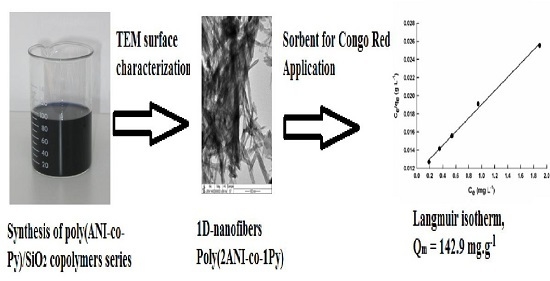
Synthesis and Characterization of Nano-Conducting Copolymer Composites: Efficient Sorbents for Organic Pollutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Nano-Copolymer Composites
2.1.1. X-ray Diffraction Structural Characterization
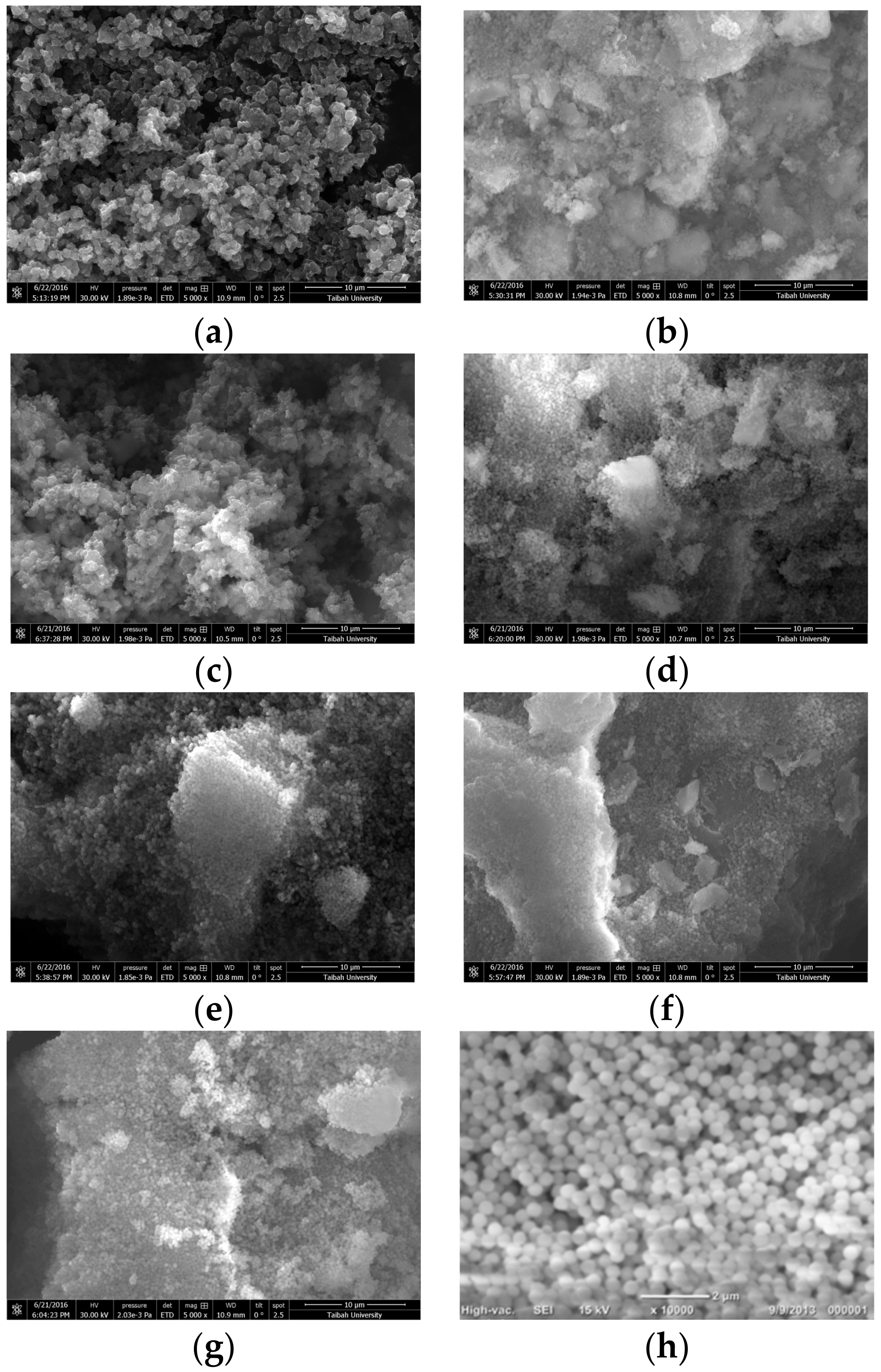
2.1.2. Surface Characterizations by Transmission and Scanning Electron Microscopes
2.1.3. Thermal Analysis
2.1.4. Fourier Transform Infrared Spectroscopy Structural Characterization
2.2. Nano-Copolymer Composites as Sorbents for CR Removal
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Nano-Silica
3.3. Preparation of Nano-Copolymer Composites
3.4. Adsorption Experiment
3.5. Characterizations of Nano-Composites
4. Conclusions
- Nano-conducting copolymer composites of ANI and Py with SiO2 have been successively prepared with different starting monomer ratios by the chemical oxidation method in the presence of CTAB.
- PANI is the major phase in PANI/SiO2, and in poly(2ANI-co-1Py)/SiO2 and poly(9ANI-co-1Py)/SiO2 samples; PPy is the major phase in PPy/SiO2, and in poly(1ANI-co-1Py)/SiO2, poly(1ANI-co-2Py)/SiO2, and poly(1ANI-co-9Py)/SiO2 samples.
- Copolymerization decreases surface area values and increases the thermal stability as compared with homopolymer composites, PANI/SiO2 and PPy/SiO2.
- Cauliflower shaped morphology can be observed for PPy/SiO2, poly(1ANI-co-9Py)/SiO2, poly(1ANI-co-2Py)/SiO2, and poly(1ANI-co-1Py)/SiO2 samples. On the other hand, hexagonal-shaped particles are assigned to PANI/SiO2 and poly(9ANI-co-1Py)/SiO2 samples. The poly(2ANI-co-1Py)/SiO2 sample offers unique 1D nano-fibers.
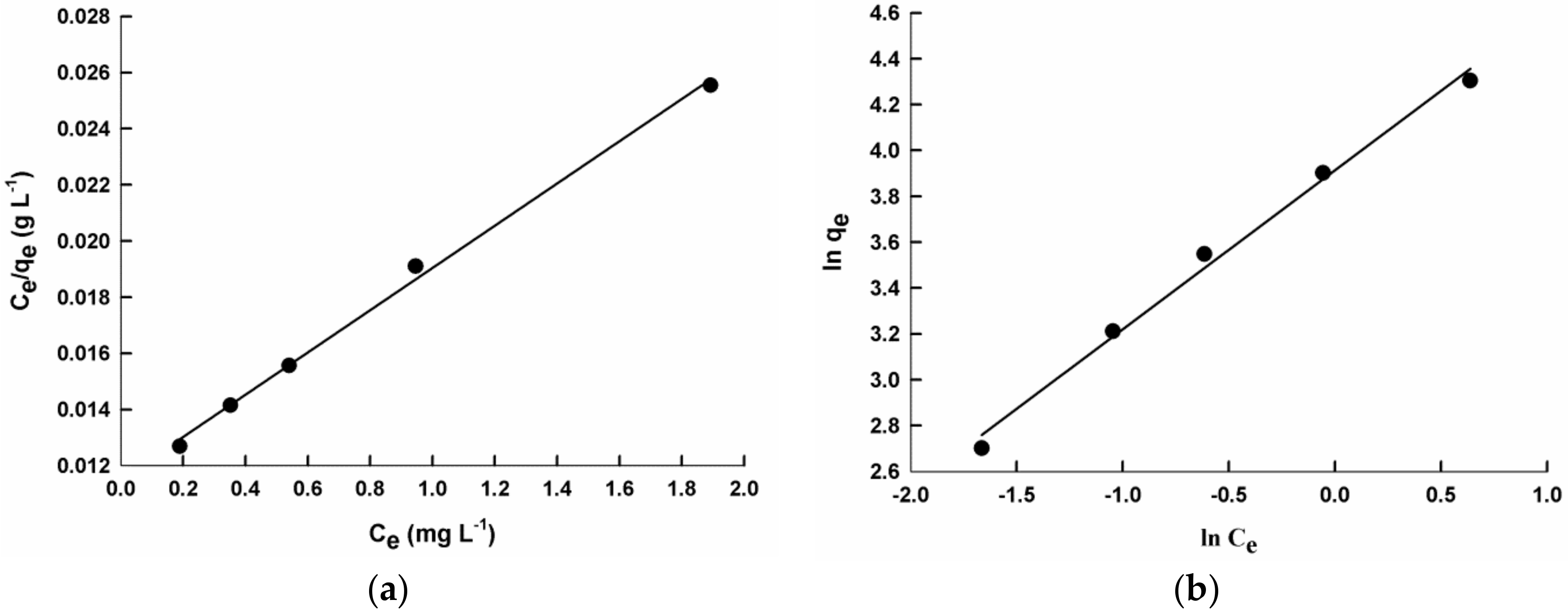
- Copolymerization decreases the sorption efficiency of PPy/SiO2 composite, except for the poly(2ANI-co-1Py)/SiO2 sample; it increases the sorption efficiency of PANI/SiO2 composite, except for the poly(1ANI-co-2Py)/SiO2 sample. The poly(2AN-co-1Py)/SiO2 sample has superior sorption capacity compared to homopolymer and other copolymer composites; the qm value is 142.9 mg g−1 due to its unique morphology which results in enhanced sorption properties.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, R.; Singh, S.; Yadav, B.C. Conducting polymers: Synthesis, properties and applications. Int. Adv. Res. J. Sci. Eng. Technol. 2015, 2, 110–124. [Google Scholar]
- Strumpler, R.; Glatz-Reichenbach, J. Conducting polymer composites. J. Electroceram. 1999, 3, 329–346. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Enhanced and selective ammonia sensing behaviour of poly(aniline-co-pyrrole) nanospheres chemically oxidative polymerized at low temperature. J. Ind. Eng. Chem. 2015, 26, 143–148. [Google Scholar] [CrossRef]
- Durairaj, S.; Vaithilingam, S. Hydrothermal assisted synthesis of zeolite based nickel deposited poly(pyrrole-co-fluoro aniline)/CuS catalyst for methanol and sulphur fuel cell applications. J. Electroanal. Chem. 2017, 787, 55–65. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, S.; Zhanga, L.; Suna, M.; Wang, W. Preparation and enhanced electrochemical properties of nano-sulfur/poly(pyrrole-co-aniline) cathode material for lithium/sulfur batteries. Electrochim. Acta 2010, 55, 4632–4636. [Google Scholar] [CrossRef]
- Liang, B.; Qin, Z.; Li, T.; Dou, Z.; Zeng, F.; Cai, Y.; Zhu, M.; Zhou, Z. Poly(aniline-co-pyrrole) on the surface of reduced graphene oxide as high-performance electrode materials for supercapacitors. Electrochim. Acta 2015, 177, 335–342. [Google Scholar] [CrossRef]
- Dhibar, S.; Bhattacharya, P.; Hatui, G.; Das, C.K. Transition metal doped poly(aniline-co-pyrrole)/multi-walled carbon nanotubes nanocomposite for high performance supercapacitor electrode materials. J. Alloys Compd. 2015, 625, 64–75. [Google Scholar] [CrossRef]
- Govindarajua, K.M.; Prakash, V.C.A. Synthesis of zinc modified poly(aniline-co-pyrrole) coatings and its anti-corrosive performance on low nickel stainless steel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 11–19. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.; Maity, A. Efficient removal of congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibres. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole–polyaniline nanofibers. Chem. Eng. J. 2012, 181–182, 323–333. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R. Adsorptive removal of Congo red a carcinogenic textile dye from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, M.R.; Anirudhan, T.S. The kinetics and thermodynamics of sorption of Cr(VI) onto the iron(III) complex of a carboxylated polymer acrylamide-grafted sawdust. Ind. Eng. Chem. Res. 2001, 40, 2693–2701. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced adsorption of Congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyltrimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for adsorption of congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.C.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (congo red) from aqueous medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V.K. Adsorptive removal of hazardous anionic dye ‘‘congo red’’ from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tor, A.; Cengeloglu, Y. Removal of congo red from aqueous solution by adsorption onto acid activated red mud. J. Hazard. Mater. 2006, 138, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.N.; Jesmeen, S.R.; Hossain, M.M. Removal of dyes from water by conducting polymeric adsorbent. Polym. Adv. Technol. 2004, 15, 633–638. [Google Scholar] [CrossRef]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J. Phys. Chem. B 2008, 112, 10153–10157. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; Abu El-Nasr, A.; Stejskal, J. Kinetics and isotherm studies of methylene blue adsorption onto polyaniline nanotubes base/silica composite. J. Ind. Eng. Chem. 2012, 18, 1964–1969. [Google Scholar] [CrossRef]
- Yao, W.; Shen, C.; Lu, Y. Fe3O4/C/polyaniline trilaminar core–shell composite microspheres as separable adsorbent for organic dye. Compos. Sci. Technol. 2013, 87, 8–13. [Google Scholar] [CrossRef]
- Jebreil, S.A. Removal of tartrazine dye form aqueous solutions by adsorption on the surface of polyaniline/iron oxide composite. Int. Sch. Sci. Res. Innov. 2014, 8, 1346–1351. [Google Scholar]
- Patil, M.R.; Shrivastava, V.S. Adsorption removal of carcinogenic acid violet19 dye from aqueous solution by polyaniline-Fe2O3 magnetic nano-composite. J. Mater. Environ. Sci. 2015, 6, 11–21. [Google Scholar]
- Patil, M.R.; Shrivastava, V.S. Adsorption of malachite green by polyaniline–nickel ferrite magnetic nanocomposite: An isotherm and kinetic study. Appl. Nanosci. 2014, 5, 809–816. [Google Scholar] [CrossRef]
- Khairy, M. Polyaniline-Zn0.2Mn0.8Fe2O4 ferrite core-shell composite: Preparation, characterization and properties. J. Alloys Compd. 2014, 608, 283–291. [Google Scholar] [CrossRef]
- Javadian, H.; Angaji, M.T.; Naushad, M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes. J. Ind. Eng. Chem. 2014, 20, 3890–3900. [Google Scholar] [CrossRef]
- Dhanavel, S.; Nivethaa, E.A.K.; Dhanapal, K.; Gupta, V.K.; Narayanan, V.; Stephen, A. α-MoO3/polyaniline composite for effective scavenging of rhodamine B, congo red and textile dye effluent. RSC Adv. 2016, 6, 28871–28886. [Google Scholar] [CrossRef]
- Bhaumik, M.; Mccrindle, R.I.; Maity, A. Enhanced adsorptive degradation of congo red in aqueous solutions using polyaniline/Fe composite nanofibers. Chem. Eng. J. 2015, 260, 716–729. [Google Scholar] [CrossRef]
- Janaki, V.; Vijayaraghavan, K.; Oh, B.; Lee, K.; Muthuchelian, K.; Ramasamy, A.K.; Kamala-Kannan, S. Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr. Polym. 2012, 90, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; El-Dib, F.I.; El-Gendy, N.S.; Sayed, W.M. Kinetic study for the removal of anionic sulphonated dye from aqueous solution using nano-polyaniline and Baker’ s yeast. Arab. J. Chem. 2012, 9, S1721–S1728. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Yan, W. Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for methylene Blue. Appl. Surf. Sci. 2013, 279, 400–408. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Feng, J.; Yan, W. Synthesis of PPy-modified TiO2 composite in H2SO4 solution and its novel adsorption characteristics for organic dyes. Chem. Eng. J. 2013, 225, 766–775. [Google Scholar] [CrossRef]
- Bagher, M.; Yamini, Y.; Dayeni, M.; Seidi, S. Adsorptive removal of alizarin red-S and alizarin yellow GG from aqueous solutions using polypyrrole-coated magnetic nanoparticles. Biochem. Pharmacol. 2015, 3, 529–540. [Google Scholar]
- Shanehsaz, M.; Seidi, S.; Ghorbani, Y.; Shoja, S.M.R.; Rouhani, S. Polypyrrole-coated magnetic nanoparticles as an efficient adsorbent for RB19 synthetic textile dye: Removal and kinetic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Emran, K.M.; Al-Oufi, A.L.L. Adsorption of organic pollutants by nano-conducting polymers composites: Effect of the supporting nano-oxide type. J. Mol. Liq. 2017, 233, 89–99. [Google Scholar] [CrossRef]
- Nittaya, T.; Apinon, N. Synthesis and characterization of nanosilica from rice husk ash prepared by precipitation method. Nat. Sci. 2008, 7, 59–65. [Google Scholar]
- Schnitzler, D.C.; Zarbin, A.J.G. Organic/Inorganic hybrid materials formed from TiO2 nanoparticles and polyaniline. J. Braz. Chem. Soc. 2004, 15, 378–384. [Google Scholar] [CrossRef]
- Wei, F.; Enhai, S.; Akihiko, F.; Hongcai, W.; Koichi, N.; Katsumi, Y. Synthesis and characterization of photoconducting polyaniline-TiO2 nanocomposite. Bull. Chem. Soc. Jpn. 2000, 73, 2627–2633. [Google Scholar]
- Partch, R.; Gangolli, S.G.; Matijevic, E.; Cai, W.; Arajst, S. Conducting polymer composites: Surface-induced polymerization of pyrrole on iron (111) and cerium(IV) oxide particles. J. Colloid Interface Sci. 1991, 144, 27–35. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Yuan, S.J.; Yi, S.P.; Yin, E.Q. Synthesis and characterization of polypyrrole doped with anionic spherical polyelectrolyte brushes. Express Polym. Lett. 2012, 6, 697–705. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Parveen, N.; Han, H.; Ansari, O. Fibrous polyaniline/manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Bhandari, H.; Sharma, C.; Khatoon, F.; Dhawan, S.K. A new smart coating of polyaniline-SiO2 composite for protection of mild steel against corrosion in strong acidic medium. Polym. Int. 2013, 62, 1192–1201. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Sun, J.; Li, Y.; Yang, X.; Wang, X.; Wang, Z.; Xia, L. Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem. Eng. J. 2014, 245, 99–106. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of nano-conducting copolymer composites, poly(ANI-co-Py)/SiO2, with different monomeric ratio, are available from the authors. |




| PANI/SiO2 Sample | PPy/SiO2 Sample | Poly(1ANIco1Py)/SiO2 Sample | Poly(1ANIco2Py)/SiO2 Sample | Poly(2ANIco1Py)/SiO2 Sample | Poly(1ANIco9Py)/SiO2 Sample | Poly(9ANIco1Py)/SiO2 Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard SiO2 | |||||||||||||||
| 2θ | I | 2θ | I | 2θ | I | 2θ | I | 2θ | I | 2θ | I | 2θ | I | 2θ | I |
| 20 | 20 | 20.4 | 24 | - | - | - | - | - | - | - | - | - | - | - | - |
| 26 | 100 | 26.0 | 24 | 26.0 | 95 | 26.1 | 76 | 26.1 | 65 | 26.1 | 79 | 26.3 | 63 | 26.0 | 45 |
| Standard PANI | |||||||||||||||
| 25 | 45 | 25.1 | 28 | - | - | - | - | - | - | - | - | - | - | ||
| 35 | 100 | 35.0 | 100 | - | - | 35.2 | 18 | No Peak | 34.9 | 100 | 34.8 | 7 | 35.1 | 100 | |
| 57 | 21 | 57.0 | 16 | - | - | - | - | - | - | - | - | 57.1 | 14 | ||
| 61 | 24 | 60.7 | 36 | - | - | - | - | - | - | 60.9 | 53 | - | - | 61.0 | 43 |
| Standard PPy | |||||||||||||||
| 24.3 | 100 | - | - | 24.3 | 100 | 24.1 | 100 | 24.1 | 100 | No peak | 24.3 | 100 | 24.5 | 35 | |
| BET surface area/m2 g−1 | 72.40 | 122.81 | 24.23 | 47.99 | 53.69 | 55.01 | 68.43 | ||||||||
| Langmuir Constants | qm (mg g−1) | KL (L mg−1) | RL | r2 | |
|---|---|---|---|---|---|
| PANI/SiO2 | 50.0 | 0.190 | 0.05–0.51 | 0.981 | |
| PPy/SiO2 | 90.9 | 0.096 | 0.09–0.68 | 0.998 | |
| Copolymers/SiO2 composites | |||||
| ANI | Py | qm (mg g−1) | KL (L/mg) | RL | r2 |
| 1 | 1 | 83.3 | 0.285 | 0.03–0.26 | 0.986 |
| 9 | 1 | 62.5 | 0.592 | 0.01–0.05 | 0.999 |
| 1 | 9 | 83.3 | 0.260 | 0.03–0.11 | 0.996 |
| 2 | 1 | 142.9 | 0.636 | 0.01–0.05 | 0.997 |
| 1 | 2 | 41.7 | 0.235 | 0.03–0.12 | 0.998 |
| Freundlich Constants | Kf (mg1−(1/n)L1/n/g) | n | r2 | ||
| PANI/SiO2 | 7.2 | 1.58 | 0.978 | ||
| PPy/SiO2 | 7.4 | 1.21 | 0.993 | ||
| Copolymers/SiO2 composites | |||||
| ANI | Py | Kf (mg1−(1/n)L1/n/g) | n | r2 | |
| 1 | 1 | 16.2 | 1.34 | 0.970 | |
| 9 | 1 | 21.8 | 2.01 | 0.960 | |
| 1 | 9 | 18.4 | 1.98 | 0.920 | |
| 2 | 1 | 50.1 | 1.44 | 0.992 | |
| 1 | 2 | 13.4 | 3.70 | 0.949 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emran, K.M.; Ali, S.M.; Al-Oufi, A.L.L. Synthesis and Characterization of Nano-Conducting Copolymer Composites: Efficient Sorbents for Organic Pollutants. Molecules 2017, 22, 772. https://doi.org/10.3390/molecules22050772
Emran KM, Ali SM, Al-Oufi ALL. Synthesis and Characterization of Nano-Conducting Copolymer Composites: Efficient Sorbents for Organic Pollutants. Molecules. 2017; 22(5):772. https://doi.org/10.3390/molecules22050772
Chicago/Turabian StyleEmran, Khadija M., Shimaa M. Ali, and Aishah L. L. Al-Oufi. 2017. "Synthesis and Characterization of Nano-Conducting Copolymer Composites: Efficient Sorbents for Organic Pollutants" Molecules 22, no. 5: 772. https://doi.org/10.3390/molecules22050772