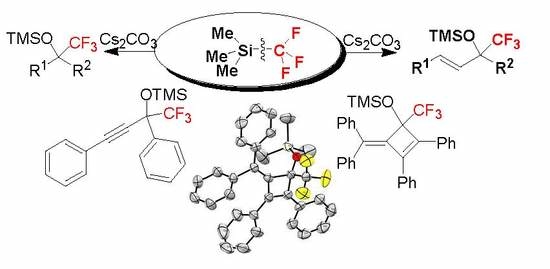
Cs2CO3-Initiated Trifluoro-Methylation of Chalcones and Ketones for Practical Synthesis of Trifluoromethylated Tertiary Silyl Ethers
Abstract
:1. Introduction
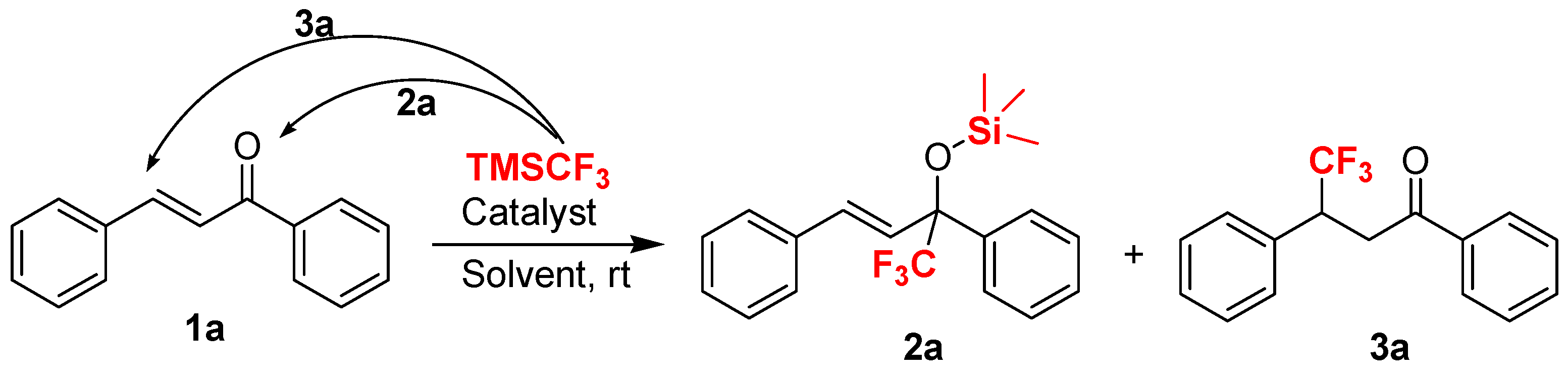
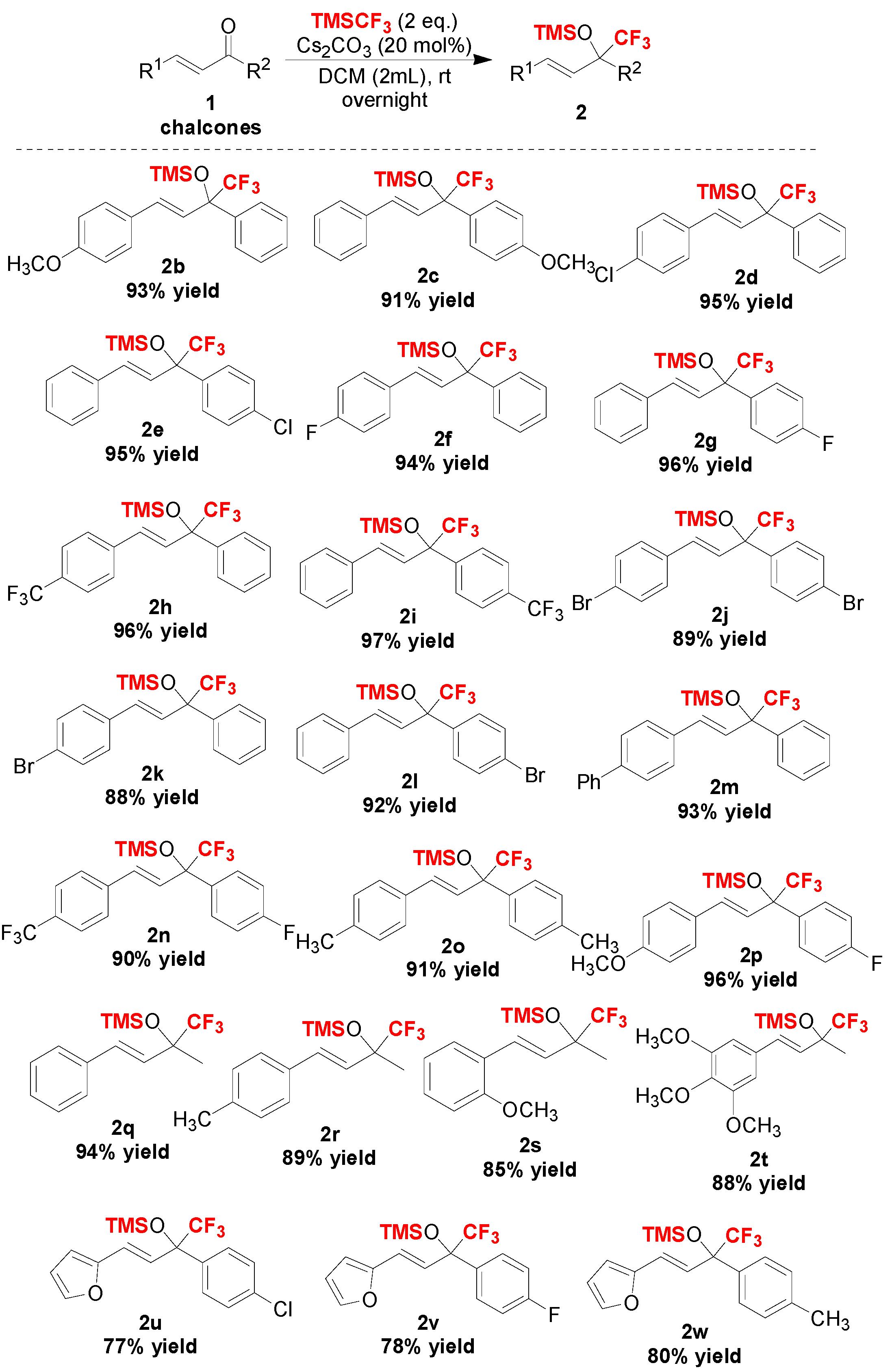
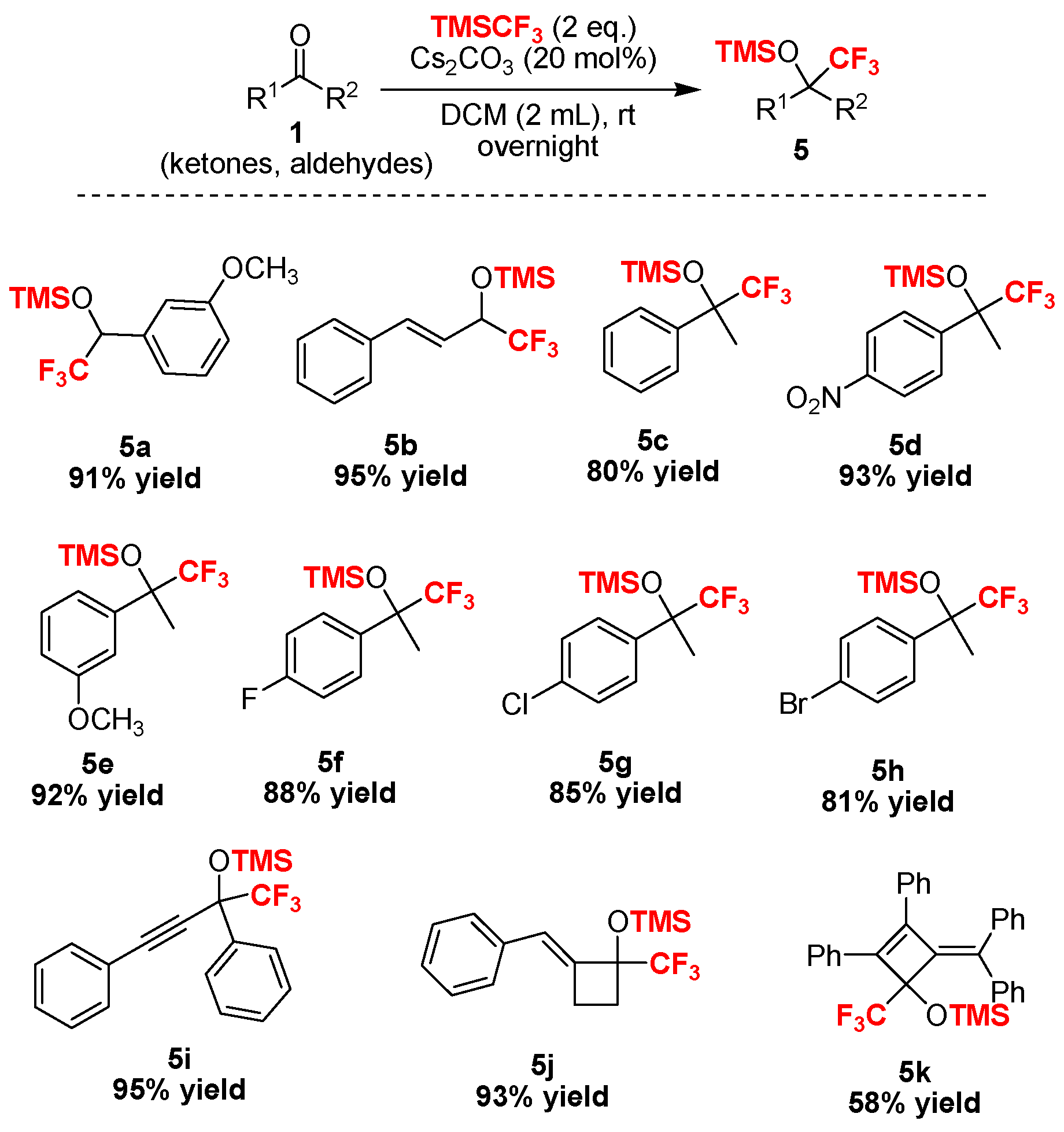
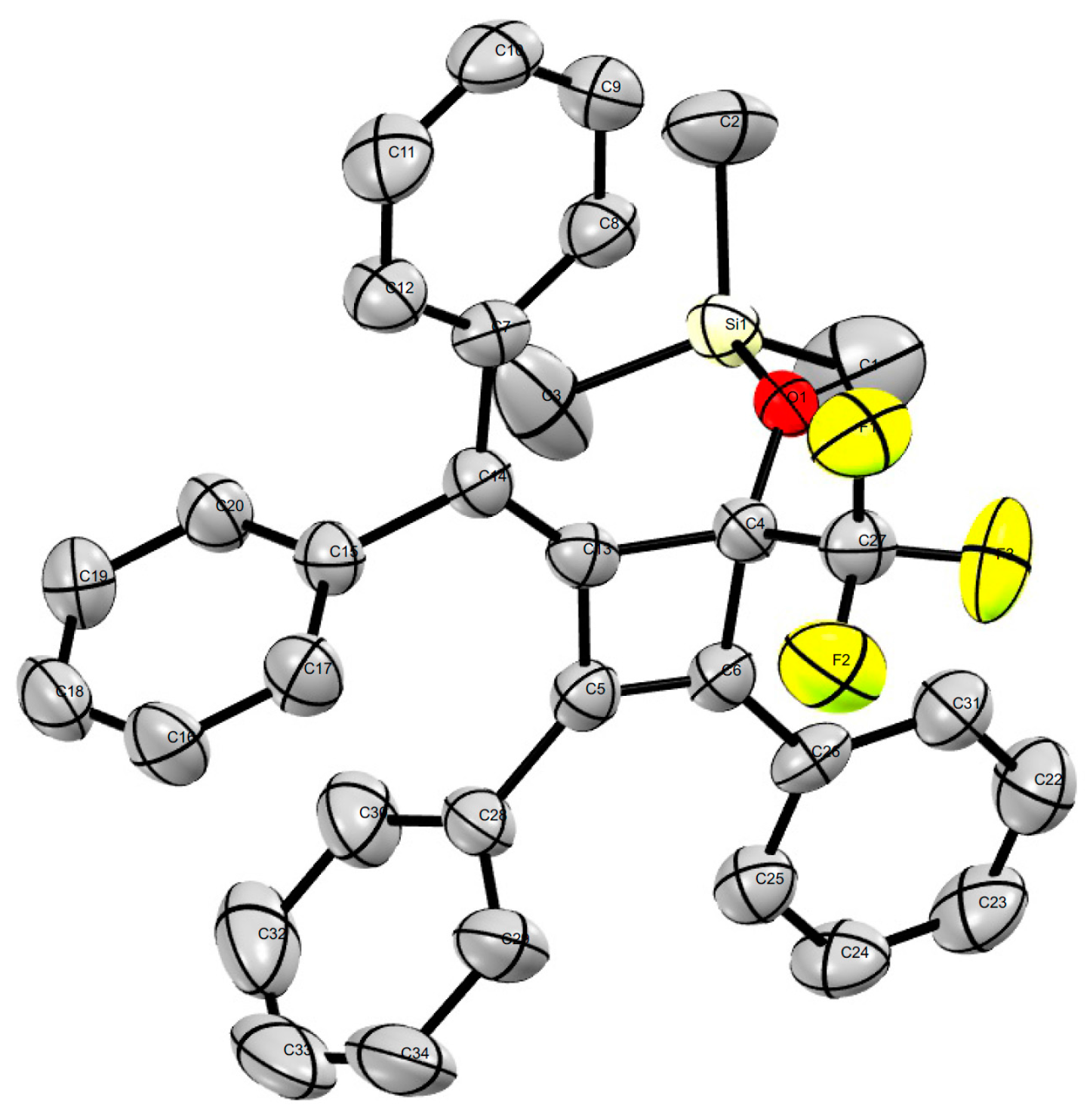
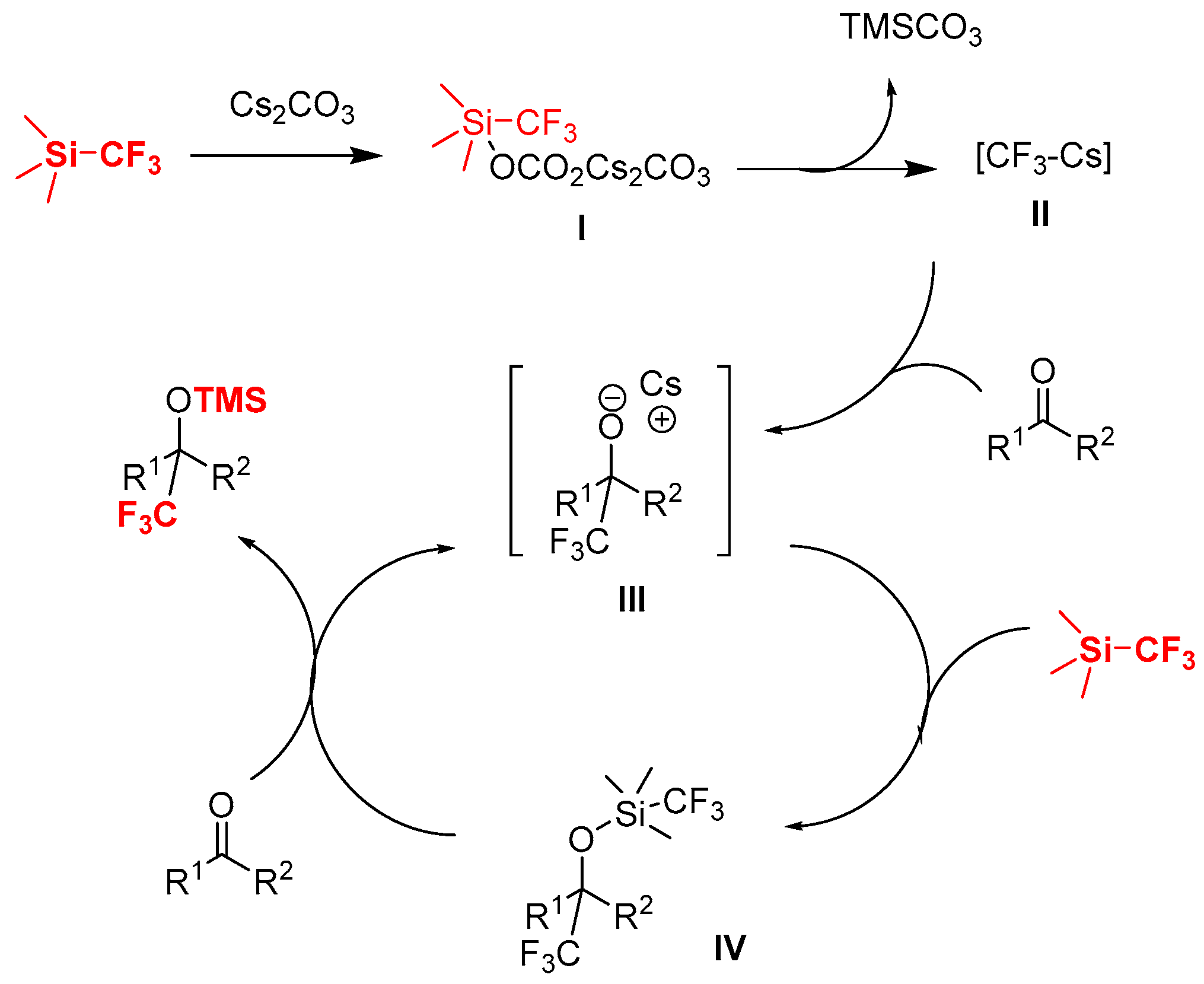
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedures
3.2.1. General Procedure for Cs2CO3-Catalyzed Addition of TMSCF3 and Chalcone 1a (Scheme 5)
3.2.2. Characterization of Compounds 2a–2w
3.2.3. Characterization of Compounds 5a–5k
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Nie, J.; Guo, H.C.; Cahard, D.; Ma, J.A. Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates. Chem. Rev. 2011, 111, 455–529. [Google Scholar] [CrossRef] [PubMed]
- Dolfen, J.; de Kimpe, N.; D’hooghe, M. Deployment of Small-Ring Azaheterocycles as Building Blocks for the Synthesis of Organofluorine Compounds. Synlett 2016, 27, 1486–1510. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Yudin, A.K. Perfluoroalkylation with Organosilicon Reagents. Chem. Rev. 1997, 97, 757–786. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.A.; Cahard, D. Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2004, 104, 6119–6146. [Google Scholar] [CrossRef]
- Caron, S.; Do, N.M.; Sieser, J.E.; Arpin, P.; Vazquez, E. Process Research and Development of an NK-1 Receptor Antagonist. Enantioselective Trifluoromethyl Addition to a Ketone in the Preparation of a Chiral Isochroman. Org. Proc. Res. Dev. 2007, 11, 1015–1024. [Google Scholar] [CrossRef]
- Boechat, N.; Bastos, M.M. Trifluoromethylation of Carbonyl Compounds. Curr. Org. Chem. 2010, 7, 403–410. [Google Scholar] [CrossRef]
- Tomashenko, O.A.; Grushin, V.V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 2011, 111, 4475–4521. [Google Scholar] [CrossRef] [PubMed]
- Konno, T. Trifluoromethylated internal alkynes: Versatile building blocks for the preparation of various fluorine-containing molecules. Synlett 2014, 25, 1350–1370. [Google Scholar] [CrossRef]
- Ma, J.A.; Cahard, D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J. Fluor. Chem. 2007, 128, 975–996. [Google Scholar] [CrossRef]
- Shibata, N.; Mizuta, S.; Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron Asymmetry 2008, 19, 2633–2644. [Google Scholar] [CrossRef]
- Nagib, D.A.; Scott, M.E.; MacMillan, D.W.C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875–10877. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; MacMillan, D.W.C. The Productive Merger of Iodonium Salts and Organocatalysis: A Non-photolytic Approach to the Enantioselective α-Trifluoromethylation of Aldehydes. J. Am. Chem. Soc. 2010, 132, 4986–4987. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Nagib, D.A.; MacMillan, D.W.C. Photoredox Catalysis: A Mild, Operationally Simple Approach to the Synthesis of α-Trifluoromethyl Carbonyl Compounds. Angew. Chem. Int. Ed. 2011, 50, 6119–6122. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Nishimine, T.; Tokunaga, E.; Hasegawa, K.; Shiro, M.; Shibata, N. Organocatalyzed Regio- and Enantioselective Allylic Trifluoromethylation of Morita-Baylis-Hillman Adducts Using Ruppert-Prakash Reagent. Org. Lett. 2011, 13, 3972–3975. [Google Scholar] [CrossRef] [PubMed]
- Matousek, V.; Togni, A.; Bizet, V.; Cahard, D. Synthesis of α-CF3-substituted carbonyl compounds with relative and absolute stereocontrol using electrophilic CF3-transfer reagents. Org. Lett. 2011, 13, 5762–5765. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Jog, P.V.; Batamack, P.T.D.; Olah, G.A. Taming of Fluoroform: Direct Nucleophilic Trifluoromethylation of Si, B, S, and C Centers. Science 2012, 338, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, R.R.; Jia, Y.X. Asymmetric friedel-crafts alkylation reaction in the construction of trifluoromethylated all-carbon quaternary stereocenters. Synlett 2014, 25, 457–460. [Google Scholar] [CrossRef]
- Liu, H.Q.; Zhang, Z.P.; Dong, W.; Luo, X.Z. A practical method for metal-free radical trifluoromethylation of styrenes with NaSO2CF3. Synlett 2014, 25, 1307–1311. [Google Scholar]
- Zhang, Z.K.; Feng, J.J.; Xu, Y.; Zhang, S.N.; Ye, X.X.; Li, T.J.; Wang, X.; Chen, J.; Zhang, Y.; Wang, J.B. Synthesis of trifluoromethylated cycloheptatrienes from N-tosylhydrazones: Transition-metal-free Buchner ring expansion. Synlett 2015, 26, 59–62. [Google Scholar] [CrossRef]
- Zhu, C.L.; Zhang, Y.Q.; Yuan, Y.A.; Xu, H. Copper-Catalyzed Aerobic C-H Trifluoromethylation of Phenanthrolines. Synlett 2015, 26, 345–349. [Google Scholar] [PubMed]
- Prakash, G.K.S.; Krishnamurti, R.; Olah, G.A. Synthetic methods and reactions. 141. Fluoride-induced trifluoromethylation of carbonyl compounds with trifluoromethyltrimethylsilane (TMS-CF3). A trifluoromethide equivalent. J. Am. Chem. Soc. 1989, 111, 393–395. [Google Scholar] [CrossRef]
- Singh, R.P.; Kirchmeier, R.L.; Shreeve, J.M. CsF-Catalyzed Nucleophilic Trifluoromethylation of trans-Enones with Trimethyl(trifluoromethyl)silane: A Facile Synthesis of trans-α-Trifluoromethyl Allylic Alcohols. Org. Lett. 1999, 1, 1047–1049. [Google Scholar] [CrossRef]
- Motherwell, W.B.; Storey, L.J. Trifluoromethylacetophenone-N,N-dimethyltrimethylsilylamine Adduct—A New Shelf Stable Reagent for Nucleophilic Trifluoromethylation. Synlett 2002, 4, 646–648. [Google Scholar] [CrossRef]
- Notte, G.T.; Sammakia, T.; Steel, P.J. Kinetic Resolution of α-Acetoxy N-Acyl Oxazolidinethiones by a Chiral O-Nucleophilic Acyl Transfer Catalyst. J. Am. Chem. Soc. 2005, 127, 13502–13503. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.; Sayalero, S.; Vicente, M.; Maestro, A. An Efficient Synthesis of Enantiomerically Enriched Trifluoromethylated 1,2-Diols and 1,2-Amino Alcohols with Quaternary Stereocenters by Diastereoselective Addition of TMSCF3 to Chiral 2-Acyl-1,3-perhydrobenzoxazines. J. Org. Chem. 2006, 71, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Shibata, N.; Akiti, S.; Fujimoto, H.; Nakamura, S.; Toru, T. Cinchona alkaloids/TMAF combination-catalyzed nucleophilic enantioselective trifluoromethylation of aryl ketones. Org. Lett. 2007, 9, 3707–3710. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Shibata, N.; Hibino, M.; Nagano, S.; Nakamura, S.; Toru, T. Ammonium bromides/KF catalyzed trifluoromethylation of carbonyl compounds with (trifluoromethyl)trimethylsilane and its application in the enantioselective trifluoromethylation reaction. Tetrahedron 2007, 63, 8521–8528. [Google Scholar] [CrossRef]
- Nonnenmacher, J.; Massicot, F.; Grellepois, F.; Portella, C. Enantiopure Quaternary α-Trifluoromethyl-α-alkoxyaldehydes from L-Tartaric Acid Derived Ketoamides. J. Org. Chem. 2008, 73, 7990–7995. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Li, W.; Lin, L.; Liu, X.; Feng, X. Cinchona alkaloid-derived quaternary ammonium salt combined with NaH: A facile catalyst system for the asymmetric trifluoromethylation of ketones. Tetrahedron Lett. 2009, 50, 4378–4380. [Google Scholar] [CrossRef]
- Kawai, H.; Tachi, K.; Tokunaga, E.; Shiro, M.; Shibata, N. Cinchona Alkaloid-Catalyzed Asymmetric Trifluoromethylation of Alkynyl Ketones with Trimethylsilyl Trifluoromethane. Org. Lett. 2010, 12, 5104–5107. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kitayama, T.; Tokunaga, E.; Shibata, N. A New Synthetic Approach to Efavirenz through Enantioselective Trifluoromethylation by Using the Ruppert-Prakash Reagent. Eur. J. Org. Chem. 2011, 2011, 5959–5961. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, W.; Wang, Q.; Chen, F.X. Asymmetric trifluoromethylation of aromatic aldehydes by cooperative catalysis with (IPr)CuF and quinidine-derived quaternary ammonium salt. Org. Biomol. Chem. 2012, 10, 9334–9337. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shreeve, J.M. Synthesis and characterization of novel trifluoromethyl-containing alcohols with Ruppert’s reagent. J. Fluor. Chem. 2012, 133, 20–26. [Google Scholar] [CrossRef]
- Kawai, H.; Yuan, Z.; Tokunaga, E.; Shibata, N. A sterically demanding organo-superbase avoids decomposition of a naked trifluoromethyl carbanion directly generated from fluoroform. Org. Biomol. Chem. 2013, 11, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, J.; Sohail, M.; Cao, C.; Chen, F.X. The enantioselective trifluoromethylation of aromatic aldehydes by quaternary ammonium bromide and (IPr)CuF at low catalyst loading. J. Fluor. Chem. 2013, 148, 19–29. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kawano, Y.; Fujisawa, H. Lithium acetate–catalyzed trifluoromethylation of carbonyl compounds with (trifluoromethyl)trimethylsilane. Chem. Lett. 2005, 34, 88–89. [Google Scholar] [CrossRef]
- Kawano, Y.; Kaneko, N.; Mukaiyama, T. Lewis Base-catalyzed perfluoroalkylation of carbonyl compounds and imines with (perfluoroalkyl)trimethylsilane. Bull. Chem. Soc. Jpn. 2006, 79, 1133–1145. [Google Scholar] [CrossRef]
- Matsukawa, S.; Saijo, M. TTMPP-catalyzed trifluoromethylation of carbonyl compounds and imines with trifluoromethylsilane. Tetrahedron Lett. 2008, 49, 4655–4657. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.X.; Pan, L.; Zhang, Q.; Liu, Q. Efficient synthesis of trifluoromethylated cyclopentadienes/fulvenes/norbornenes from divinyl ketones. Org. Biomol. Chem. 2013, 11, 6703–6706. [Google Scholar] [CrossRef] [PubMed]
- Iakovenko, R.O.; Kazakova, A.N.; Muzalevskiy, V.M.; Ivanov, A.Y.; Boyarskaya, I.A.; Chicca, A.; Petrucci, V.; Gertsch, J.; Krasavin, M.; Starova, G.L.; et al. Reactions of CF3-enones with arenes under superelectrophilic activation: A pathway to trans-1,3-diaryl-1-CF3-indanes, new cannabinoid receptor ligands. Org. Biomol. Chem. 2015, 13, 8827–8842. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Takahashi, S.; Takahashi, H. TBD-Catalyzed Trifluoromethylation of Carbonyl Compounds with (Trifluoromethyl)-Trimethylsilane. Synth. Commun. 2013, 43, 1523–1529. [Google Scholar] [CrossRef]
- Iwanami, K.; Oriyama, T. A New and Efficient Method for the Trifluoromethylation of Carbonyl Compounds with Trifluoromethyltrimethylsilane in DMSO. Synlett 2006, 1, 112–114. [Google Scholar] [CrossRef]
- Kusuda, A.; Kawai, H.; Nakamura, S.C.; Shibata, N. Solkane R 365mfc is an environmentally benign alternative solvent for trifluoromethylation reactions. Green Chem. 2009, 11, 1733–1735. [Google Scholar] [CrossRef]
- Nagao, H.; Yamane, Y.; Mukaiyama, T. Asymmetric Trifluoromethylation of Ketones with (Trifluoromethyl)trimethylsilane Catalyzed by Chiral Quaternary Ammonium Phenoxides. Chem. Lett. 2007, 36, 666–667. [Google Scholar] [CrossRef]
- Surya Prakash, G.K.; Panja, C.; Vaghoo, H.; Surampudi, V.; Kultyshev, R.; Mandal, M.; Rasul, G.; Mathew, T.; Olah, G.A. Facile Synthesis of TMS-Protected Trifluoromethylated Alcohols Using Trifluoromethyltrimethylsilane (TMSCF3) and Various Nucleophilic Catalysts in DMF. J. Org. Chem. 2006, 71, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yue, Z.; Zhang, J.L. A highly efficient one-pot trifluoromethylation/ cyclization reaction of electron-deficient 1,3-conjugated enynes: Modular access to trifluoromethylated furans and 2,3-dihydrofurans. Org. Chem. Front. 2016, 3, 1416–1419. [Google Scholar] [CrossRef]
- Yang, W.; Cui, Y.M.; Zhou, W.; Li, L.; Yang, K.F.; Zheng, Z.J.; Lu, Y.X.; Xu, L.W. Enantioselective primary amine catalyzed aldol-type construction of trifluoromethylated tertiary alcohols. Synlett 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- Shang, J.Y.; Li, L.; Lu, Y.X.; Yang, K.F.; Xu, L.W. Enantioselective Fluorination Reaction of β-Ketoester-Catalyzed Chiral Primary Amine-Based Multifunctional Catalyst Systems. Synth. Commun. 2014, 44, 101–114. [Google Scholar] [CrossRef]
- Wang, H.; Yang, K.F.; Li, L.; Bai, Y.; Zheng, Z.J.; Zhang, W.Q.; Gao, Z.W.; Xu, L.W. Modulation of Silver-Titania Nanoparticles on Polymethylhydrosiloxane-based Semi-Interpenetrating Networks for Catalytic Alkynylation of Trifluoromethyl Ketones and Aromatic Aldehydes in Water. ChemCatChem 2014, 6, 580–591. [Google Scholar] [CrossRef]
- Zheng, L.S.; Wei, Y.L.; Jiang, K.Z.; Deng, Y.; Zheng, Z.J.; Xu, L.W. Enantioselective Fluorination of β-Ketoamides Catalyzed by Ar-BINMOL-derived Salan-Copper Complex. Adv. Synth. Catal. 2014, 356, 3769–3776. [Google Scholar] [CrossRef]
- Okusu, S.; Sugita, Y.; Tokunaga, E.; Shibata, N. Regioselective 1,4-trifluoromethylation of α,β-unsaturated ketones via a S-(trifluoromethyl)diphenylsulfonium salts/copper system. Beilstein J. Org. Chem. 2013, 9, 2189–2193. [Google Scholar] [CrossRef] [PubMed]
- Sosnovskikh, V.Y.; Sevenard, D.V.; Usachev, B.I.; Röschenthaler, G.V. The first example of a preparative 1,4-perfluoroalkylation using (perfluoroalkyl)trimethylsilanes. Tetrahedron Lett. 2003, 44, 2097–2099. [Google Scholar] [CrossRef]
- The polysubstituted alkylidenecyclobutenone 1k was easily achieved in good yield by a formal [2 + 2] cycloaddition reaction of ynamide with propargyl silyl ether. See [55].
- Chen, L.; Cao, J.; Xu, Z.; Zheng, Z.J.; Cui, Y.M.; Xu, L.W. Lewis acid catalyzed [2+2] cycloaddition of ynamides and propargyl silyl ethers: Synthesis of alkylidenecyclobutenones and their reactivity in ring-opening and ring expansion. Chem. Commun. 2016, 52, 9574–9577. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurohara, T.; Shibuya, M. CF3-Substituted semisquarate: A pluripotent building block for the divergent synthesis of trifluoromethylated functional molecules. Chem. Commun. 2015, 51, 16357–16360. [Google Scholar] [CrossRef] [PubMed]
- Chuit, C.; Corriu, R.J.P.; Reye, C.; Young, J.C. Reactivity of penta- and hexacoordinate silicon compounds and their role as reaction intermediates. Chem. Rev. 1993, 93, 1371–1448. [Google Scholar] [CrossRef]
- Holmes, R.R. Comparison of Phosphorus and Silicon: Hypervalency, Stereochemistry, and Reactivity. Chem. Rev. 1996, 96, 927–950. [Google Scholar] [CrossRef] [PubMed]
- Dilman, A.D.; Loffe, S.L. Carbon-carbon bond forming reactions mediated by silicon Lewis acids. Chem. Rev. 2003, 103, 733–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Li, L.; Li, F.; Jiang, K.Z.; Shang, J.Y.; Lai, G.Q.; Xu, L.W. Silicon-based Lewis acid assisted cinchona alkaloid catalysis: Highly enantioselective aza-Michael reaction under solvent-free conditions. Org. Lett. 2011, 13, 6508–6511. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Deng, Y.; Jiang, K.Z.; Lai, G.Q.; Ni, Y.; Yang, K.F.; Jiang, J.X.; Xu, L.W. Neighboring Acetal-Assisted Brønsted-Acid-Catalyzed Si–H Bond Activation: Divergent Synthesis of Functional Siloxanes through Silylation and Hydrolytic Oxidation of Organosilanes. Eur. J. Org. Chem. 2011, 2011, 1736–1742. [Google Scholar] [CrossRef]
- Rubiales, G.; Alonso, C.; Martínez de Marigorta, E.; Palacios, F. Nucleophilic trifluoromethylation of carbonyl compounds and derivatives. Arkivoc 2014, 2, 362–405. [Google Scholar]
- Rew, Y.; DeGraffenreid, M.; He, X.; Jaen, J.C.; McMinn, D.L.; Sun, D.; Tu, H.; Ursu, S.; Powers, J.P. Discovery and optimization of benzenesulfonanilide derivatives as a novel class of 11b-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3786–3790. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a–2w and 5a–5k are available from the authors. |
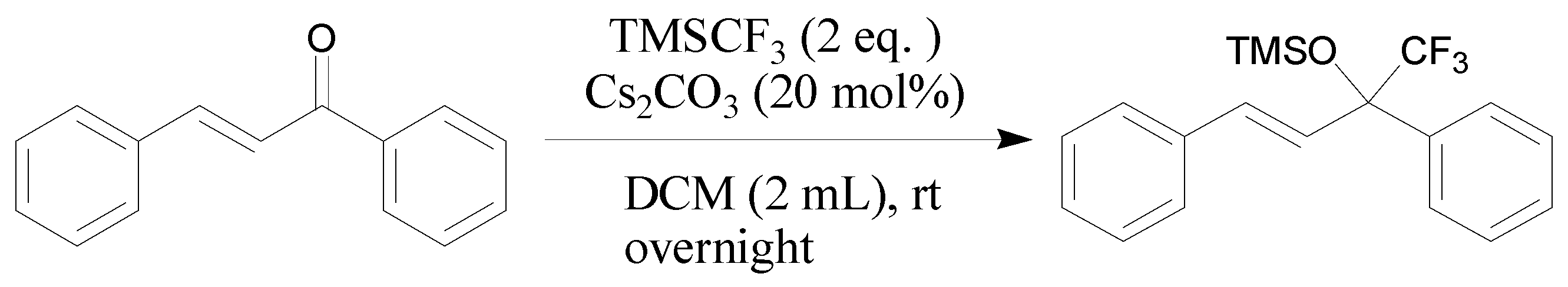

| Entry | Copper | Base | Yield of 2a (%) a |
|---|---|---|---|
| 1 | Cu2(OH)2CO3 | - | NR |
| 2 | Cu2(OH)2CO3 | K2CO3 | <5 |
| 3 | Cu2(OH)2CO3 | KOAc | NR |
| 4 | Cu2(OH)2CO3 | KH2PO4 | NR |
| 5 | Cu2(OH)2CO3 | KHF2 | 57 |
| 6 | Cu2(OH)2CO3 | KF | <5 |
| 7 | Cu2(OH)2CO3 | KCl | NR |
| 8 | Cu2(OH)2CO3 | KBr | NR |
| 9 | Cu2(OH)2CO3 | KOH | 52 |
| 10 | Cu2(OH)2CO3 | t-BuOK | 55 |
| 11 | Cu2(OH)2CO3 | Cs2CO3 | 60 |
| 12 | Cu2(OH)2CO3 | LiOH | <5 |
| 13 | - | Cs2CO3 | 94 |
| 14 | - | CaF2 | 37 |
| 15 | - | KF | NR |
| 16 | - | BaF2 | NR |
| 17 | - | K2CO3 | 72 b |
| 18 | - | KOAc | NR |
| 19 | - | KOH | 83 |
| 20 | - | t-BuOK | 71 |

| Entry | Solvent | Yield of 2a (%) a | Yield of 4a (%) a |
|---|---|---|---|
| 1 | DCM | 94 | 0 |
| 2 | DCE | NR | NR |
| 3 | THF | 36 | 51 |
| 4 | DMSO | NR | NR |
| 5 | MeCN | NR | NR |
| 6 | toluene | 15 | 0 |
| 7 | dioxane | NR | NR |
| 8 | Et2O | 60 | 0 |
| 9 | CHCl3 | NR | NR |
| 10 | DMF | 0 | 83 |
| 11 | DMA | 0 | 81 |
| 12 | TMSOTMS | 68 | 0 |
| 13 | n-Hexane | 61 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Bai, X.-F.; Lv, J.-Y.; Cui, Y.-M.; Cao, J.; Zheng, Z.-J.; Xu, L.-W. Cs2CO3-Initiated Trifluoro-Methylation of Chalcones and Ketones for Practical Synthesis of Trifluoromethylated Tertiary Silyl Ethers. Molecules 2017, 22, 769. https://doi.org/10.3390/molecules22050769
Dong C, Bai X-F, Lv J-Y, Cui Y-M, Cao J, Zheng Z-J, Xu L-W. Cs2CO3-Initiated Trifluoro-Methylation of Chalcones and Ketones for Practical Synthesis of Trifluoromethylated Tertiary Silyl Ethers. Molecules. 2017; 22(5):769. https://doi.org/10.3390/molecules22050769
Chicago/Turabian StyleDong, Cheng, Xing-Feng Bai, Ji-Yuan Lv, Yu-Ming Cui, Jian Cao, Zhan-Jiang Zheng, and Li-Wen Xu. 2017. "Cs2CO3-Initiated Trifluoro-Methylation of Chalcones and Ketones for Practical Synthesis of Trifluoromethylated Tertiary Silyl Ethers" Molecules 22, no. 5: 769. https://doi.org/10.3390/molecules22050769