Antibacterial Activity of 7-Epiclusianone and Its Novel Copper Metal Complex on Streptococcus spp. Isolated from Bovine Mastitis and Their Cytotoxicity in MAC-T Cells
Abstract
:1. Introduction
2. Results and Discussion
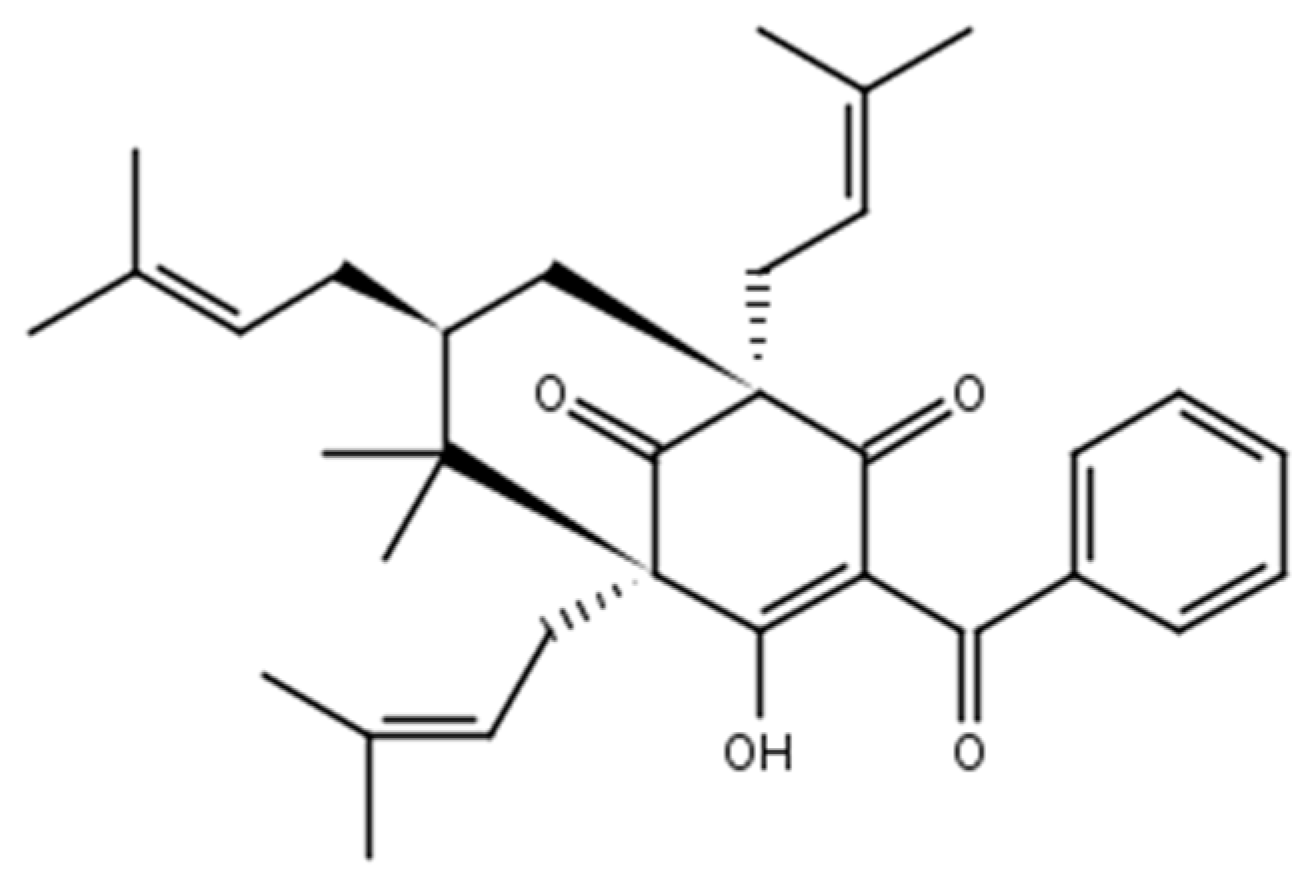
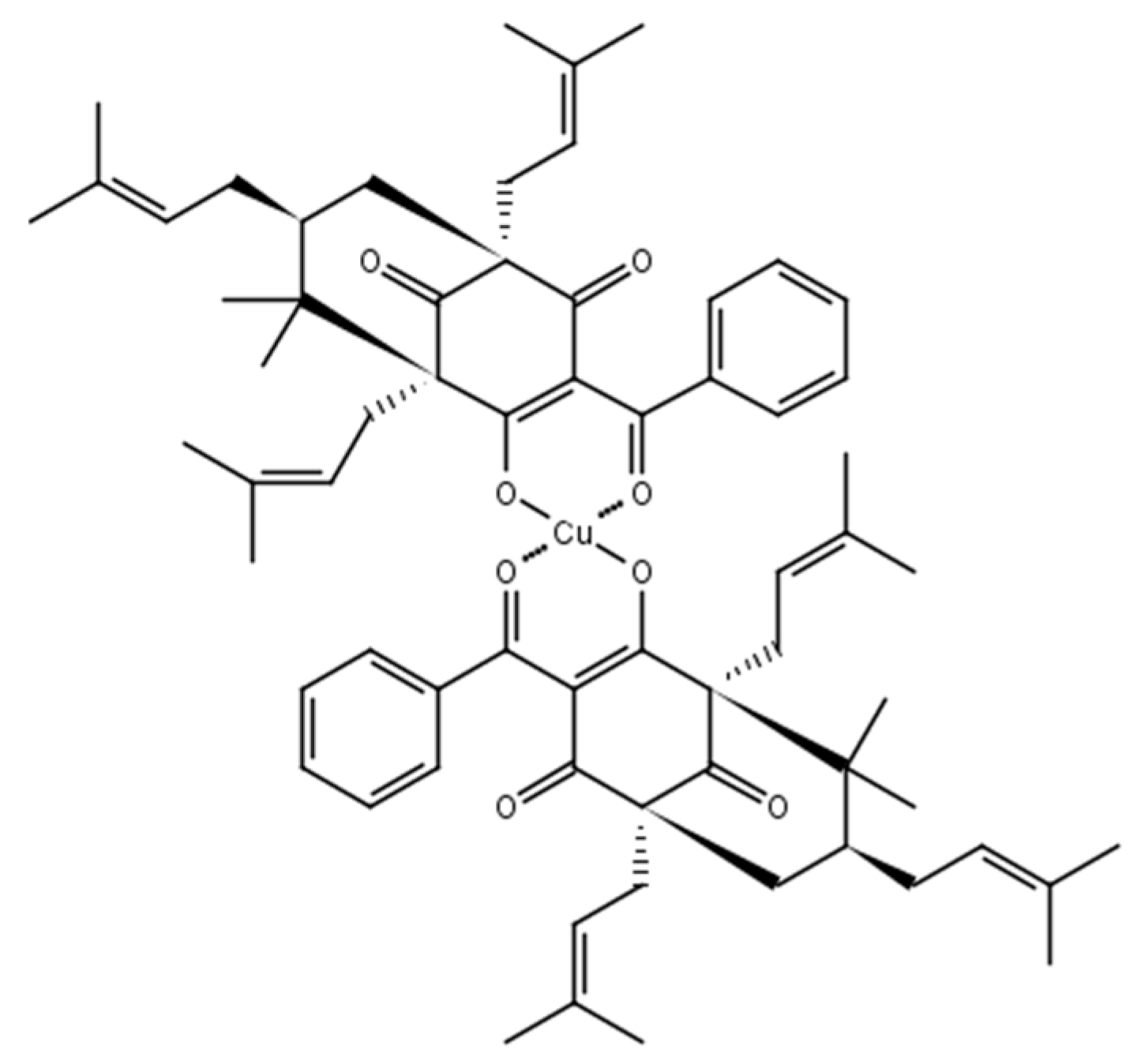
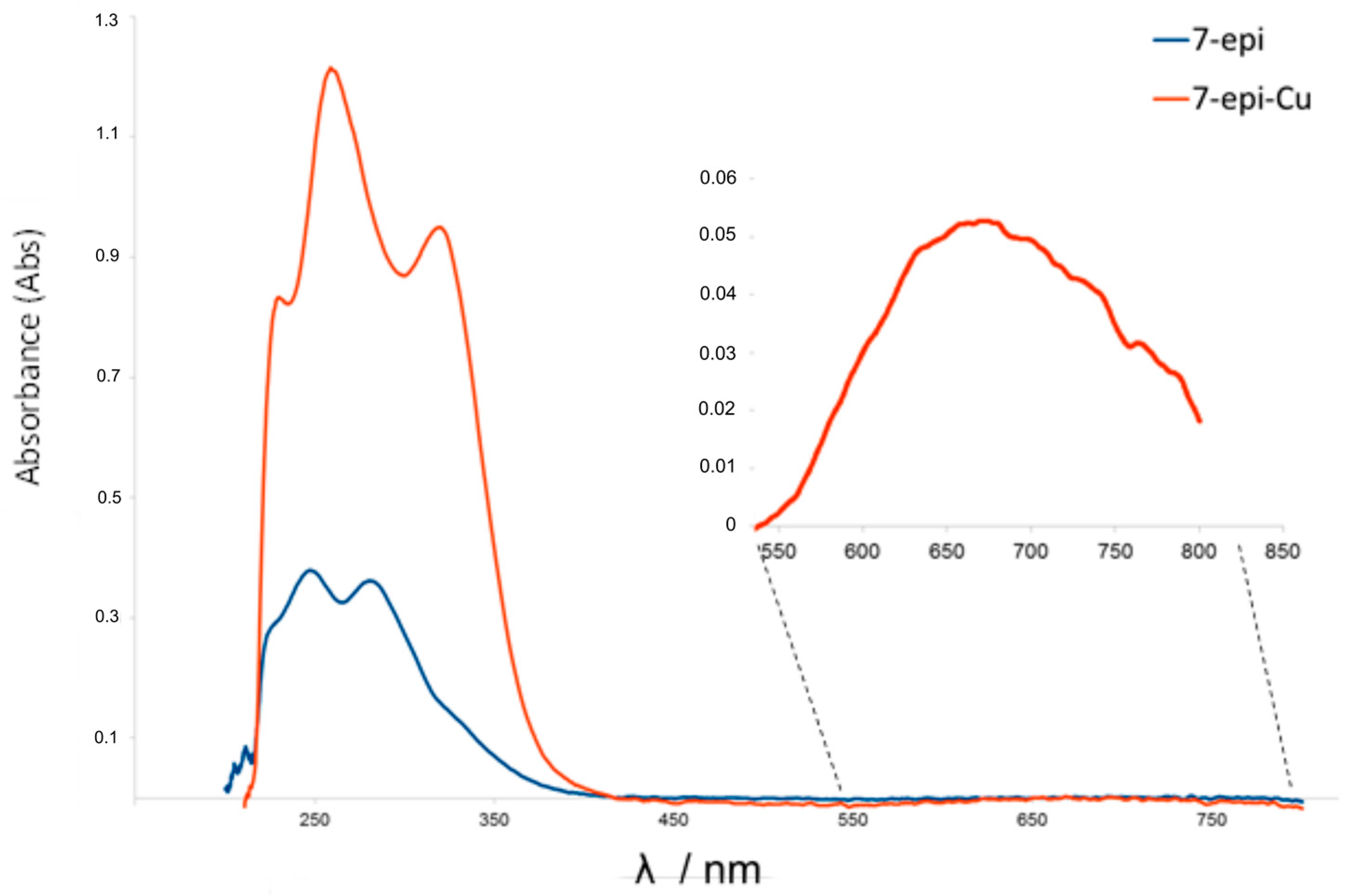
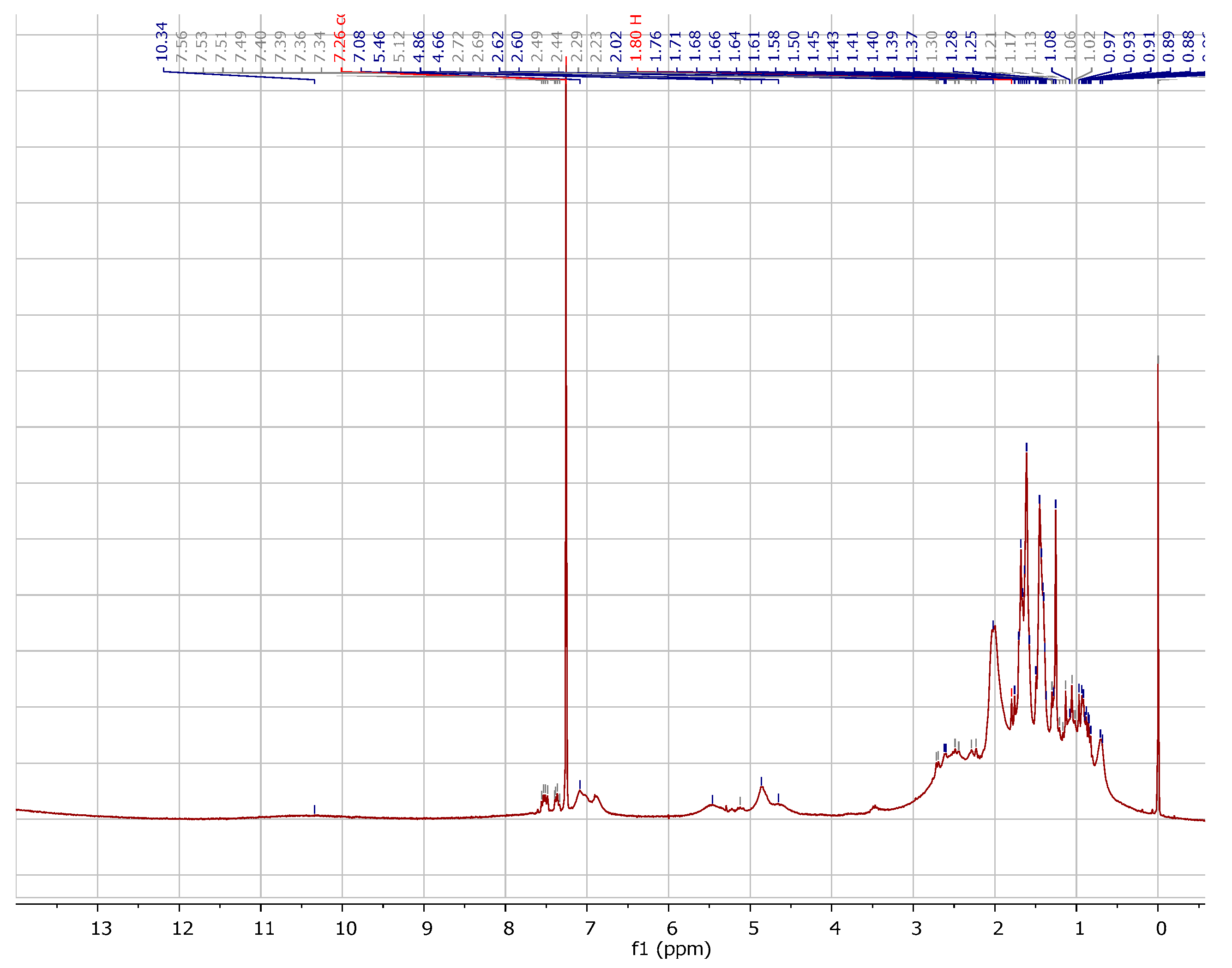
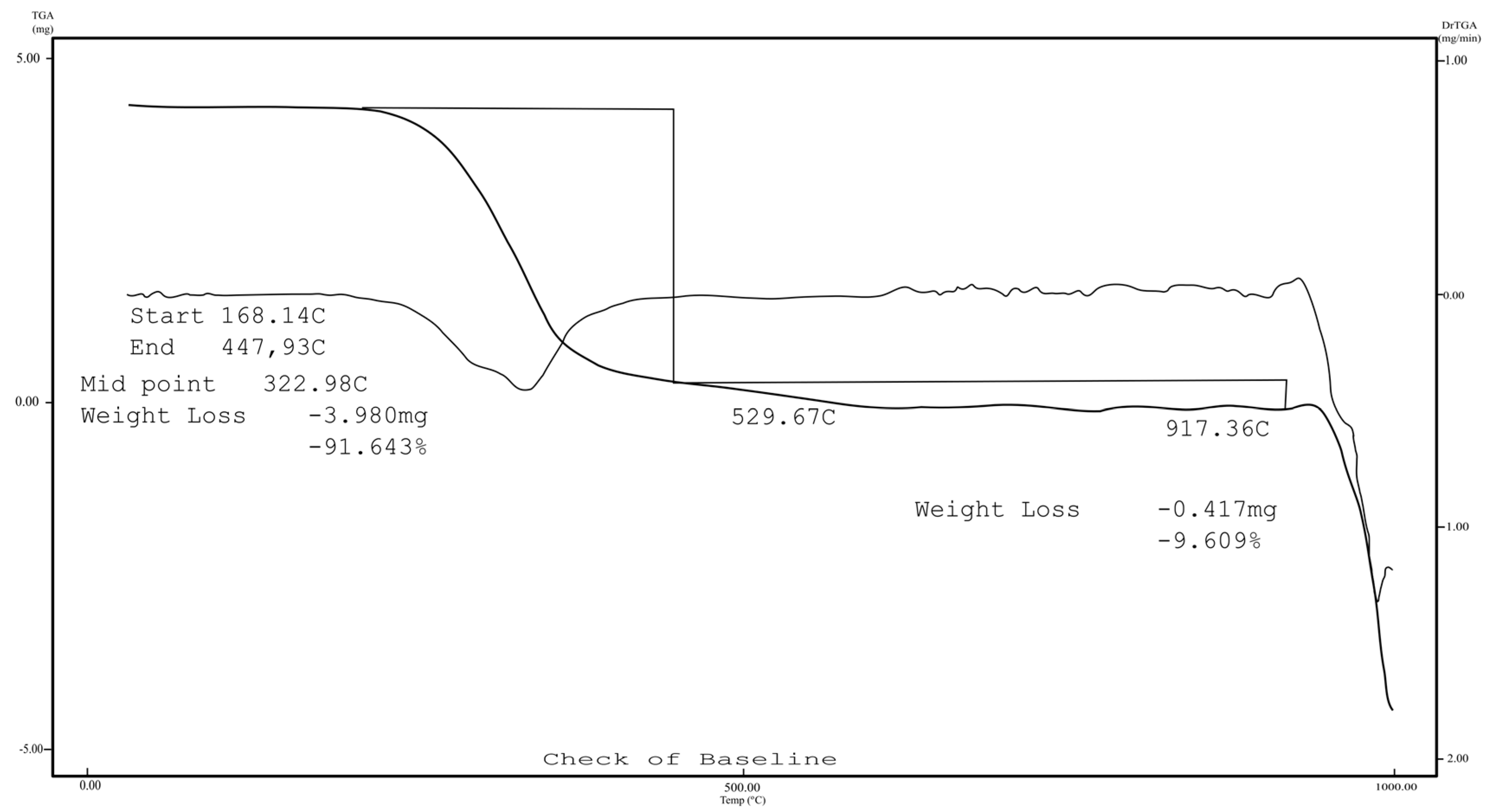
2.1. Chemistry
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
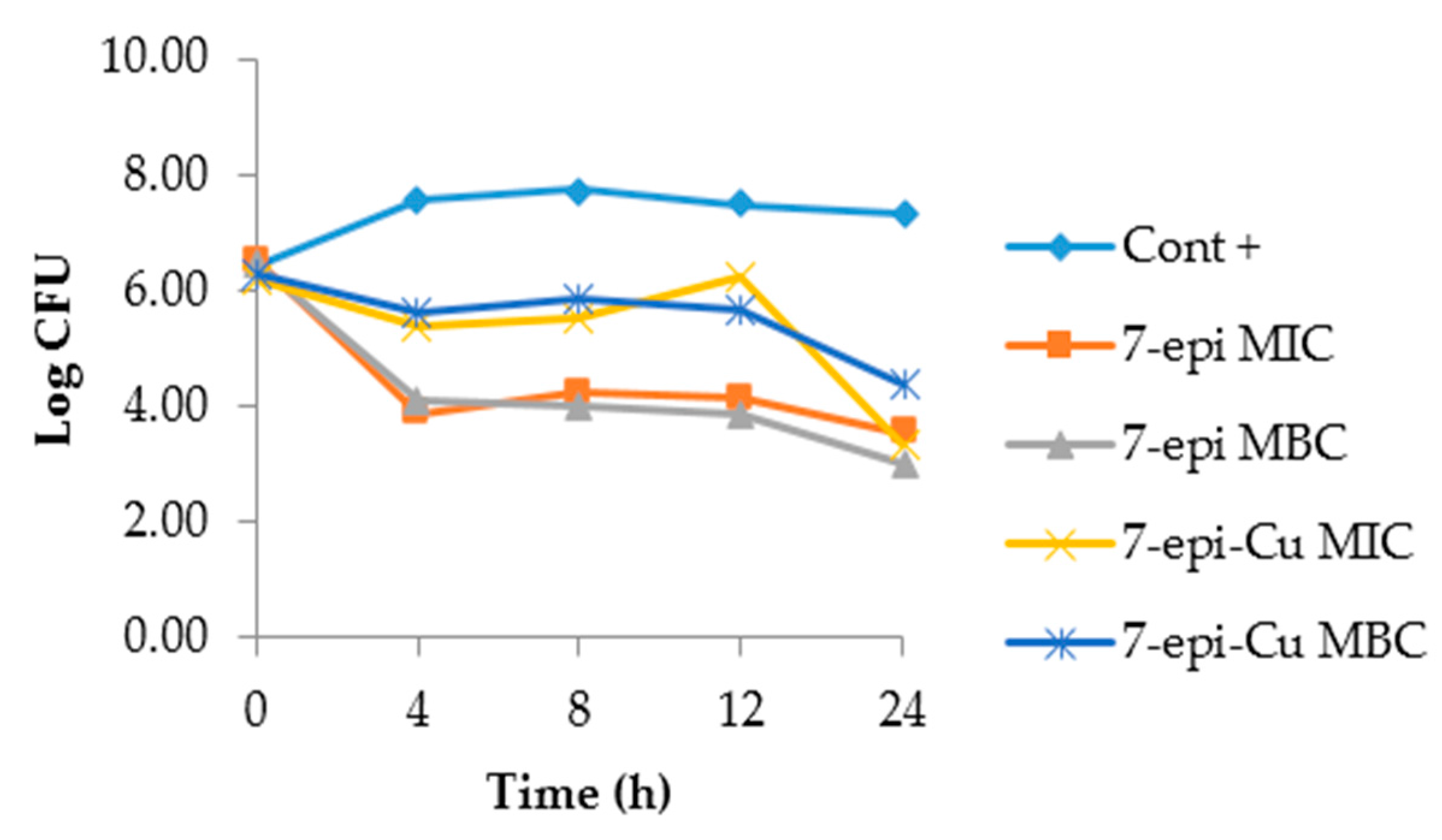
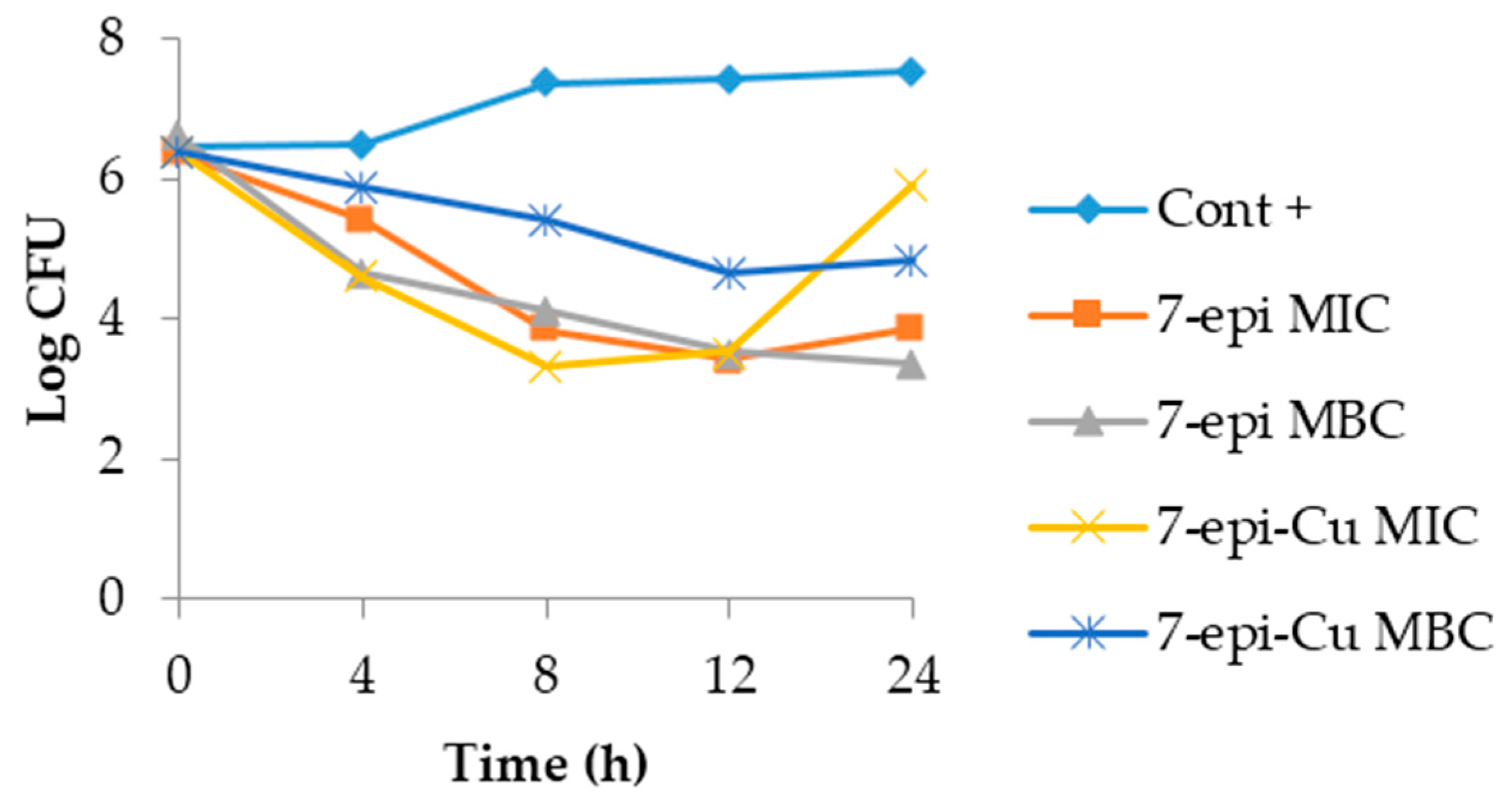
2.3. Time-Kill Curve
2.4. Protein Leakage Assay
2.5. Adherence Assessment
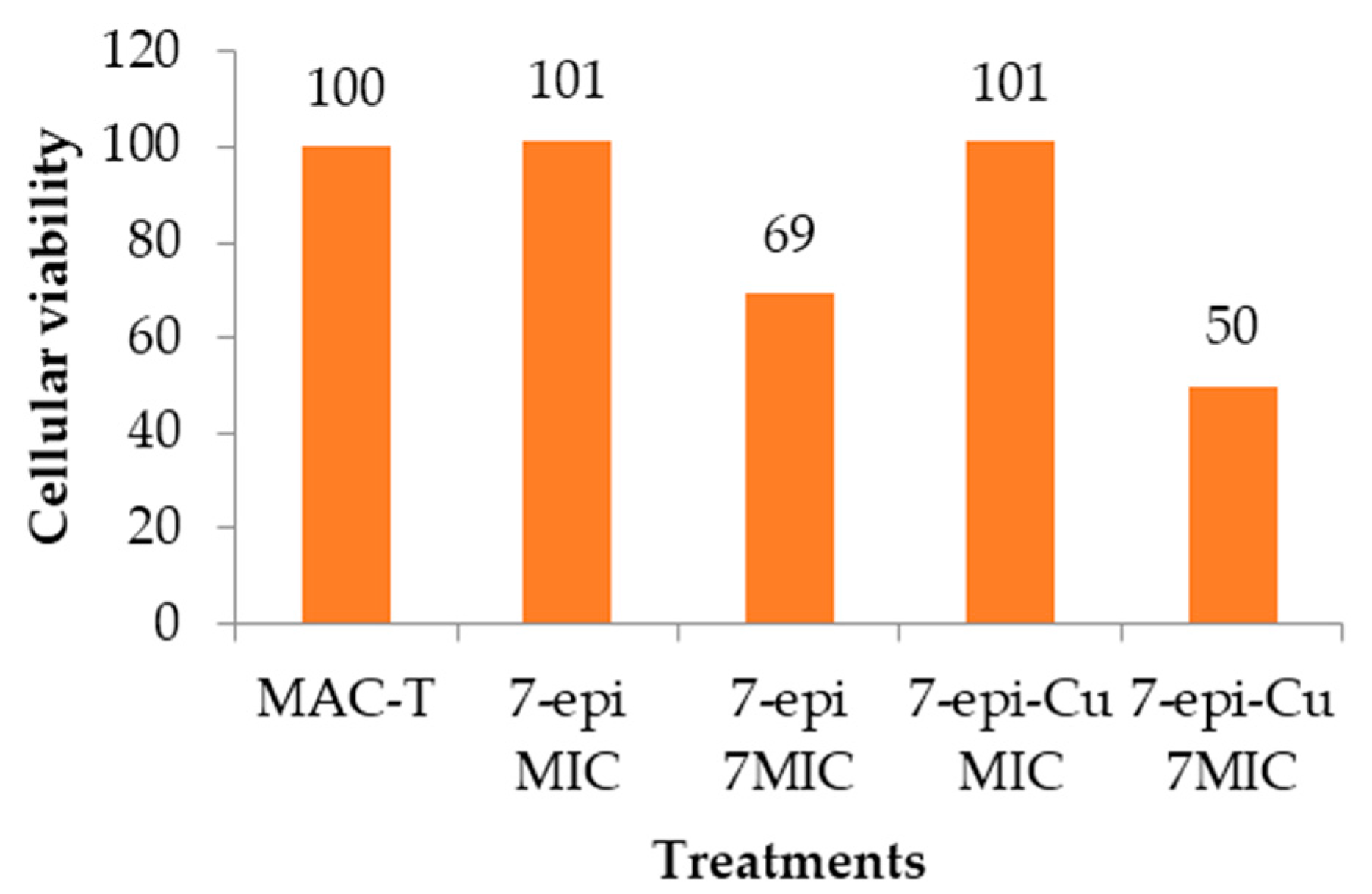
2.6. Cytotoxic Effect
3. Materials and Methods
3.1. Chemistry, Strains, and Culture Medium
3.1.1. Instrumentation General Methods
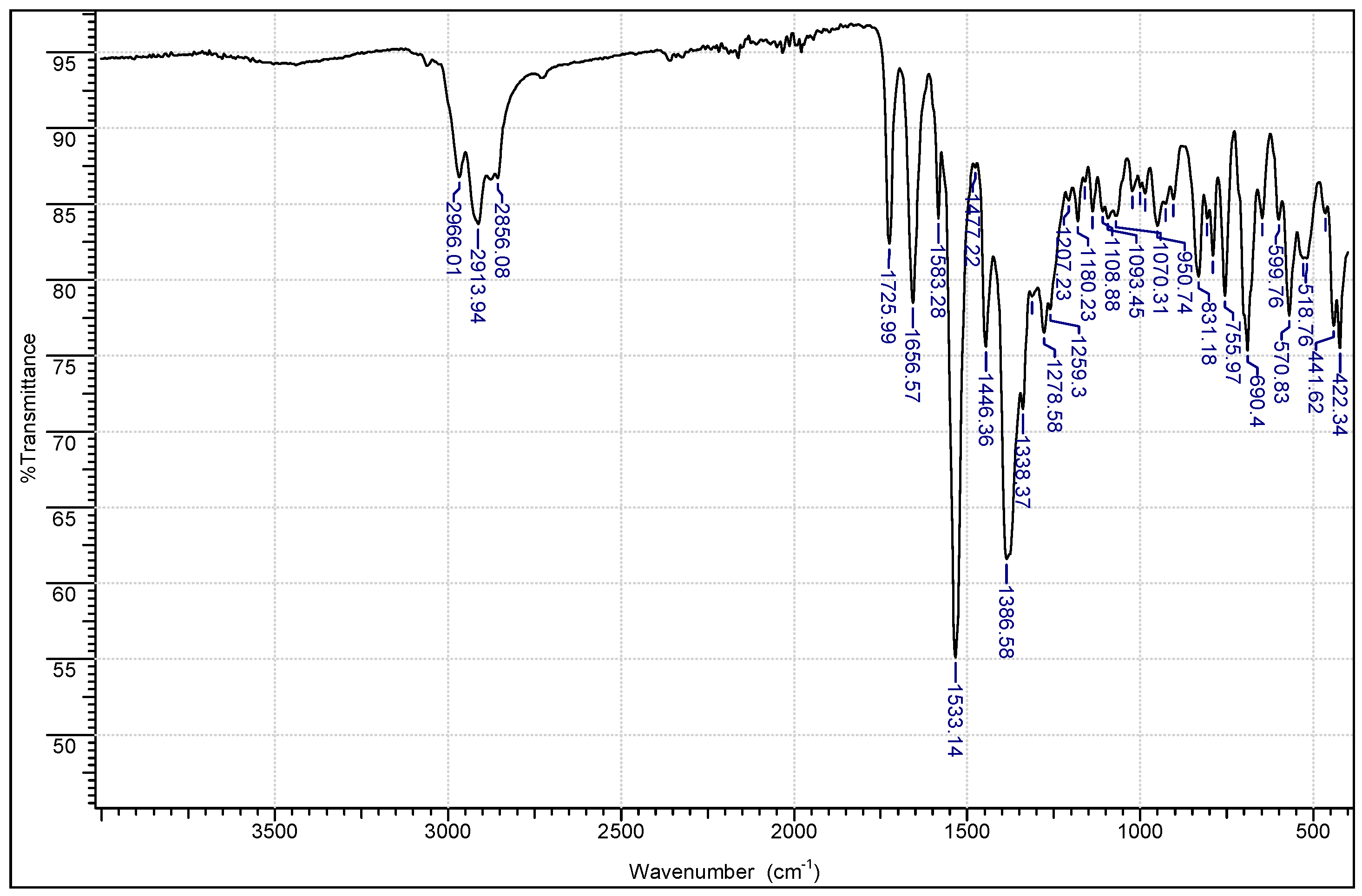
3.1.2. 7-Epiclusianone-Cu Physical Data
3.2. Minimum Inhibitory Concentration (MIC)
3.3. Minimum Bactericidal Concentration (MBC)
3.4. Time Kill Curve
3.5. Protein Leakage Assay
3.6. Adherence Assessment
3.7. Cytotoxic Effect in MAC-T
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maciel, M.A.M.; Pinto, A.C.; Veiga, V.F., Jr.; Grynberg, N.F.; Echevarria, A. Plantas medicinais: A necessidade de estudos multidisciplinares. Quim. Nova 2002, 25, 429–438. [Google Scholar] [CrossRef]
- Sponchiado, G.; Adam, M.L.; Silva, C.D.; Soley, B.S.; Mello-Sampayo, C.; Cabrini, D.A.; Correr, C.J.; Otuki, M.F. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. J. Ethnopharmacol. 2016, 178, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological sudies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 436, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ionta, M.; Ferreira-Silva, G.A.; Niero, E.L.; Costa, É.D’M.; Martens, A.A.; Rosa, W.; Soares, M.G.; Machado-Santelli, G.M.; Lago, J.H.G.; Santos, M.H. 7-Epiclusianone, a Benzophenone Extracted from Garcinia brasiliensis (Clusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells. Molecules 2015, 20, 12804–12816. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Silva, L.B.; Oliveira, M.V.; Gontijo, V.S.; Oliveira, W.F.; Derogis, P.B.M.C.; Stringheta, P.C.; Nagem, T.J.; Brigagão, M.R.P.L.; dos Santos, M.H. Antioxidant, cytotoxic and antimutagenic activities of 7-epi-clusianone obtained from pericarp of Garcinia brasiliensis. Food Res. Int. 2012, 48, 180–186. [Google Scholar] [CrossRef]
- Santos, M.H.; Nagem, T.J.; Oliveira, T.T.; Braz-Filho, R. 7-Epiclusianona, a nova benzofenona tetraprenilada e outros constituintes químicos dos frutos de Rheedia gardneriana. Quim. Nova 1999, 22, 654–660. [Google Scholar] [CrossRef]
- Alves, T.M.A.; Alves, R.O.; Romanha, A.J.; Santos, M.H.; Nagem, T.J.; Zani, C.L. Biological Activities of 7-Epiclusianone. J. Nat. Prod. 1999, 62, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.J.; Lemos, V.S.; dos Santos, M.H.; Nagem, T.J.; Cortes, S.F. Vascular effects of 7-epiclusianone, a prenylated benzophenone from Rheedia gardneriana, on the rat aorta. Phytomedicine 2006, 13, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Coelho, L.P.; Cordeiro, R.S.B.; Veloso, M.P.; Silva, P.M.R.; Santos, M.H.; Martins, M.A. Antianaphylactic properties of 7-epiclusianone, a tetraprenylated benzophenone isolated from Garcinia brasiliensis. Planta Med. 2007, 73, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Cuesta-Rubio, O.; Chica, M.B.; Mahmood, N.; Pagano, B.; Pavone, M.; Barone, V.; Rastrelli, L. Structural revision of clusianone and 7-epi-clusianone and anti-HIV activity of polyisoprenylated benzophenones. Tetrahedron 2005, 61, 8206–8211. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Freitas, L.A.S.; Vilela, F.C.; Veloso, C.C.; da Rocha, C.Q.; Moreira, M.E.C.; Dias, D.F.; Giusti-Paiva, A.; Santos, M.H. Antinociceptive and anti-inflammatory properties of 7-epiclusianone, a prenylated benzophenone from Garcinia brasiliensis. Eur. J. Pharmacol. 2011, 670, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.T.; Maia, J.R.S.; Vilela, M.J.; Ardisson, J.D.; Santos, M.H.; Oliveira, T.T.; Nagem, T.J. Spectroscopic Investigation of Organotin (IV) Derivatives of 7-Epiclusianone : A preliminary in vitro Antitumor Evaluation of the HN-5 Human Carcinoma Cell. Main Group Met. Chem. 2009, 32, 235–246. [Google Scholar] [CrossRef]
- Santi, E.; Facchin, G.; Faccio, R.; Barroso, R.P.; Costa-Filho, A.J.; Borthagaray, G.; Torre, M.H. Antimicrobial evaluation of new metallic complexes with xylitol active against P. aeruginosa and C. albicans: MIC determination, post-agent effect and Zn-uptake. J. Inorg. Biochem. 2016, 155, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarritan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.H.B.; de Paiva, R.E.F.; Cuin, A.; Ferreira, A.M.C.; Lustri, W.R.; Corbi, P.P. Synthesis, spectroscopic characterization, crystallographic studies and antibacterial assays of new copper(II) complexes with sulfathiazole and nimesulide. J. Mol. Struct. 2016, 1112, 14–20. [Google Scholar] [CrossRef]
- Silva, P.B.; Bonifácio, B.V.; Frem, R.C.G.; Godoy Netto, A.V.; Mauro, A.E.; Ferreira, A.M.C.; Lopes, E.O.; Raddi, M.S.G.; Bauab, T.M.; Pavan, F.R.; et al. Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes. Molecules 2015, 20, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.E.; El-Khaiat, H.M. The Importance of Copper and the Effects of Its Deficiency and Toxicity in Animal Health. Int. J. Livest. Res. 2015, 5, 1. [Google Scholar] [CrossRef]
- Humann-Ziehank, E. Selenium, copper and iron in veterinary medicine—From clinical implications to scientific models. J. Trace Elem. Med. Biol. 2016, 37, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Richards, V.P.; Lefébure, T.; Bitar, P.D.P.; Dogan, B.; Simpson, K.W.; Schukken, Y.H.; Stanhope, M.J. Genome Based Phylogeny and Comparative Genomic Analysis of Intra-Mammary Pathogenic Escherichia coli. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, K.R.; Trajcev, M.; Buneski, G. A review of the factors affecting the costs of bovine mastitis. J. S. Afr. Vet. Assoc. 2006, 77, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.B.; Vanderlei, D.R.; Moraes, W.S.; Brandespim, D.F.; Mota, R.A.; Oliveira, A.A.F.; Medeiros, E.S.; Pinheiro Júnior, J.W. Fatores de risco associados à mastite bovina na microrregião Garanhuns, Pernambuco. Pesqui. Vet. Bras. 2012, 32, 391–395. [Google Scholar] [CrossRef]
- Altalhi, A.D.; Hassan, S.A. Bacterial quality of raw milk investigated by Escherichia coli and isolates analysis for specific virulence-gene markers. Food Control 2009, 20, 913–917. [Google Scholar] [CrossRef]
- Carvalho, I.A.; Pietralonga, P.A.G.; Schwarz, D.G.G.; Faria, A.C.S.; Moreira, M.A.S. Short communication: Recovery of viable Mycobacterium avium subspecies paratuberculosis from retail pasteurized whole milk in Brazil. J. Dairy Sci. 2012, 95, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, H.; Khan, A.A.; Banday, M.T.; Ashraf, I.; Handoo, N.; Bashir, A.; Hamadani, A. Bovine Mastitis—A Disease of Serious Concern for Dairy Farmers. Int. J. Livest. Res. 2013, 3, 42–55. [Google Scholar] [CrossRef]
- Wang, W.; Song, Y.; Petrovski, K.; Eats, P.; Trott, D.J.; Wong, H.S.; Page, S.W.; Perry, J.; Garg, S. Development of intramammary delivery systems containing lasalocid for the treatment of bovine mastitis: Impact of solubility improvement on safety, efficacy, and milk distribution in dairy cattle. Drug Des. Dev. Ther. 2015, 9, 631–642. [Google Scholar]
- Nani, J.P.; Raschia, M.A.; Carignano, H.; Poli, M.A.; Calvinho, L.F.; Amadio, A.F. Single nucleotide polymorphisms in candidate genes and their relation with somatic cell scores in Argentinean dairy cattle. J. Appl. Genet. 2015, 56, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wójcik, A. Antimicrobial properties of gold, silver, copper and platinum against selected microorganisms isolated from cases of mastitis in cattle. Med. Weter. 2014, 70, 4. [Google Scholar]
- Nielsen, E.I.; Viberg, A.; Löwdin, E.; Cars, O.; Karlsson, M.O.; Sandström, M. Semimechanistic Pharmacokinetic/Pharmacodynamic Model for Assessment of Activity of Antibacterial Agents from Time-Kill Curve Experiments. Antimicrob. Agents Chemother. 2007, 51, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.; Oesch, G.; Kuster, S.P. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2015, 70, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Brötz-Oesterhelt, H.; Brunner, N.A. How many modes of action should an antibiotic have? Curr. Opin. Pharmacol. 2008, 8, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial Adhesion and Entry into Host Cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.M.; Espechit, I.F.; Pieri, F.A; Benjamin, L.A.; Moreira, M.A.S. Increased production of biofilms by Escherichia coli in the presence of enrofloxacin. Vet. Microbiol. 2012, 160, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.J.; Green, M.J. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J. Dairy Sci. 2009, 92, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Soares, L.O.; Silva Júnior, A.; Mantovani, H.C.; Chang, Y.-F.; Moreira, M.A.S. Biofilm Formation on Biotic and Abiotic Surfaces in the Presence of Antimicrobials by Escherichia coli Isolates from Cases of Bovine Mastitis. Appl. Environ. Microbiol. 2014, 80, 6136–6145. [Google Scholar] [PubMed]
- Odenholt, I. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int. J. Antimicrob. Agents 2001, 17, 1–8. [Google Scholar] [PubMed]
- Wallinga, D.; Burch, D.G.S. Does adding routine antibiotics to animal feed pose a serious risk to human health? BMJ 2013, 347, 9–11. [Google Scholar]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [PubMed]
- Almeida, R.A.; Dego, O.K.; Headrick, S.I.; Lewis, M.J.; Oliver, S.P. Role of Streptococcus uberis adhesion molecule in the pathogenesis of Streptococcus uberis mastitis. Vet. Microbiol. 2015, 179, 332–335. [Google Scholar] [PubMed]
- Chuzeville, S.; Dramsi, S.; Madec, J.-Y.; Haenni, M.; Payot, S. Antigen I/II encoded by integrative and conjugative elements of Streptococcus agalactiae and role in biofilm formation. Microb. Pathog. 2015, 88, 1–9. [Google Scholar] [PubMed]
- Nobbs, A.H.; Jenkinson, H.F.; Everett, D.B. Generic determinants of Streptococcus colonization and infection. Infect. Genet. Evol. 2015, 33, 361–370. [Google Scholar] [PubMed]
- Dunne, W.M., Jr. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [PubMed]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [PubMed]
- Melchior, M.B.; Vaarkamp, H.; Fink-Gremmels, J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006, 171, 398–407. [Google Scholar] [PubMed]
- Santos, M.H.; Speziali, N.L.; Nagem, T.J.; Oliveira, T.T. Epiclusianone: A New Natural Product Derivative of Bicyclo[3.3.1]nonane-2,4,9-trione. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 1990–1992. [Google Scholar] [CrossRef]
- Costa, É.D’M.; Lemes, N.H.T.; Santos, M.H.; Braga, J.P. Uso de redes neurais recorrentes na determinação das constantes de acidez para a 7-epiclusianona em misturas etanol-água. Quim. Nova 2012, 35, 91–96. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, 9th ed.; Clinical and Laboratorial Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and γ-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- Bhande, R.M.; Khobragade, C.N.; Mane, R.S.; Bhande, S. Enhanced synergism of antibiotics with zinc oxide nanoparticles against extended spectrum β-lactamase producers implicated in urinary tract infections. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M. Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2). Pharm. Biol. 2015, 54, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Canteri, M.G.; Althaus, R.A.; Virgens Filho, J.S.; Giglioti, E.A.; Godoy, C.V. SASM—Agri: Sistema para análise e separação de médias em experimentos agrícolas pelos métodos Scoft—Knott, Tukey e Duncan. Rev. Bras. Agrocomputação 2001, 1, 18–24. [Google Scholar]
Sample Availability: Samples of the compounds 7-epiclusianone and 7-epiclusianone-copper are available from the authors. |

| Strains | MIC | MBC | ||
|---|---|---|---|---|
| Compounds (µg mL−1/µM) | ||||
| 7-epi | 7-epi-Cu | 7-epi | 7-epi-Cu | |
| SA3930 | - | - | - | - |
| SA4038 | 7.8/15.5 | 7.8/7.3 | 15.6/31.1 | 31.3/29.3 |
| SU959 | 7.8/15.5 | 7.8/7.3 | 31.3/62.2 | 31.3/29.3 |
| SU3580 | 7.8/15.5 | 7.8/7.3 | 31.3/62.2 | 31.3/29.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Barros, M.; Perciano, P.G.; Dos Santos, M.H.; De Oliveira, L.L.; Costa, É.D.; Moreira, M.A.S. Antibacterial Activity of 7-Epiclusianone and Its Novel Copper Metal Complex on Streptococcus spp. Isolated from Bovine Mastitis and Their Cytotoxicity in MAC-T Cells. Molecules 2017, 22, 823. https://doi.org/10.3390/molecules22050823
De Barros M, Perciano PG, Dos Santos MH, De Oliveira LL, Costa ÉD, Moreira MAS. Antibacterial Activity of 7-Epiclusianone and Its Novel Copper Metal Complex on Streptococcus spp. Isolated from Bovine Mastitis and Their Cytotoxicity in MAC-T Cells. Molecules. 2017; 22(5):823. https://doi.org/10.3390/molecules22050823
Chicago/Turabian StyleDe Barros, Mariana, Pedro Griffo Perciano, Marcelo Henrique Dos Santos, Leandro Licursi De Oliveira, Éderson D’Martin Costa, and Maria Aparecida Scatamburlo Moreira. 2017. "Antibacterial Activity of 7-Epiclusianone and Its Novel Copper Metal Complex on Streptococcus spp. Isolated from Bovine Mastitis and Their Cytotoxicity in MAC-T Cells" Molecules 22, no. 5: 823. https://doi.org/10.3390/molecules22050823






