Application of Soluplus to Improve the Flowability and Dissolution of Baicalein Phospholipid Complex
Abstract
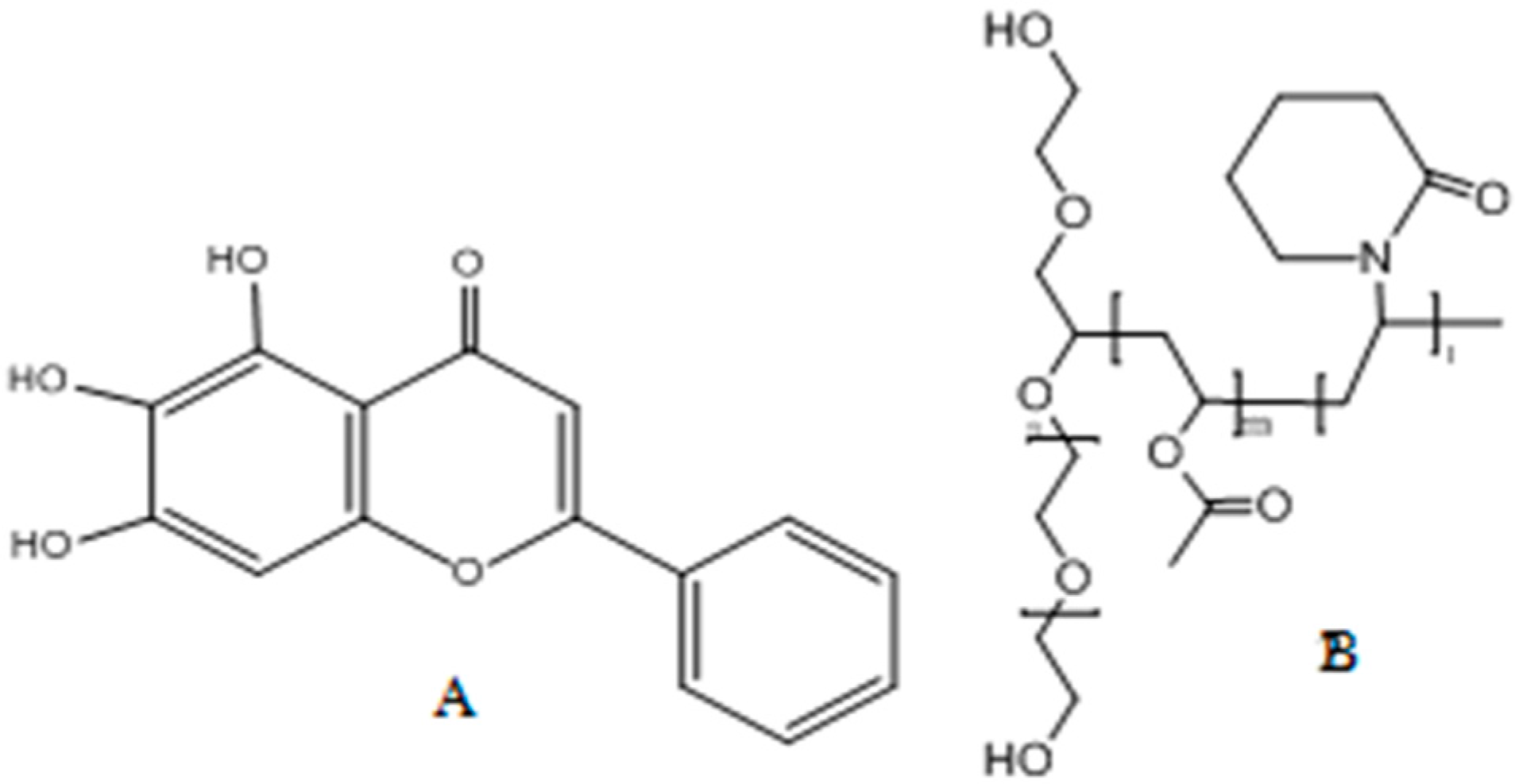
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Samples
Preparation of BPC
Preparation of TCS
Preparation of Physical Mixture (PM) of TCS
2.2.2. Characterization of the Sample
Differential Scanning Calorimetry (DSC)
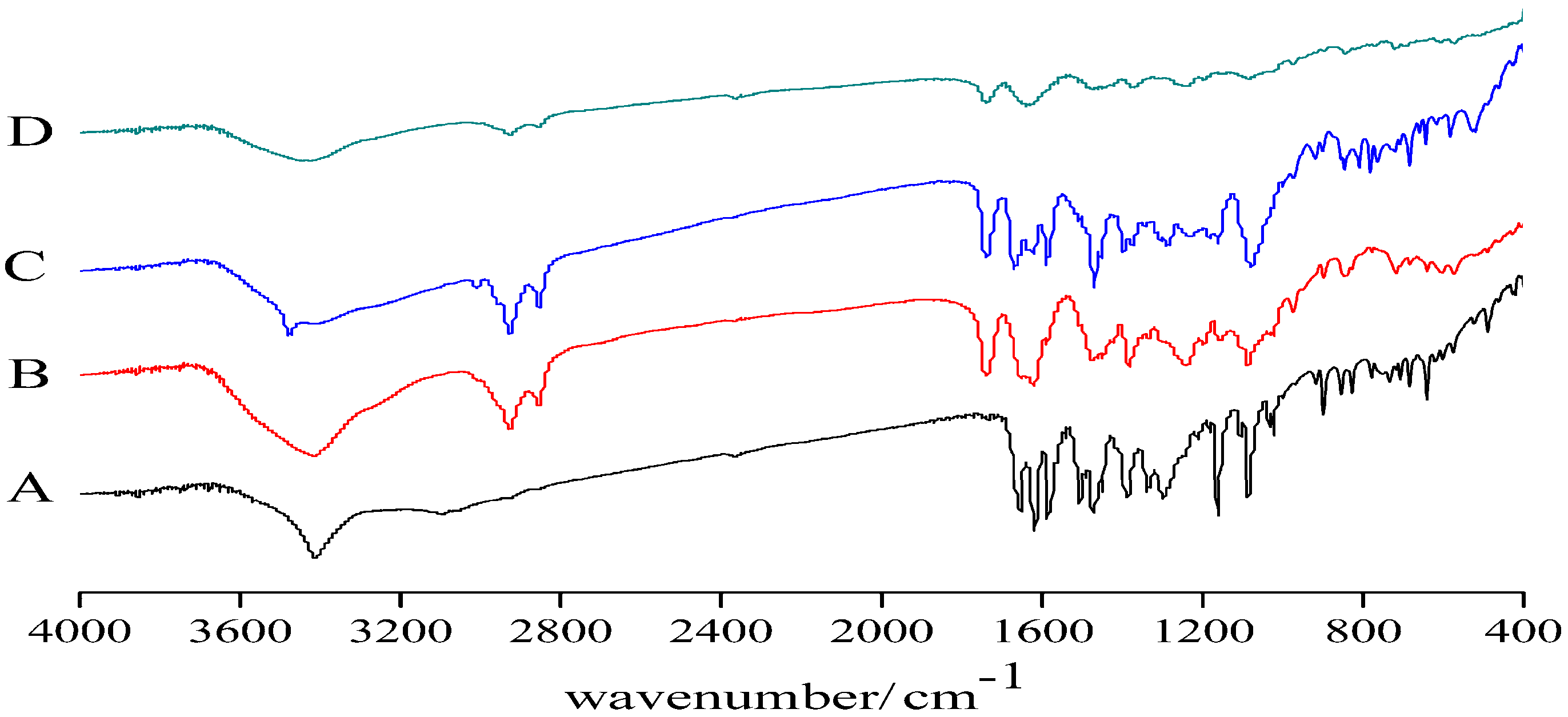
Infrared Spectroscopy (IR)
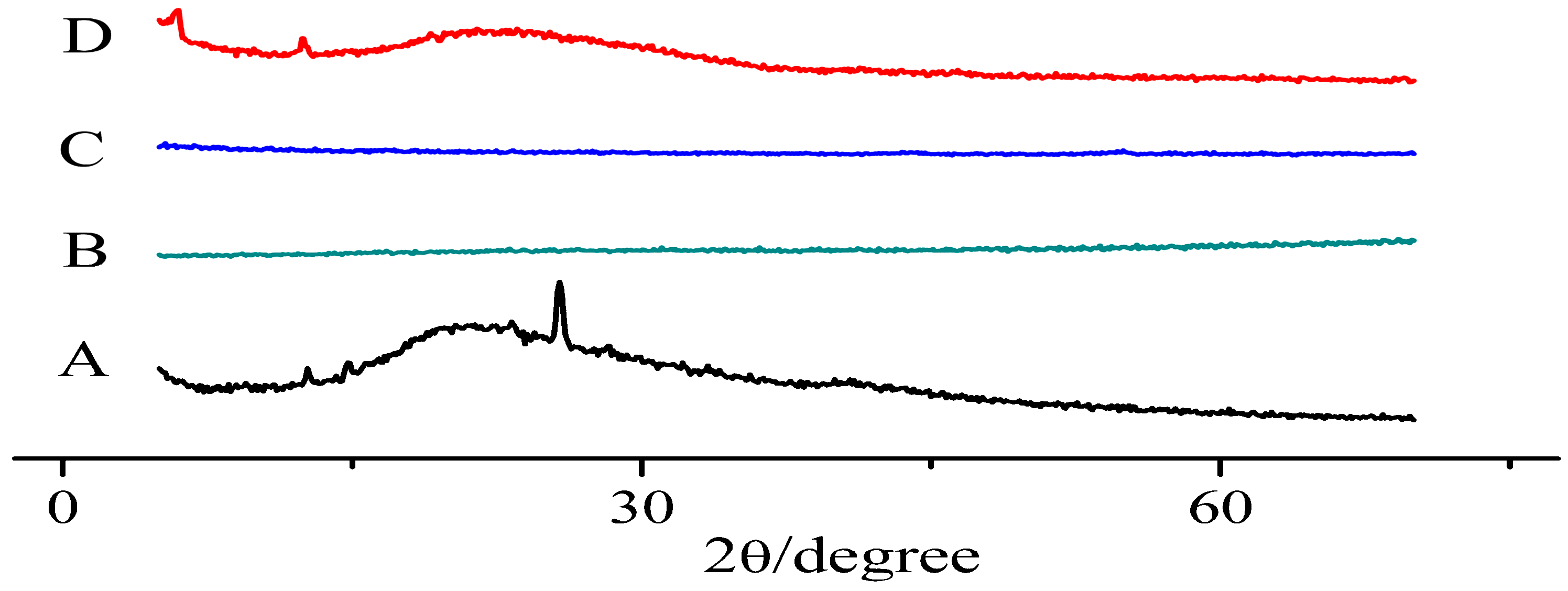
Powder X-ray Diffraction (PXRD)
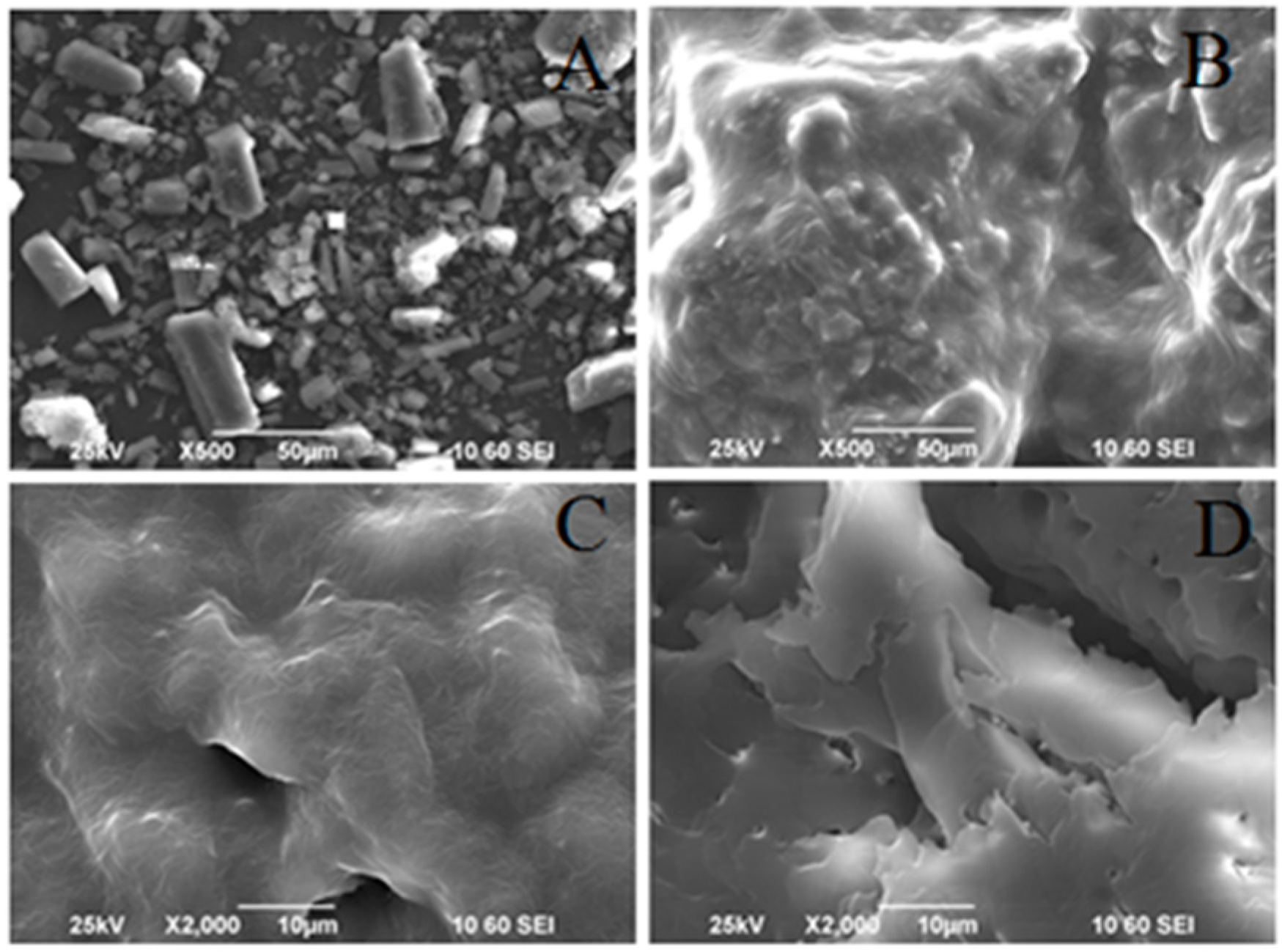
Scanning Electron Microscopy (SEM)
2.2.3. Flowability
2.2.4. Solubility
2.2.5. Oil–Water Partition Coefficient Studies
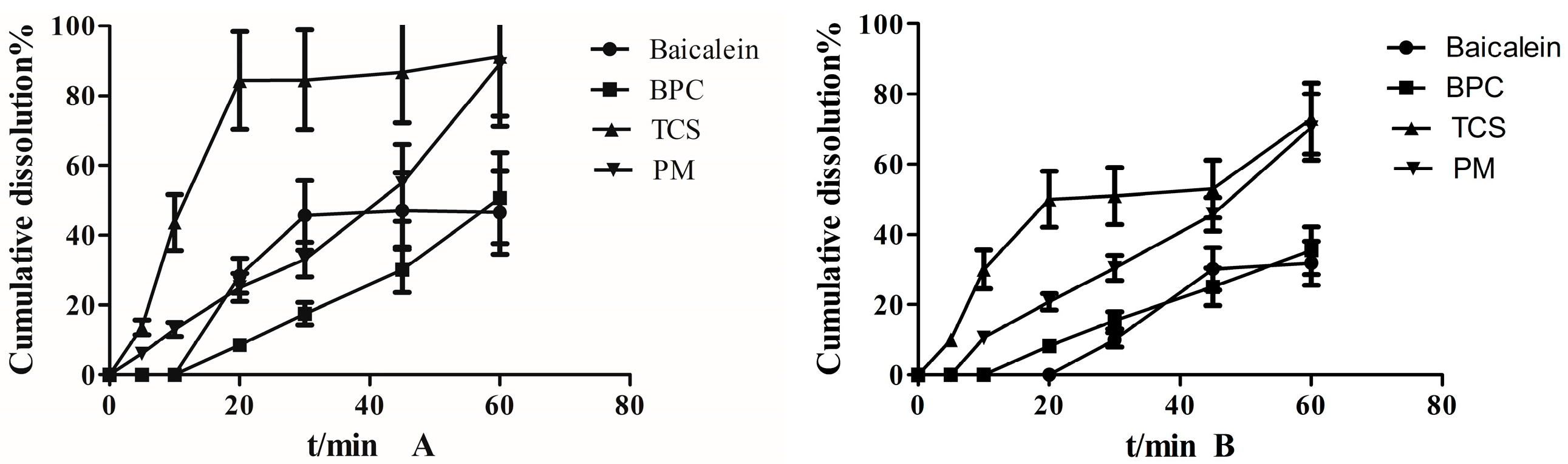
2.2.6. In Vitro Dissolution Studies
Chromatographic Conditions
Dissolution
2.2.7. Pharmacokinetic Study In Vivo
Animals
Plasma Sample Preparations
Plasma Sample Handing
Analysis of Date
3. Results and Discussion
3.1. Particle Morphology
3.2. Infrared Spectroscopy
3.3. X-ray Diffraction Pattern
3.4. Differential Scanning Calorimetry
3.5. Flowability of TCS
3.6. Solubility and Oil–Water Partition Coefficient
3.7. Dissolution Study
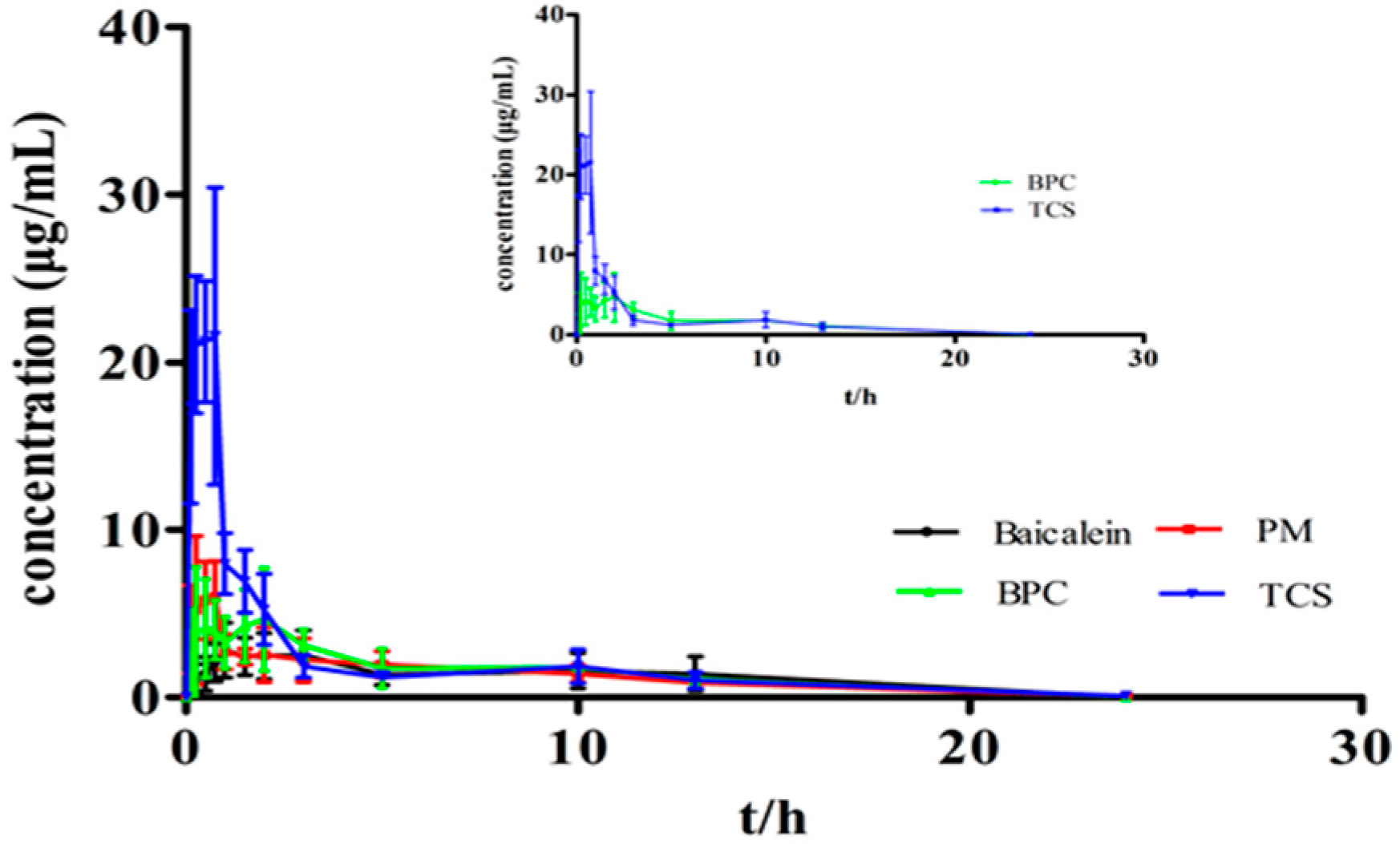
3.8. Bioavailability Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, T.Y.; Gong, W.; Tan, Z.J. Baicalein inhibits progression of gallbladder cancer cells by downregulating ZFX. PLoS ONE 2015, 10, e0114851. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Lee, Y.J.; Baik, J.S. Baicalein inhibits agonist and tumor cell-induced platelet aggregation while suppressing pulmonary tumor metastasis via cAMP-mediated VASP phosphorylation along with impaired MAPKs and PI3K-Akt activation. Biochem. Pharmacol. 2014, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.D.; Wang, W.J.; Fang, H.S. Baicalein protects against polymicrobial sepsis-induced liverinjury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. 2015, 748, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Cha, S.M.; Choi, S.M. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral Biol. 2014, 59, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Choi, J.S.; Burm, J.P. Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: A possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol. Rep. 2011, 63, 1066–1073. [Google Scholar] [CrossRef]
- Ma, X.C.; Yan, W.J.; Dai, Z.J.; Gao, X.Y.; Ma, Y.N.; Xu, Q.T.; Jiang, J.T; Zhang, S.Q. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Tang, J.J.; Chen, J.; Zhang, P.; Wang, T.; Chen, T.Y.; Yan, B.; Huang, B.; Wang, L. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des. Dev. Ther. 2015, 9, 5169–5183. [Google Scholar]
- Wu, H.Y.; Chen, L. Phospholipid complex and its effect on membrane transport of active constituents of Chinese materia medica. Chin. Tradit. Herb. Drugs 2012, 43, 393–398. [Google Scholar]
- Guo, B.; Liu, H.Z.; Li, Y.; Zhao, J.H. Application of phospholipid complex technique to improve the dissolution and pharmacokinetic of probucol by solvent-evaporation and co-grinding methods. Int. J. Pharm. 2014, 474, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Chen, Y.; Peng, J. Solid dispersion of berberine-phospholipid complex/TPGS 1000/Sio2: Preparation, characterization and in vivo studies. Int. J. Pharm. 2014, 465, 306–316. [Google Scholar] [CrossRef]
- Sunil, K.J. Development of tamoxifen-phospholipid complex: Novel approach for improving solubility and bioavailability. Int. J. Pharm. 2014, 473, 1–9. [Google Scholar]
- Devendra, S.R.; Bandana, K.; Thakur. Baicalein-Phospholipid Complex: A Novel Drug Delivery Technology for Phytotherapeutics. Curr. Drug Discov. Technol. 2013, 10, 224–232. [Google Scholar]
- Jang, S.W.; Han, S.D.; Jung, S.W. Preparation of solid dispersion of dronedarone hydrochloride with soluplus by hot melt extrusion technique for enhanced drug release. Chem. Pharm. Bull. 2015, 63, 295–299. [Google Scholar]
- Piao, J.; Lee, J.Y.; Weon, J.B. Angelica gigas Nakai and Soluplus-Based Solid Formulations Prepared by Hot-Melting Extrusion: Oral Absorption Enhancing and Memory Ameliorating Effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Parikh, T.; Meena, A.K.; Mahajan, N.; Vitez, I.; Serajuddin, A.T. Effect of carbamazepine on viscoelastic properties and hot melt extrudability of Soluplus®. Int. J. Pharm. 2015, 478, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Jae, Y.L.; Kang, W.S.; Piao, J.P. Soluplus®/TPGS-based solid dispersions prepared by hot-melt extrusion equipped with twin-screw systems for enhancing oral bioavailability of valsartan. Drug Des. Dev. Ther. 2015, 9, 2745–2756. [Google Scholar]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Afrasiabi, G.H. Preparation and Characterization of Celecoxib Dispersions in Soluplus®: Comparison of Spray Drying and Conventional Methods. Iran. J. Pharm. Res. 2015, 14, 35–50. [Google Scholar] [PubMed]
- Ian, S.; Nicolas, T. Spray-dried solid dispersions of nifedipine and vinylcaprolactam/vinylacetate/PEG6000 for compacted oral formulations. Int. J. Pharm. 2015, 481, 140–147. [Google Scholar]
- Yang, H.; Teng, F.; Wang, P.X. Investigation of a nanosuspension stabilized by Soluplus® to improve bioavailability. Int. J. Pharm. 2014, 477, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Sadeghi, F.; Varshosaz, J.; Afrasiabi, G.H.; Nokhodchi, A. Promising dissolution enhancement effect of soluplus on crystallizedcelecoxib obtained through antisolvent precipitation and highpressure homogenization techniques. Colloids Surf. B Biointerfaces 2014, 10, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Andres, L.; Clare, J.S.; Peep, V. Amorphous solid dispersions of piroxicam and Soluplus1: Qualitative and quantitative analysis of piroxicam recrystallization during storage. Int. J. Pharm. 2015, 486, 306–314. [Google Scholar]
- Liu, J.W.; Zou, M.J.; Piao, H.Y.; Liu, Y.; Tang, B.; Gao, Y.; Ma, N.; Cheng, G. Characterization and Pharmacokinetic Study of Aprepitant Solid Dispersions with Soluplus®. Molecules 2015, 20, 11345–11356. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, Y.; Xiang, Z.; Wang, C.J.; Zhou, H.; Liu, L. Simultaneous Determination of Multiple Components in Guanjiekang in Rat Plasma via the UPLC–MS/MS Method and Its Application in Pharmacokinetic Study. Molecules 2016, 21, 1732. [Google Scholar] [CrossRef] [PubMed]
- Lacramioara, O.; Cristian, G.; Marcel, P.; Iulian, S.; Corneliu, M.; Daniel, T.; Lenuta, P.; Anca, G.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Luo, R.; Chen, Y. Application of Carrier and Plasticizer to Improve the Dissolution and Bioavailability of Poorly Water-Soluble Baicalein by Hot Melt Extrusion. AAPS PharmSciTech 2014, 15, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Li, P.; Ding, L. Tissue distribution and excretion of baicalein and its main metabolite in rats by LC-MS/MS. J. China Pharm. Univ. 2009, 40, 348–352. [Google Scholar]
- Philip, F.; Builders, P.A.; Anwunobi; Chukwuemeka, C.; Mbah; Michael, U.; Adikwu. New Direct Compression Excipient from Tigernut Starch: Physicochemical and Functional Properties. AAPS PharmSciTech 2013, 14, 818–827. [Google Scholar]
- Che, Q.M.; Yang, L.; Chen, Y. Comparison of pharmacokinetics between different doses of baicalein in rats. Chin. J. New Drugs 2007, 116, 604–606. [Google Scholar]
- Gong, M.T.; Li, F.Y.; Cgen, Q.H. Pharmacokinetical study of baicalein and baicalin after intragastric administration in rats. Chin. Tradit. Herb. Drugs 2009, 40, 392–394. [Google Scholar]
- He, X.Q.; Pei, L.X.; Wang, Y.T. Study on pharmacokinetics of baicalein by HPLC-UV after intragastric administration in rats. Chin. J. Hosp. Pharm. 2010, 130, 1901–1905. [Google Scholar]
- Lee, D.H.; Yeom, D.W.; Song, Y.S.; Cho, H.R.; Choi, Y.S.; Kang, M.J.; Choi, Y.W. Improved oral absorption of dutasteride via Soluplus1-based supersaturable self-emulsifying drug delivery system (S-SEDDS). Int. J. Pharm. 2015, 478, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, H.; Luo, Q.; Yang, S.; Lin, X.; Zhang, Y.; Tian, B.; Tang, X. Increased dissolution and oral absorption of itraconazole / Soluplus extrudate compared with itraconazole nanosuspension. Eur. J. Pharm. Biopharm. 2013, 85, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

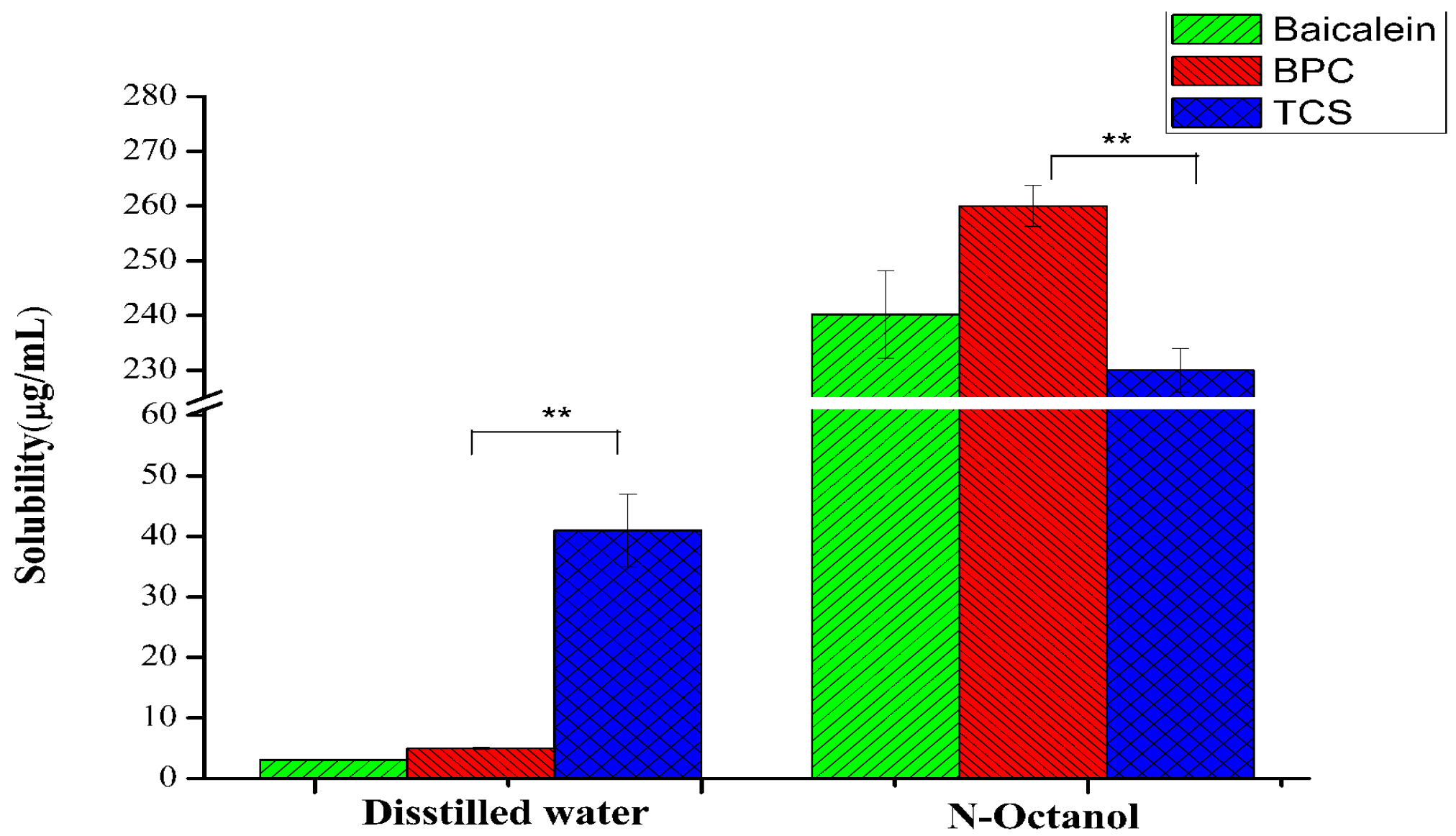

| Sample | Baicalein Concentration in Aqueous Phase Cw (μg/mL) | Baicalein Concentration in Organic Phase Co (μg/mL) | Partition Coefficient (Co/Cw) | Log p (Co/Cw) |
|---|---|---|---|---|
| Baicalein | 0.17 ± 0.03 | 2.01 ± 0.05 | 11.82 | 1.07 |
| BPC | 0.13 ± 0.02 | 25.08 ± 1.02 | 109.04 | 2.04 |
| TCS | 0.25 ± 0.01 | 26.01 ± 0.95 | 104.04 | 2.01 |
| Parameters | Baicalein | PM | BPC | TCS |
|---|---|---|---|---|
| Cmax (μg/mL) | 2.87 ± 1.82 | 8.67 ± 2.04 | 6.05 ± 3.02 | 25.55 ± 1.11 ** |
| Tmax (h) | 1.44 ± 1.05 | 0.75 ± 0.26 | 1.01 ± 0.74 | 0.63 ± 0.25 |
| AUC0–24 h (μg·h/mL) | 24.93 ± 13.13 | 30.01 ± 8.44 | 38.40 ± 10.32 | 53.16 ± 6.71 * |
| AUC0–∞ (μg·h/mL) | 40.99 ± 13.35 | 43.25 ± 8.50 | 50.48 ± 10.34 | 62.47 ± 7.11 * |
| t1/2 (h) | 2.32 ± 0.33 | 3.05 ± 0.77 | 2.24 ± 0.22 | 3.09 ± 0.65 |
| MRT (0–∞) | 7.43 ± 1.04 | 6.48 ± 0.49 | 6.80 ± 0.97 | 5.04 ± 1.11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Dai, Y.; Shen, H.; Ju, J.; Zhao, Z. Application of Soluplus to Improve the Flowability and Dissolution of Baicalein Phospholipid Complex. Molecules 2017, 22, 776. https://doi.org/10.3390/molecules22050776
Fan J, Dai Y, Shen H, Ju J, Zhao Z. Application of Soluplus to Improve the Flowability and Dissolution of Baicalein Phospholipid Complex. Molecules. 2017; 22(5):776. https://doi.org/10.3390/molecules22050776
Chicago/Turabian StyleFan, Junting, Yunhao Dai, Hongxue Shen, Jianming Ju, and Zhiying Zhao. 2017. "Application of Soluplus to Improve the Flowability and Dissolution of Baicalein Phospholipid Complex" Molecules 22, no. 5: 776. https://doi.org/10.3390/molecules22050776





