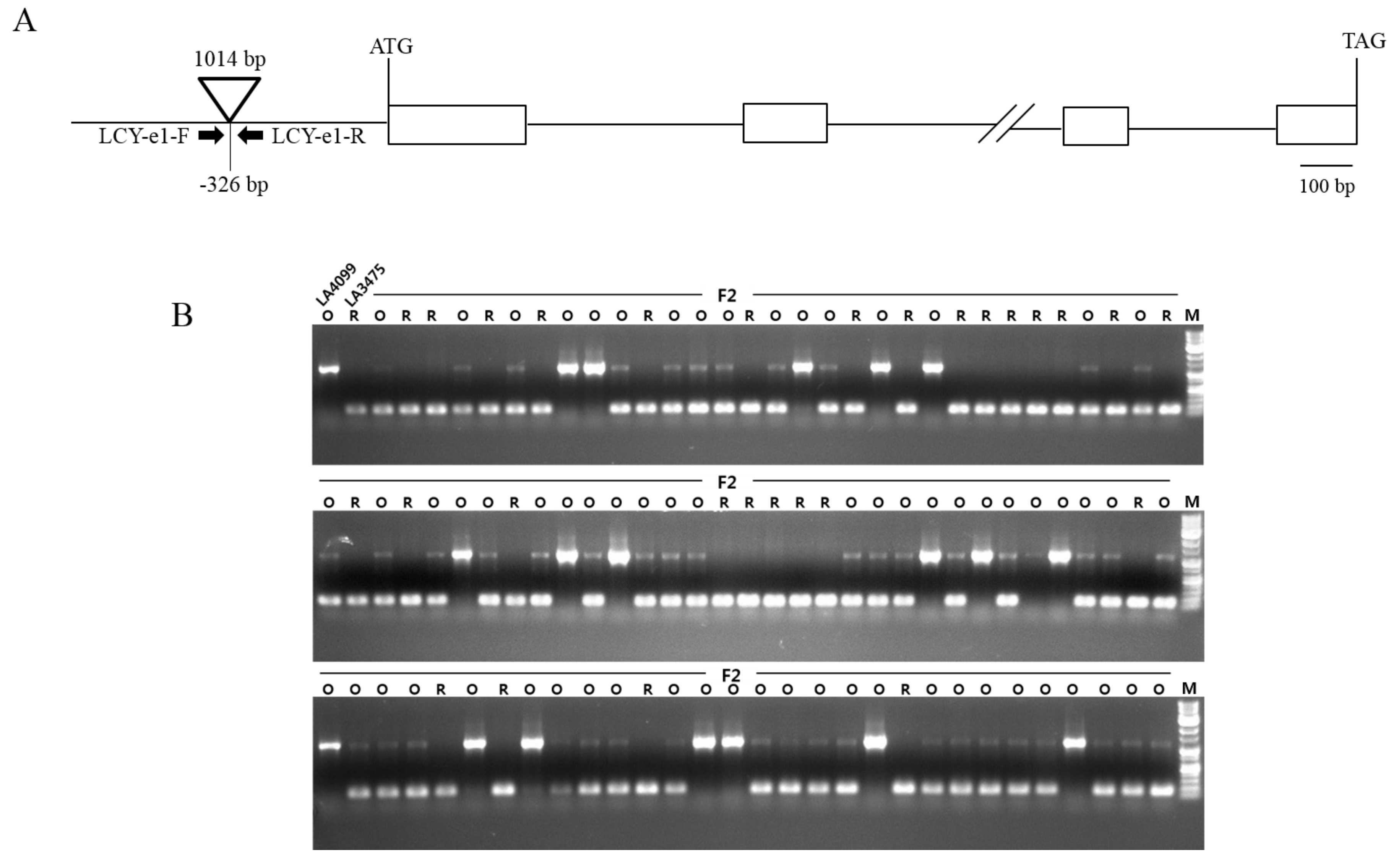
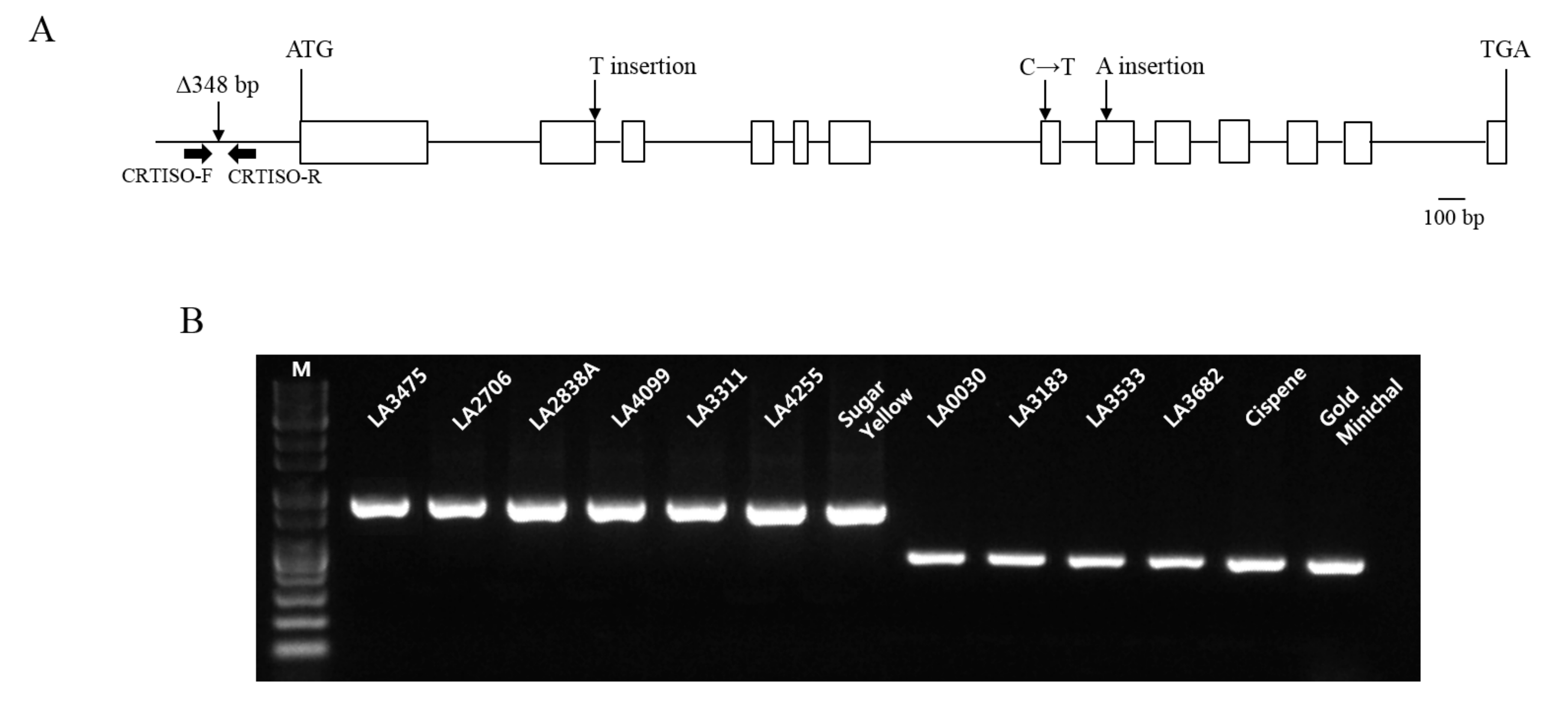
2.3. Association Analysis between HPLC Profiles and Genotype Screening for Carotenoid Accumulation
3. Materials and Methods
3.1. Plant Materials
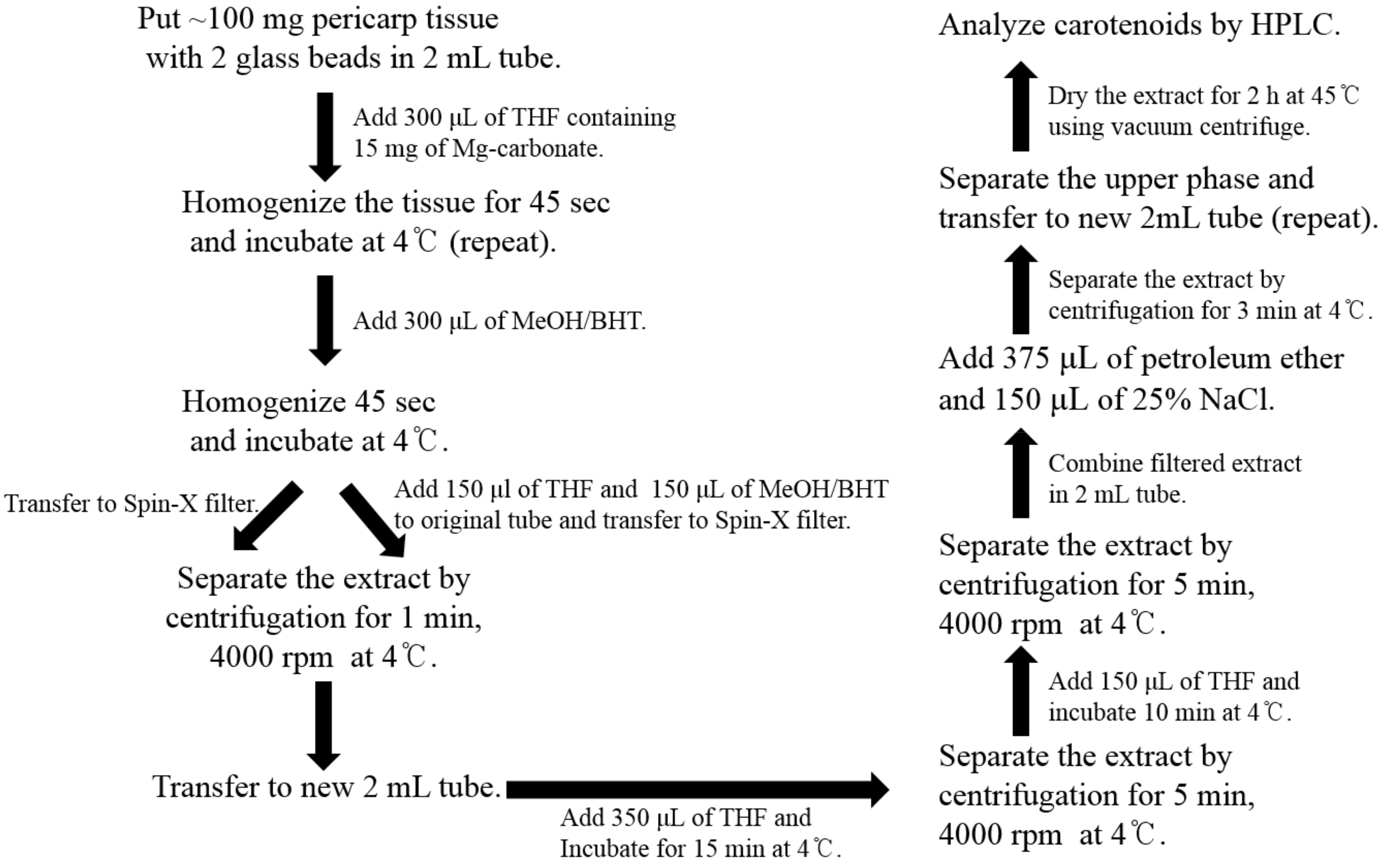
3.2. Carotenoid Extraction
3.3. Carotenoid Analysis Using HPLC
3.4. DNA Extraction
3.5. Sequence Analysis and DNA Marker Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell 1993, 5, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Gómez-Gómez, L.; Rodrigo, M.; Avalos, J.; Limón, M. Carotenoid Cleavage Oxygenases from Microbes and Photosynthetic Organisms: Features and Functions. Int. J. Mol. Sci. 2016, 17, e1781. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.A.; Erdman, J.W. Are lycopene metabolites metabolically active. Acta Biochim. Pol. 2012, 59, 1–4. [Google Scholar] [PubMed]
- Cookson, P.J.; Kiano, J.W.; Shipton, C.A.; Fraser, P.D.; Romer, S.; Schuch, W.; Bramley, P.M.; Pyke, K.A. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta 2003, 217, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Enfissi, E.M.; Halket, J.M.; Truesdale, M.R.; Yu, D.; Gerrish, C.; Bramley, P.M. Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 2007, 19, 3194–3211. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Fray, R.G.; Grierson, D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from Tomato Reveals a Carotenoid Isomerase Essential for the Production of β-Carotene and Xanthophylls in Plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ohad, I.; Beyer, P.; Hirschberg, J. Analysis in Vitro of the Enzyme CRTISO Establishes a Poly-cis-Carotenoid Biosynthesis Pathway in Plants. Plant Physiol. 2004, 136, 4246–4255. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Li, J.; Yang, C.; Wang, T.; Ouyang, B.; Li, H.; Giovannoni, J.; Ye, Z. A STAY-GREEN protein SlSGR1 regulates lycopene and beta-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol. 2013, 198, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Cohen, M.; Zamir, D.; Hirschberg, Z. Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999, 17, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Rosati, C.; Aquilani, R.; Dharmapuri, S.; Pallara, P.; Marusic, C.; Tavazza, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kachanovsky, D.E.; Filler, S.; Isaacson, T.; Hirschberg, J. Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc. Natl. Acad. Sci. USA 2012, 109, 19021–19026. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-Localized Phytochromes Regulate Lycopene Accumulation Independently of Ethylene Production in Tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Schofield, A.; Paliyath, G. Modulation of carotenoid biosynthesis during tomato fruit ripening through phytochrome regulation of phytoene synthase activity. Plant Physiol. Biochem. 2005, 43, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Cocaliadis, M.F.; Fernández-Muñoz, R.; Pons, C.; Orzaez, D.; Granell, A. Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J. Exp. Bot. 2014, 65, 4589–4598. [Google Scholar] [CrossRef] [PubMed]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidantcontent of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Martel, C.; Vrebalov, J.; Tafelmeyer, P.; Giovannoni, J.J. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol. 2011, 157, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Vrebalov, J.; Pan, I.L.; Arroyo, A.J.; McQuinn, R.; Chung, M.; Poole, M.; Rose, J.; Seymour, G.; Grandillo, S.; Giovannoni, J.; et al. Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1. Plant Cell 2009, 21, 3041–3062. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; van Tuinen, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Joung, J.G.; McQuinn, R.; Chung, M.Y.; Fei, Z.; Tieman, D.; Klee, H.; Giovannoni, J. Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J. 2012, 70, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Apel, W.; Bock, R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol. 2009, 151, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Vrebalov, J.; Alba, R.; Lee, J.; McQuinn, R.; Chung, J.-D.; Klein, P.; Giovannoni, J. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 2010, 64, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Menda, N.; Semel, Y.; Peled, D.; Eshed, Y.; Zamir, D. In silico screening of a saturated mutation library of tomato. Plant J. 2004, 38, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A Novel Tomato Mutant Database Distributing Micro-Tom Mutant Collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Matsuda, F. Metabolomics for Functional Genomics, Systems Biology, and Biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Benning, C. Genetic mutant screening by direct metabolite analysis. Anal. Biochem. 2004, 332, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Lee, S.; Rim, Y.; Kumar, R.; Han, X.; Lee, S.Y.; Lee, C.H.; Kim, J.Y. De-novo RNA Sequencing and Metabolite Profiling to Identify Genes Involved in Anthocyanin Biosynthesis in Korean Black Raspberry (Rubus coreanus Miquel). PLoS ONE 2014, 9, e88292. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Sérino, S.; Laurent, G.; Costagliola, G.; Gautier, H. HPLC Assay of Tomato Carotenoids: Validation of a Rapid Microextraction Technique. J. Agric. Food Chem. 2009, 57, 8753–8760. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Pinto, M.E.S.; Holloway, D.E.; Bramley, P.M. Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000, 24, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. General carotenoid methods. Methods Enzymol. 1985, 3, 113–149. [Google Scholar]
- Pankratov, I.; McQuinn, R.; Schwartz, J.; Bar, E.; Fei, Z.; Lewinsohn, E.; Zamir, D.; Giovannoni, J.J.; Hirschberg, J. Fruit carotenoid-deficient mutants in tomato reveal a function of the plastidial isopentenyl diphosphate isomerase (IDI1) in carotenoid biosynthesis. Plant J. 2016, 88, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Giorio, G.; Yildirim, A.; Stigliani, A.L.; D’Ambrosio, C. Elevation of lutein content in tomato: A biochemical tug-of-war between lycopene cyclases. Metab. Eng. 2013, 20, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Boileau, T.W.M.; Boileau, A.C.; John, W.; Erdman, J. Bioavailability of all-trans and cis–Isomers of Lycopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef]
- Burri, B.J.; Chapman, M.H.; Neidlinger, T.R.; Seo, J.S.; Ishida, B.K. Tangerine tomatoes increase total and tetra-cis-lycopene isomer concentrations more than red tomatoes in healthy adult humans. Int. J. Food Sci. Nutr. 2009, 60, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the tomatoes are available from the authors. |


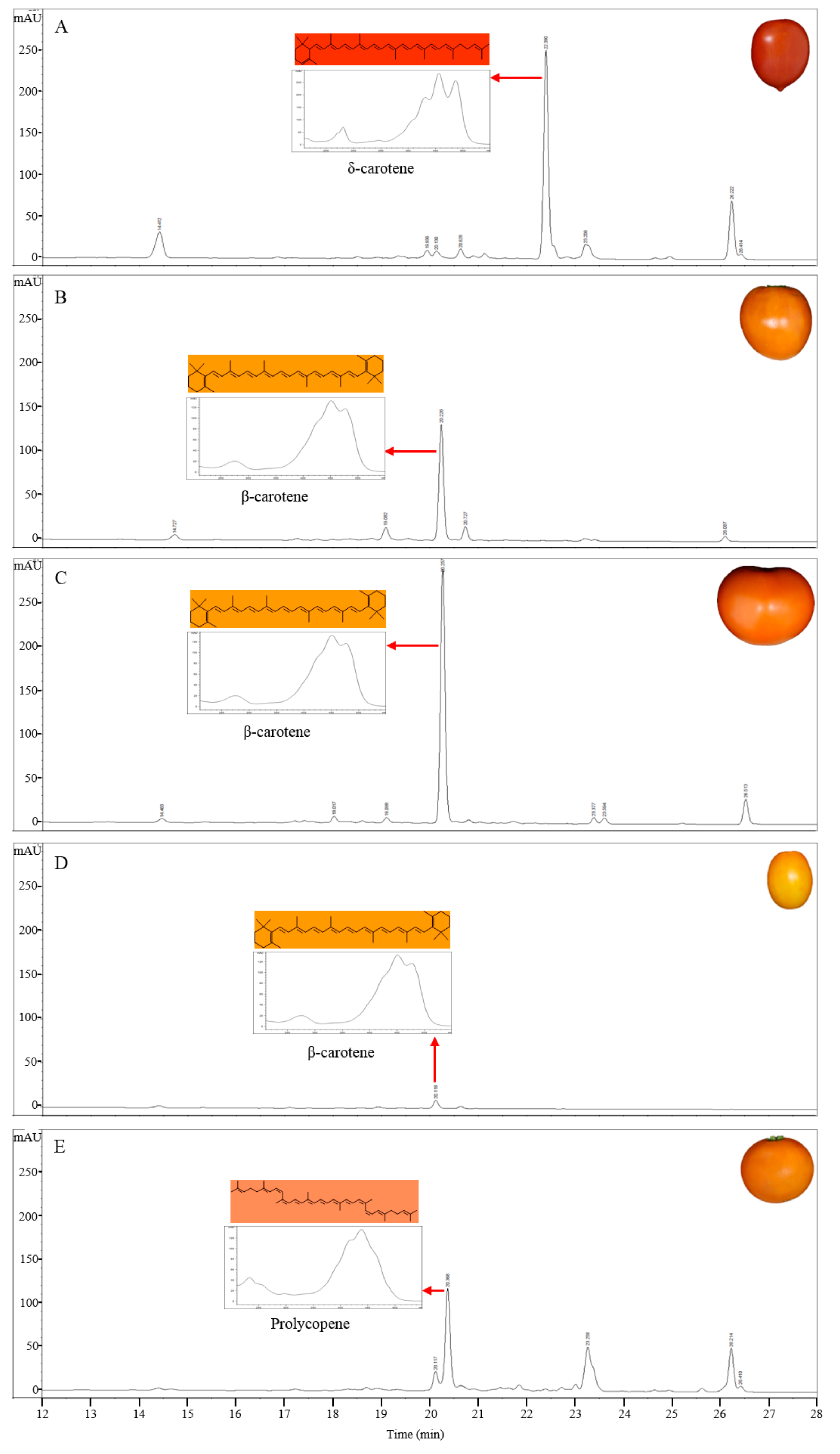
| Compound | RT (min) | λ (nm) | Observed Wavelength (nm) | |||||
|---|---|---|---|---|---|---|---|---|
| Observed | Reported | |||||||
| Phytoene | 16.9 | (274) * | 286 | 298 | (276) | 286 | 297 | 286 |
| Phytofluene | 17.6 | 330 | 350 | 366 | 331 | 348 | 367 | 348 |
| 18.4 | ||||||||
| Lutein | 14.3 | (418) | 442 | 470 | (421) | 445 | 474 | 434 |
| β-carotene | 20.3 | (422) | 450 | 478 | (425) | 449 | 476 | 450 |
| Prolycopene | 20.6 | 414 | 438 | (466) | 414 | 436 | 463 | 450 |
| δ-carotene | 22.3 | 430 | 454 | 486 | 431 | 456 | 489 | 450 |
| γ-carotene | 23.5 | 438 | 466 | 494 | 437 | 462 | 494 | 450 |
| cis-lycopene | 25.1 | 446 | 470 | 502 | 446 | 472 | 503 | 471 |
| trans-lycopene | 26.4 | 446 | 470 | 502 | 444 | 470 | 502 | 471 |
| Marker Name | Primer Name | Sequence (5′–3′) | Tm (°C) |
|---|---|---|---|
| LCY-e1 | LCY-e1-F | CATGTTTGAAAACAAGCCAATATTG | 56.4 |
| LCY-e1-R | TGCTGGAGTTATTTCATCTTGAC | 57.0 | |
| CRTISO | CRTISO-F | AGTCGAATCAATCTGAATTCACCT | 57.5 |
| CRTISO-R | GGTCAAAACAAGAACTTCTCTGTT | 57.5 | |
| Primers for CRTISO cloning | CRTISO-gDNA-1F | TCTTGGGTTTCCAGCAATTTAAAG | 57.5 |
| CRTISO-gDNA-1R | AACTTCTAATTTACGTCCTACTGC | 57.5 | |
| CRTISO-gDNA-2F | TACTATTGTATATGGTCTGCAGTG | 57.5 | |
| CRTISO-gDNA-2R | AGAATTACCATGCTTGCATTGATC | 57.5 | |
| CRTISO-gDNA-3F | TTGCTGTCTTTTATAGATGCAGAG | 57.5 | |
| CRTISO-gDNA-3R | ATACTGGCAAAGACAACATTGAAG | 57.5 | |
| CRTISO-gDNA-4F | TCAGTTACATCCCTGAAGTTATTC | 57.5 | |
| CRTISO-gDNA-4R | GTAAACATAGATGGTGTAACTCCA | 57.5 | |
| CRTISO-gDNA-5F | CTGAAAGGATTATAAGCAGACTTG | 57.5 | |
| CRTISO-gDNA-5R | AATCTGTGATTTCTATACTGGAGG | 57.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.J.; Park, W.J.; Lee, G.-M.; Oh, C.-S.; Yeam, I.; Won, D.-C.; Kim, C.K.; Lee, J.M. Inferring the Genetic Determinants of Fruit Colors in Tomato by Carotenoid Profiling. Molecules 2017, 22, 764. https://doi.org/10.3390/molecules22050764
Yoo HJ, Park WJ, Lee G-M, Oh C-S, Yeam I, Won D-C, Kim CK, Lee JM. Inferring the Genetic Determinants of Fruit Colors in Tomato by Carotenoid Profiling. Molecules. 2017; 22(5):764. https://doi.org/10.3390/molecules22050764
Chicago/Turabian StyleYoo, Hee Ju, Woo Jung Park, Gyu-Myung Lee, Chang-Sik Oh, Inhwa Yeam, Dong-Chan Won, Chang Kil Kim, and Je Min Lee. 2017. "Inferring the Genetic Determinants of Fruit Colors in Tomato by Carotenoid Profiling" Molecules 22, no. 5: 764. https://doi.org/10.3390/molecules22050764