Identification and Validation of SAA4 as a Rheumatoid Arthritis Prescreening Marker by Liquid Chromatography Tandem-mass Spectrometry
Abstract
:1. Introduction
2. Results
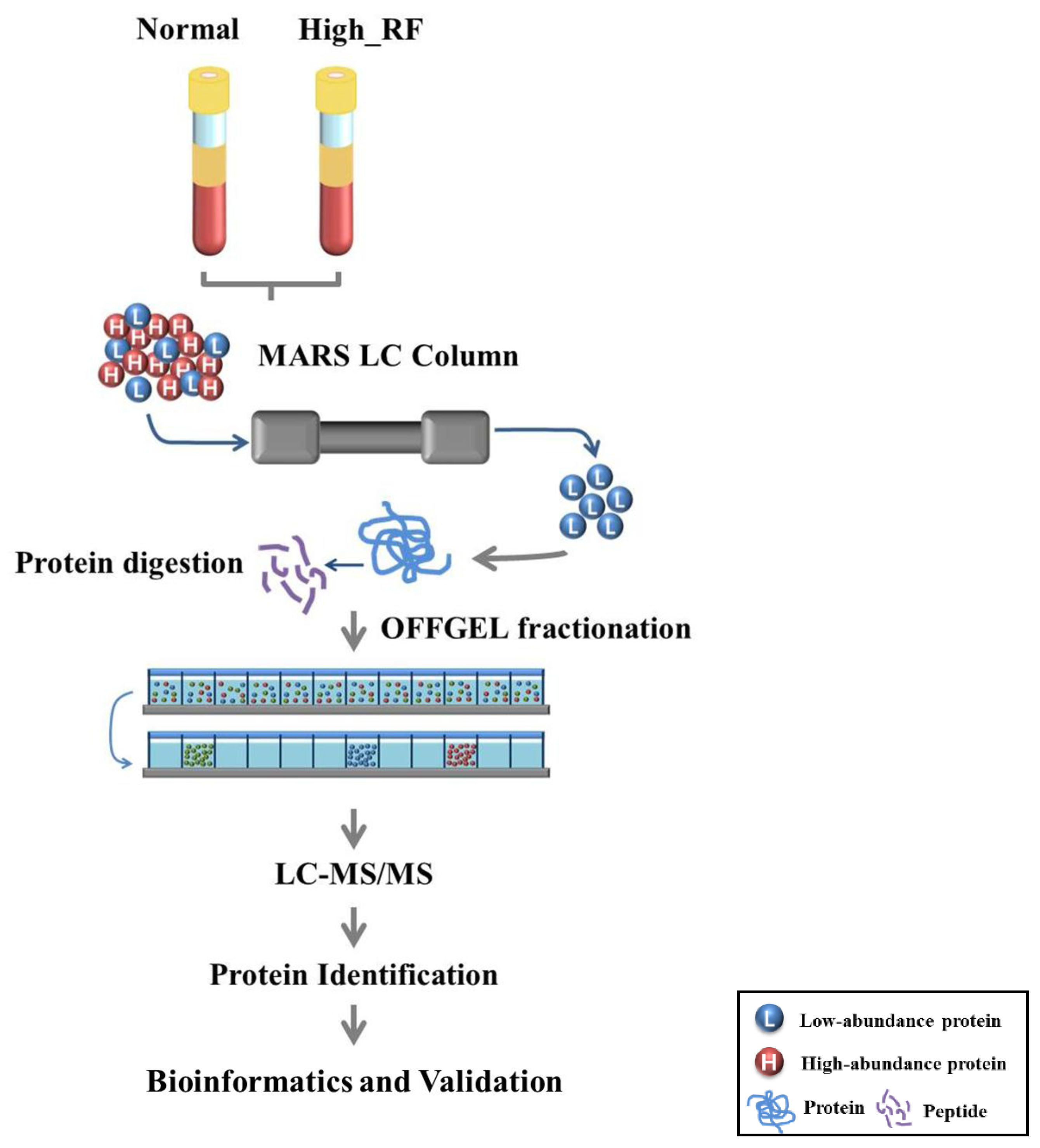
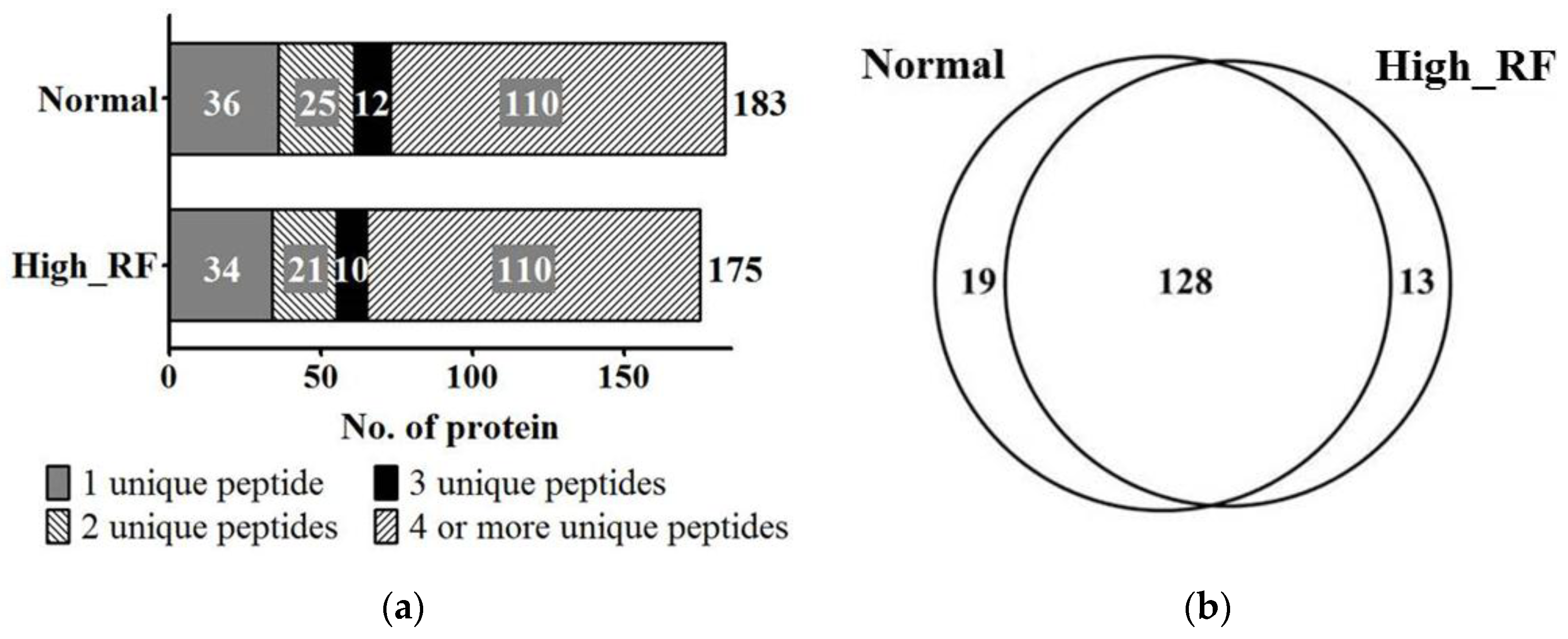
2.1. Identification of Proteins in Normal and RA Sera
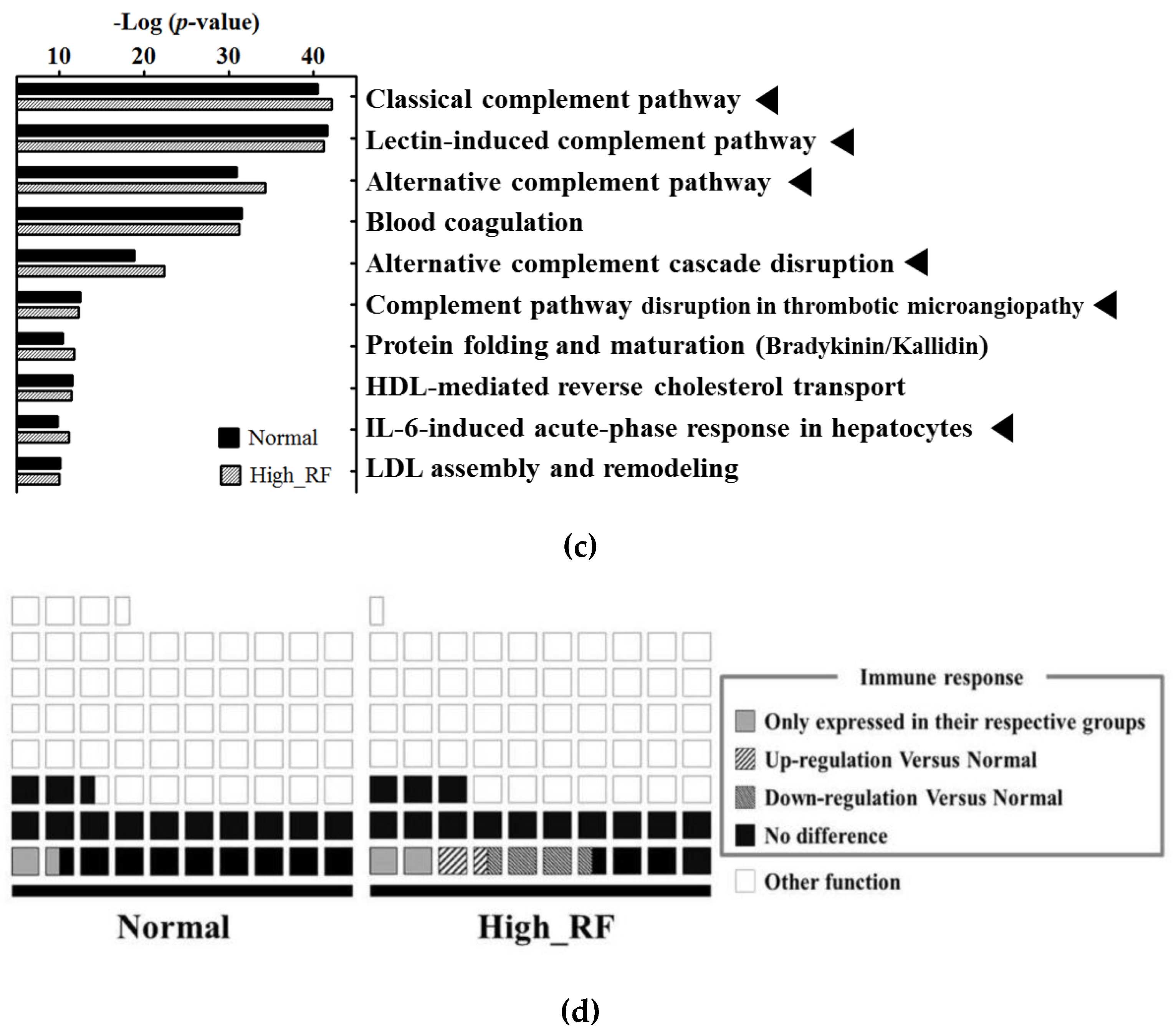
2.2. Protein Network and Pathway Analysis
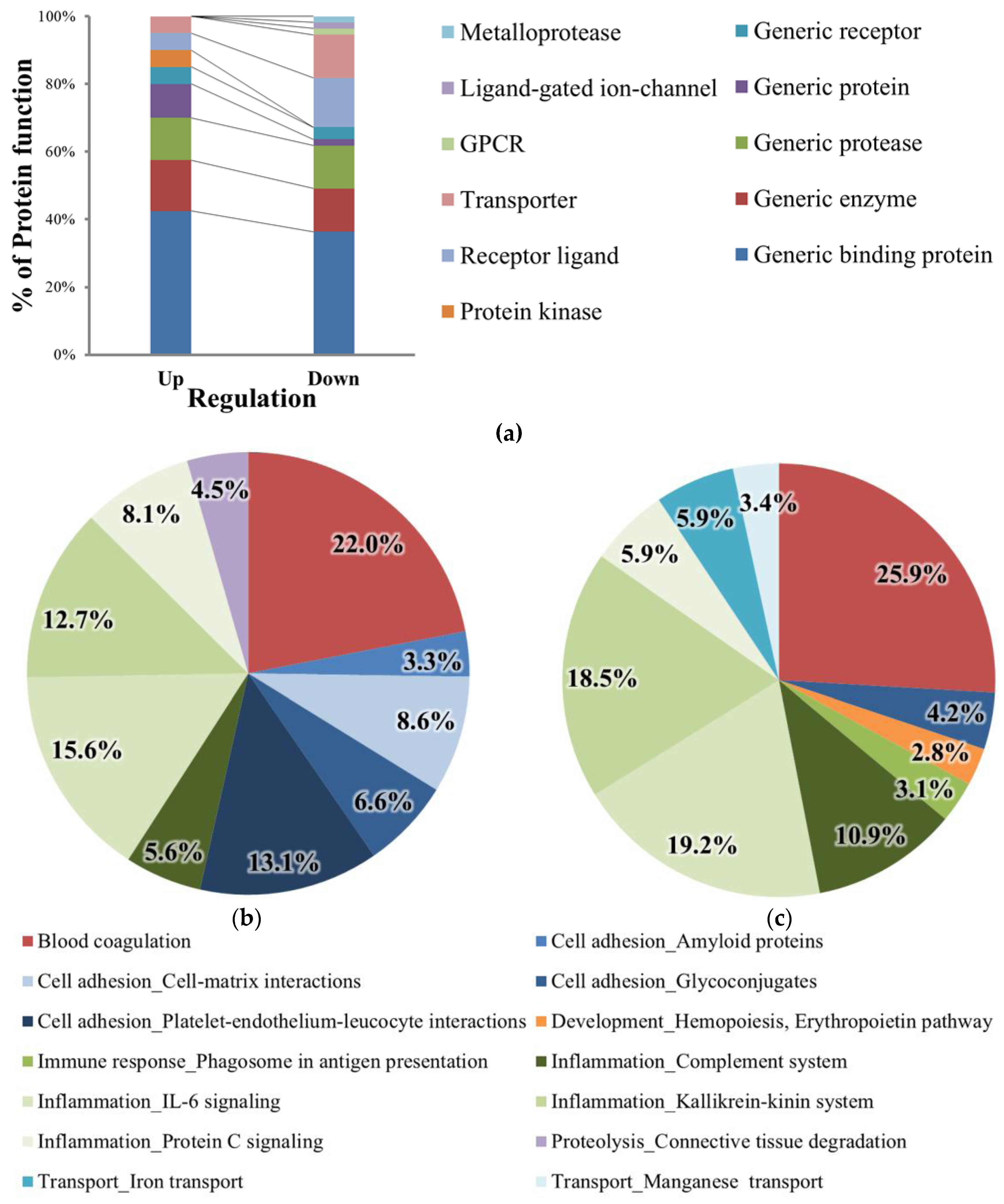
2.3. Comparative Analysis of the Expression Levels of the Identified Proteins
2.4. Protein Network Process Analysis and Marker Candidate Selection
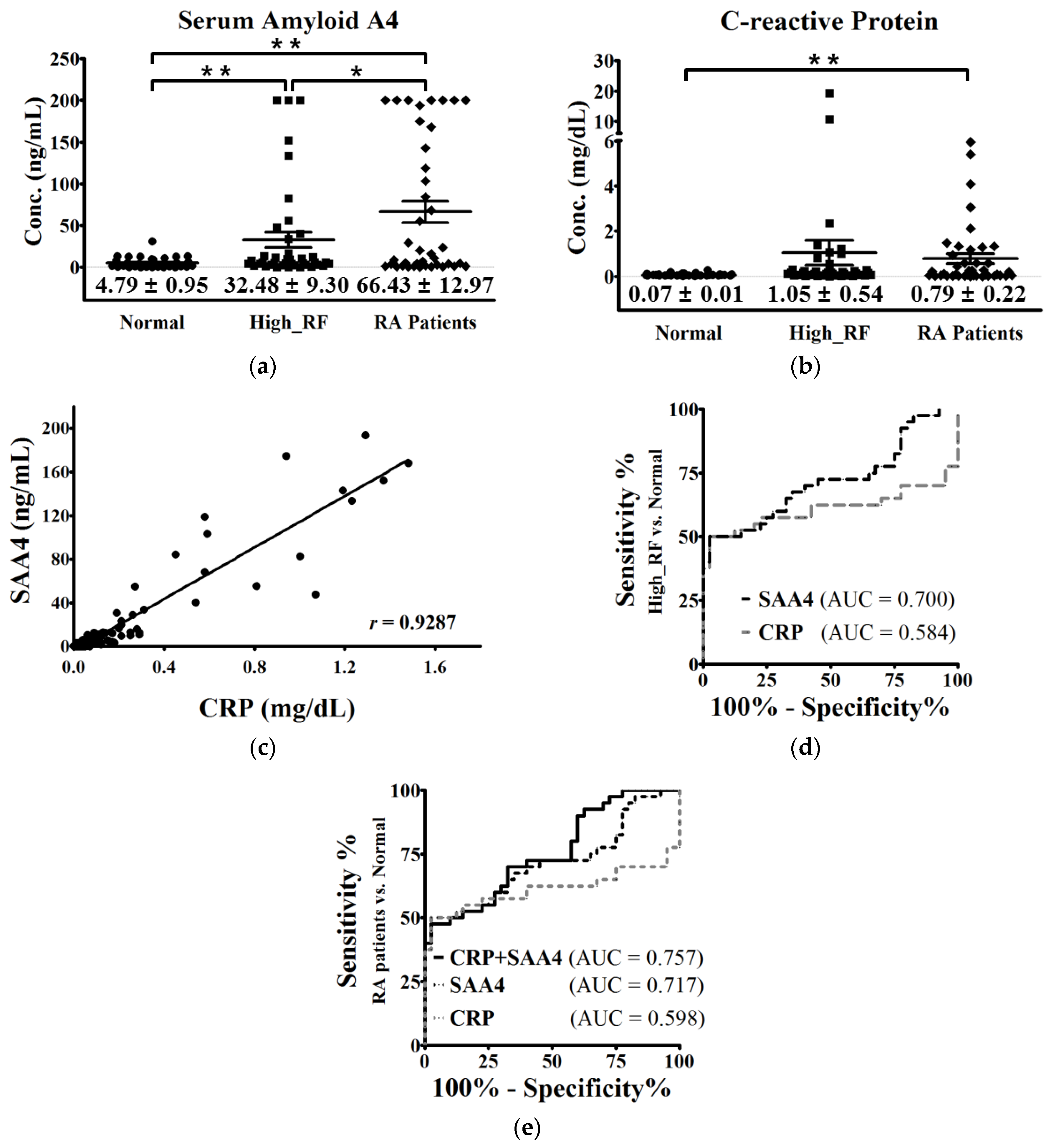
2.5. Validation of SAA4 by Individual Serum Enzyme-linked Immunosorbent Assay (ELISA)
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Serum Collection
4.2. Serum Depletion Using a Multiple Affinity Removal System LC Column
4.3. MS/MS Sample Preparation and Tryptic Digestion
4.4. Peptide Fractionation by OFFGEL Electrophoresis
4.5. Nano-LC-MS/MS
4.6. Protein Identification
4.7. Label-free Quantification and Bioinformatics Analysis
4.8. ELISA
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scott, D. Prognostic factors in early rheumatoid arthritis. Rheumatology (Oxford, England) 2000, 39, 24–29. [Google Scholar] [CrossRef]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Wells, G.A. Patient-driven outcomes in rheumatoid arthritis. J. Rheumatol. 2009, 82, 33–38. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, L.; Peene, I.; Hoffman, I.; Kruithof, E.; Union, A.; Meheus, L.; Lebeer, K.; Wyns, B.; Vincent, C.; Mielants, H. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: Diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann. Rheum. Dis. 2004, 63, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Alamanos, Y.; Drosos, A.A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 2005, 4, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.H.; Verstappen, S.M.; Hyrich, K.L.; Chipping, J.R.; Marshall, T.; Symmons, D.P. The incidence of rheumatoid arthritis in the uk: Comparisons using the 2010 acr/eular classification criteria and the 1987 acr classification criteria. Results from the norfolk arthritis register. Ann. Rheum. Dis. 2013, 72, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, G.A.; Visser, H.; De Jong, B.A.; Van Den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; Van Venrooij, W.J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Crowson, C.S.; Kremers, H.M.; Doran, M.F.; Turesson, C.; O'Fallon, W.M.; Matteson, E.L. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum. 2003, 48, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Mitchell, D.M.; Sibley, J.T.; Fries, J.F.; Bloch, D.A.; Williams, C.A.; Spitz, P.W.; Haga, M.; Kleinheksel, S.M.; Cathey, M.A. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994, 37, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Icen, M.; Kremers, H.M.; Crowson, C.S.; Davis, J.M.; Therneau, T.M.; Roger, V.L.; Gabriel, S.E. Mortality trends in rheumatoid arthritis: The role of rheumatoid factor. J. Rheum. 2008, 35, 1009–1014. [Google Scholar] [PubMed]
- Dörner, T.; Egerer, K.; Feist, E.; Burmester, G.R. Rheumatoid factor revisited. Curr. Opin. Rheum. 2004, 16, 246–253. [Google Scholar] [CrossRef]
- Song, Y.; Kang, E. Autoantibodies in rheumatoid arthritis: Rheumatoid factors and anticitrullinated protein antibodies. Qjm 2010, 103, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D. 2010 Rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-Reactive protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, C.; Amarillo, T. The erythrocyte sedimentation rate: Old and new clinical applications. Women 1998, 4, 11. [Google Scholar] [CrossRef]
- Wolfe, F. Comparative usefulness of c-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J. Rheum. 1997, 24, 1477–1485. [Google Scholar] [PubMed]
- Suzuki, K.; Sawada, T.; Murakami, A.; Matsui, T.; Tohma, S.; Nakazono, K.; Takemura, M.; Takasaki, Y.; Mimori, T.; Yamamoto, K. High diagnostic performance of elisa detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand. J. Rheum. 2009, 32. [Google Scholar] [CrossRef]
- Wordsworth, B.; Lanchbury, J.; Sakkas, L.; Welsh, K.; Panayi, G.; Bell, J. Hla-dr4 subtype frequencies in rheumatoid arthritis indicate that drb1 is the major susceptibility locus within the hla class ii region. Proc. Natl. Acad. Sci. USA 1989, 86, 10049–10053. [Google Scholar] [CrossRef] [PubMed]
- Rønningen, K.S.; Spurkland, A.; Egeland, T.; Iwe, T.; Munthe, E.; Vartdal, F.; Thorsby, E. Rheumatoid arthritis may be primarily associated with hla-dr4 molecules sharing a particular sequence at residues 67–74. Tissue Antigens 1990, 36, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S.M.; Pitteri, S.J.; Faca, V.M. Mining the plasma proteome for cancer biomarkers. Nature 2008, 452, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, A.Y.; Loriaux, P.; Wollscheid, B.; Zhou, Y.; Watts, J.D.; Aebersold, R. Mass spectrometric detection of tissue proteins in plasma. Mol. Cell. Proteom. 2007, 6, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Schiess, R.; Wollscheid, B.; Aebersold, R. Targeted proteomic strategy for clinical biomarker discovery. Mol. Oncol. 2009, 3, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Wu, J.; Karl, J.; Liao, H.; Zolg, W.; Guild, B. Quantification of c-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13c-labeled peptide standards. Proteomics 2004, 4, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wu, J.; Kuhn, E.; Chin, W.; Chang, B.; Jones, M.D.; O'Neil, S.; Clauser, K.R.; Karl, J.; Hasler, F. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004, 50, 3792–3803. [Google Scholar] [CrossRef] [PubMed]
- Serada, S.; Fujimoto, M.; Ogata, A.; Terabe, F.; Hirano, T.; Iijima, H.; Shinzaki, S.; Nishikawa, T.; Ohkawara, T.; Iwahori, K. Itraq-based proteomic identification of leucine-rich α-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann. Rheum. Dis. 2010, 69, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Nakarai, H.; Yamashita, A.; Nagayasu, S.; Iwashita, M.; Kumamoto, S.; Ohyama, H.; Hata, M.; Soga, Y.; Kushiyama, A.; Asano, T. Adipocyte-macrophage interaction may mediate lps-induced low-grade inflammation: Potential link with metabolic complications. Innate Immun. 2012, 18, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, C.; Windpassinger, C.; Brunner, D.; Kovacevic, A.; Schweighofer, N.; Malli, R.; Schuligoi, R.; Prokesch, A.; Kluve-Beckerman, B.; Graier, W.F.; et al. Characterization of rat serum amyloid a4 (saa4): A novel member of the saa superfamily. Biochem. Biophys. Res. Commun. 2014, 450, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Targońska-Stępniak, B.; Majdan, M. Serum amyloid a as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mullan, R.H.; Bresnihan, B.; Golden-Mason, L.; Markham, T.; O'Hara, R.; FitzGerald, O.; Veale, D.J.; Fearon, U. Acute-phase serum amyloid a stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an nf-κb-dependent signal transduction pathway. Arthritis Rheum. 2006, 54, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.G.; Balasubramani, G.K.; Metes, I.D.; Levesque, M.C.; Bridges, S.L.; Moreland, L.W. Differential response of serum amyloid a to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res. Ther. 2016, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Mullan, R.H.; McCormick, J.; Matthews, C.; Sullivan, O.; Kennedy, A.; FitzGerald, O.; Poole, A.R.; Bresnihan, B.; Veale, D.J. Acute-phase serum amyloid a regulates tumor necrosis factor α and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012, 64, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Shen, C.; Ding, H.; Huang, S.; Mu, Y.; Su, H.; Wei, W.; Ma, J.; Zheng, F. An involvement of sr-b1 mediated p38 mapk signaling pathway in serum amyloid a-induced angiogenesis in rheumatoid arthritis. Mol. Immun. 2015, 66, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Vallon, R.; Freuler, F.; Desta-Tsedu, N.; Robeva, A.; Dawson, J.; Wenner, P.; Engelhardt, P.; Boes, L.; Schnyder, J.; Tschopp, C. Serum amyloid a (aposaa) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J. Immun. 2001, 166, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Beguin, Y.; Leprince, P.; Willems, E.; Baron, F.; Deroyer, C.; Seidel, L.; Cavalier, E.; de Seny, D.; Malaise, M.; et al. Comprehensive plasma profiling for the characterization of graft-versus-host disease biomarkers. Talanta 2014, 125, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Kristjansdottir, B.; Partheen, K.; Fung, E.T.; Marcickiewicz, J.; Yip, C.; Brännström, M.; Sundfeldt, K. Ovarian cyst fluid is a rich proteome resource for detection of new tumor biomarkers. Clin. Proteom. 2012, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Erlinger, T.P.; Platz, E.A.; Rifai, N.; Helzlsouer, K.J. C-Reactive protein and the risk of incident colorectal cancer. Jama 2004, 291, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, K.; Ebrahim, S.; Lawlor, D.A. A systematic review of the association between circulating concentrations of c reactive protein and cancer. J. Epidemiol. Commun. Health 2007, 61, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Milstoc, M. Cholinesterase activity in patients with rheumatoid arthritis. Am. J. Clin. Pathol. 1970, 53, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.J.; Kang, E.-J.; Lee, S.-W.; Lee, S.-K.; Park, Y.-B.; Song, J.-S.; Choi, S.T. Usefulness of serum leucine-rich alpha-2 glycoprotein as a disease activity biomarker in patients with rheumatoid arthritis. J. Korean Med. Sci. 2014, 29, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
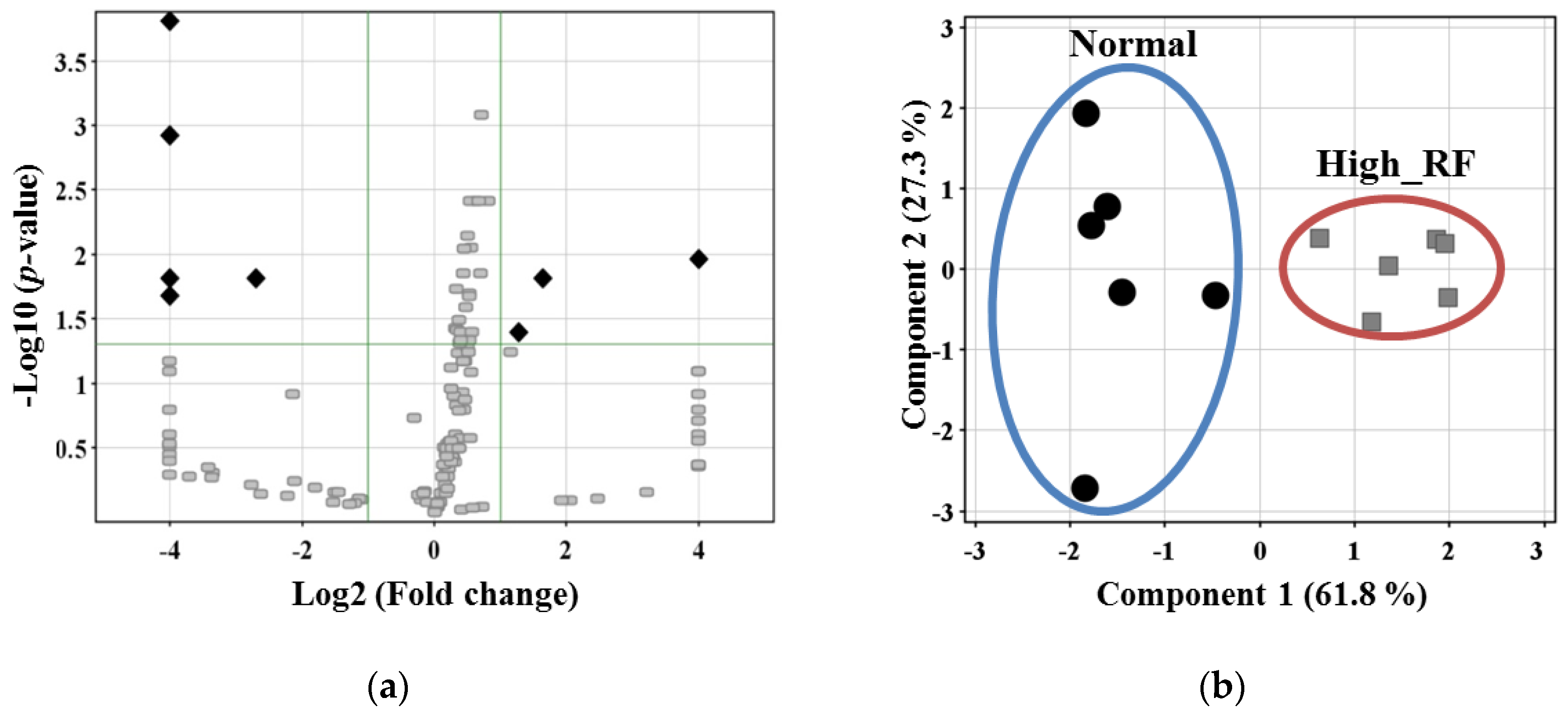
| Compound | p-value | Regulation | FC | Mass | Swiss-Prot ID | |
|---|---|---|---|---|---|---|
| Ig lambda-2 chain C regions | 0.0000 | down | −268306.0 | 11464.5 | P0CG05 | |
| √ | Cholinesterase | 0.0000 | down | −209972.4 | 68987.3 | P06276 |
| Immunoglobulin lambda-like polypeptide 5 | 0.0018 | down | −16107.3 | 23405.2 | B9A064 | |
| Hemoglobin subunit alpha | 0.0029 | down | −11740.1 | 15314.3 | P69905 | |
| Ig mu chain C region | 0.0017 | down | −6.5 | 49990.3 | P01871 | |
| √ | Leucine-rich alpha-2-glycoprotein | 0.0078 | up | 2.4 | 38405.4 | P02750 |
| √ | Serum amyloid A-4 protein | 0.0017 | up | 3.1 | 14860.5 | P35542 |
| √ | C-reactive protein | 0.0009 | up | 55962.2 | 25209.3 | P02741 |
| Subject Group | Rheumatoid Factor (Mean ± SE) | N | Sex (M/F) | Age (Mean ± SE) | |
|---|---|---|---|---|---|
| Discovery set | Normal (RF < 18 IU/mL) | 6.54 ± 0.88 | 18 | 4/14 | 65.06 ± 3.34 |
| High_RF (RF > 18 IU/mL) | 65.69 ± 8.00 | 36 | 10/26 | 61.58 ± 2.41 | |
| Validation set | Normal (RF < 18 IU/mL) | 5.74 ± 0.60 | 40 | 12/28 | 57.38 ± 1.85 |
| High_RF (RF > 18 IU/mL) | 77.69 ± 18.20 | 40 | 12/28 | 57.03 ± 2.00 | |
| RA patients | 65.15 ± 9.28 | 40 | 6/34 | 55.43 ± 1.81 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, A.; Lee, H.-J.; Lee, S.; Lee, J.; Mun, S.; Park, A.; Chun, Y.-T.; Lee, J.-H.; Lim, H.-J.; Kang, H.-G. Identification and Validation of SAA4 as a Rheumatoid Arthritis Prescreening Marker by Liquid Chromatography Tandem-mass Spectrometry. Molecules 2017, 22, 805. https://doi.org/10.3390/molecules22050805
Seok A, Lee H-J, Lee S, Lee J, Mun S, Park A, Chun Y-T, Lee J-H, Lim H-J, Kang H-G. Identification and Validation of SAA4 as a Rheumatoid Arthritis Prescreening Marker by Liquid Chromatography Tandem-mass Spectrometry. Molecules. 2017; 22(5):805. https://doi.org/10.3390/molecules22050805
Chicago/Turabian StyleSeok, AeEun, Hyun-Jung Lee, Sungeun Lee, Jiyeong Lee, Sora Mun, Arum Park, Yeon-Tae Chun, Jae-Hyeon Lee, Hee-Joung Lim, and Hee-Gyoo Kang. 2017. "Identification and Validation of SAA4 as a Rheumatoid Arthritis Prescreening Marker by Liquid Chromatography Tandem-mass Spectrometry" Molecules 22, no. 5: 805. https://doi.org/10.3390/molecules22050805






