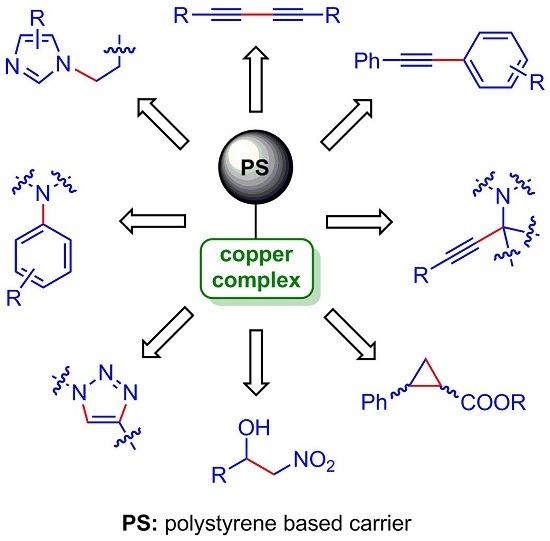
Recent Advances in C–C and C–N Bond Forming Reactions Catalysed by Polystyrene-Supported Copper Complexes
Abstract
:1. Introduction
2. Carbon–Carbon Forming Reactions
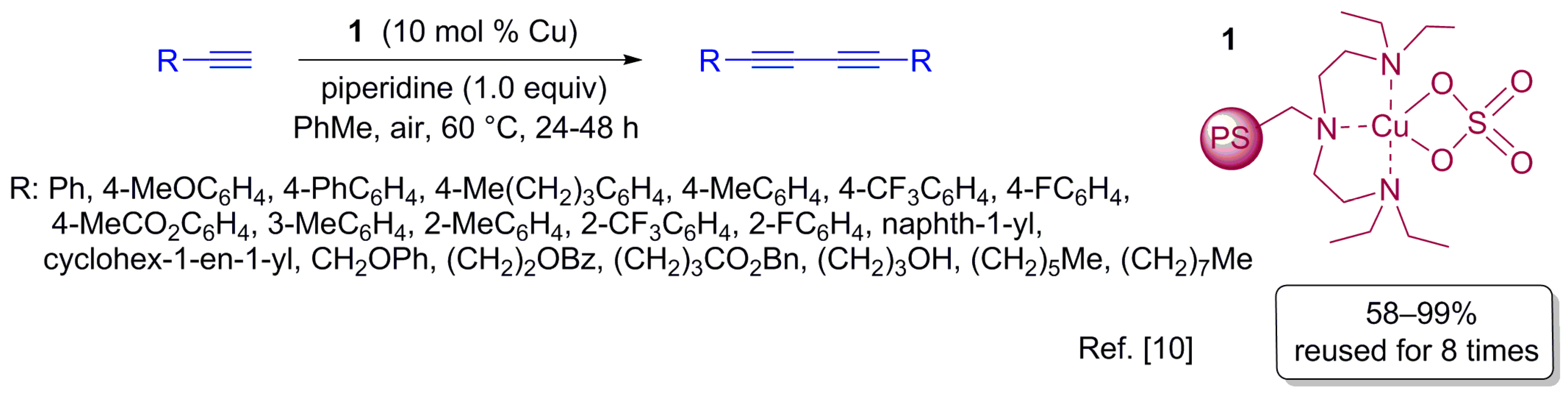
2.1. Oxidative Homocoupling of Terminal Alkynes
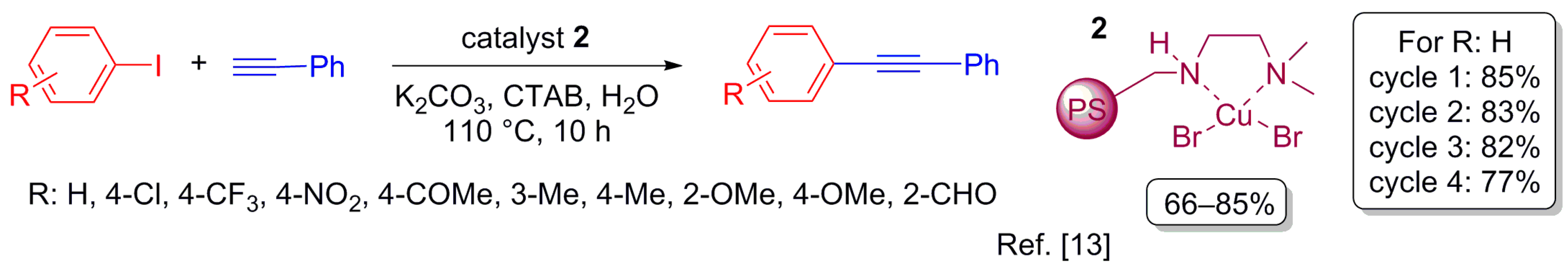
2.2. Sonogashira Cross-Coupling Reaction
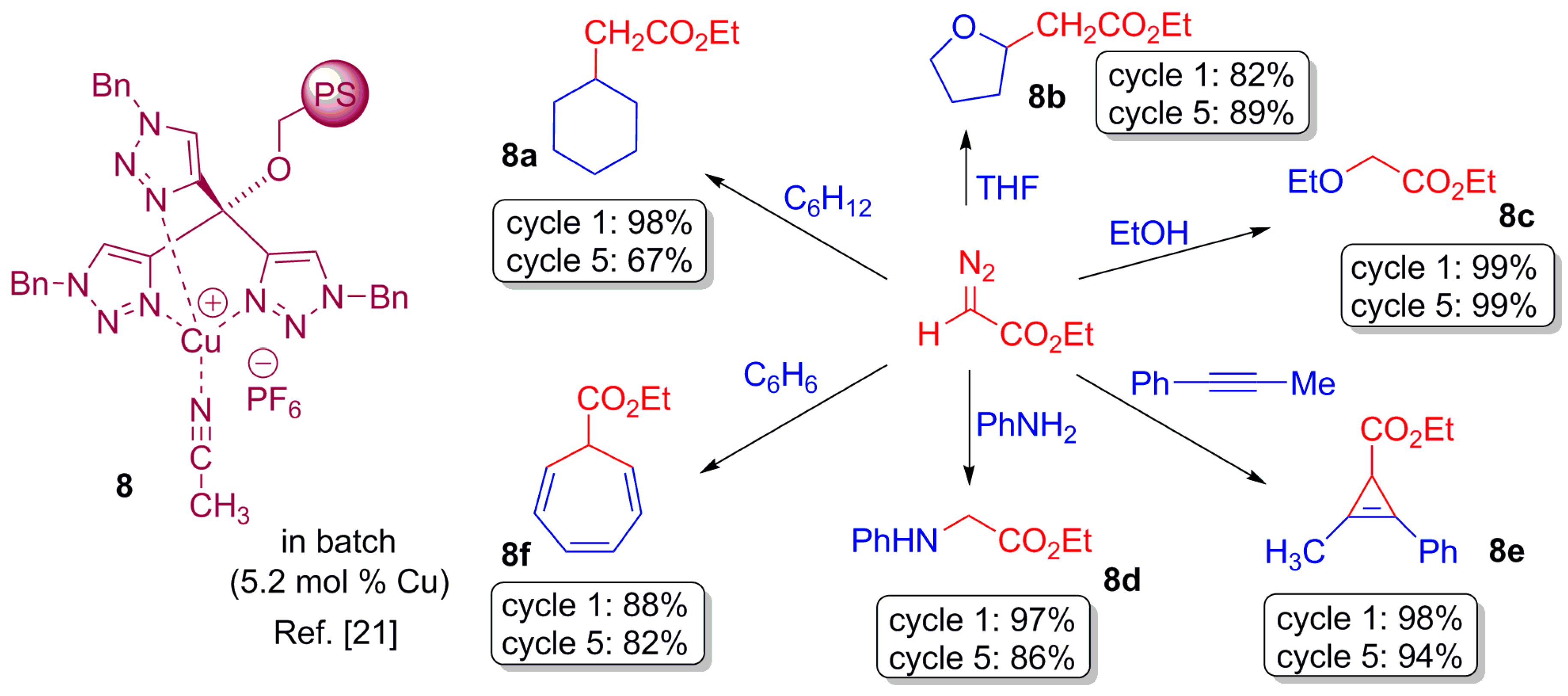
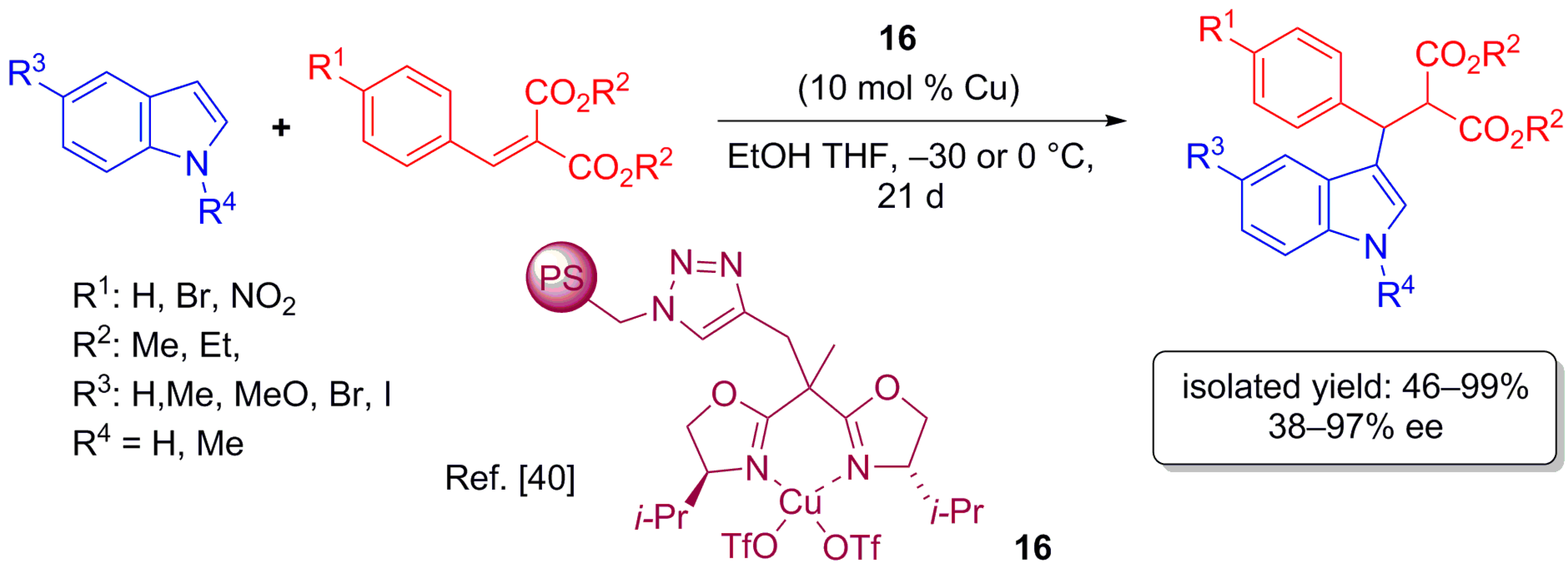
2.3. Carbene Transfer Reaction
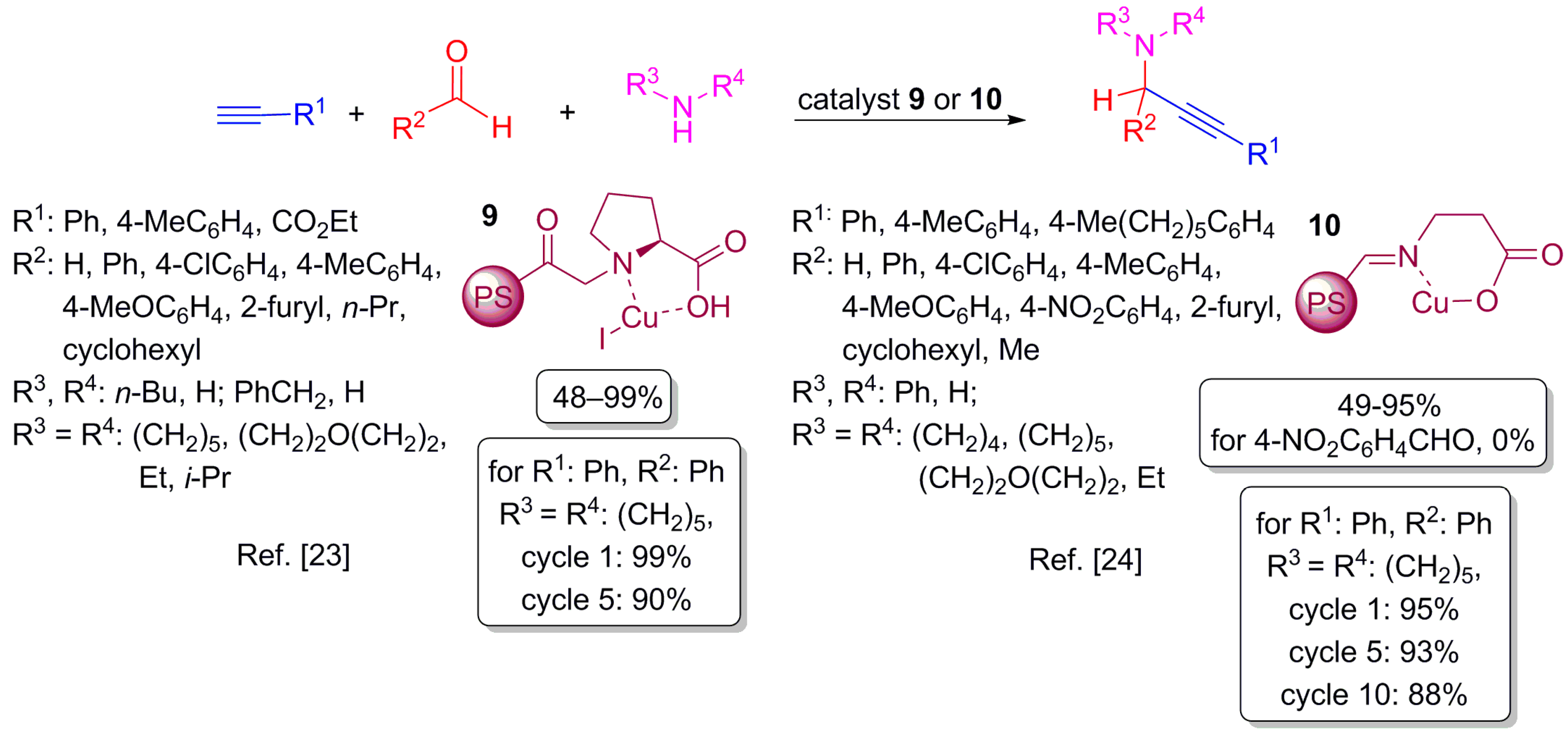
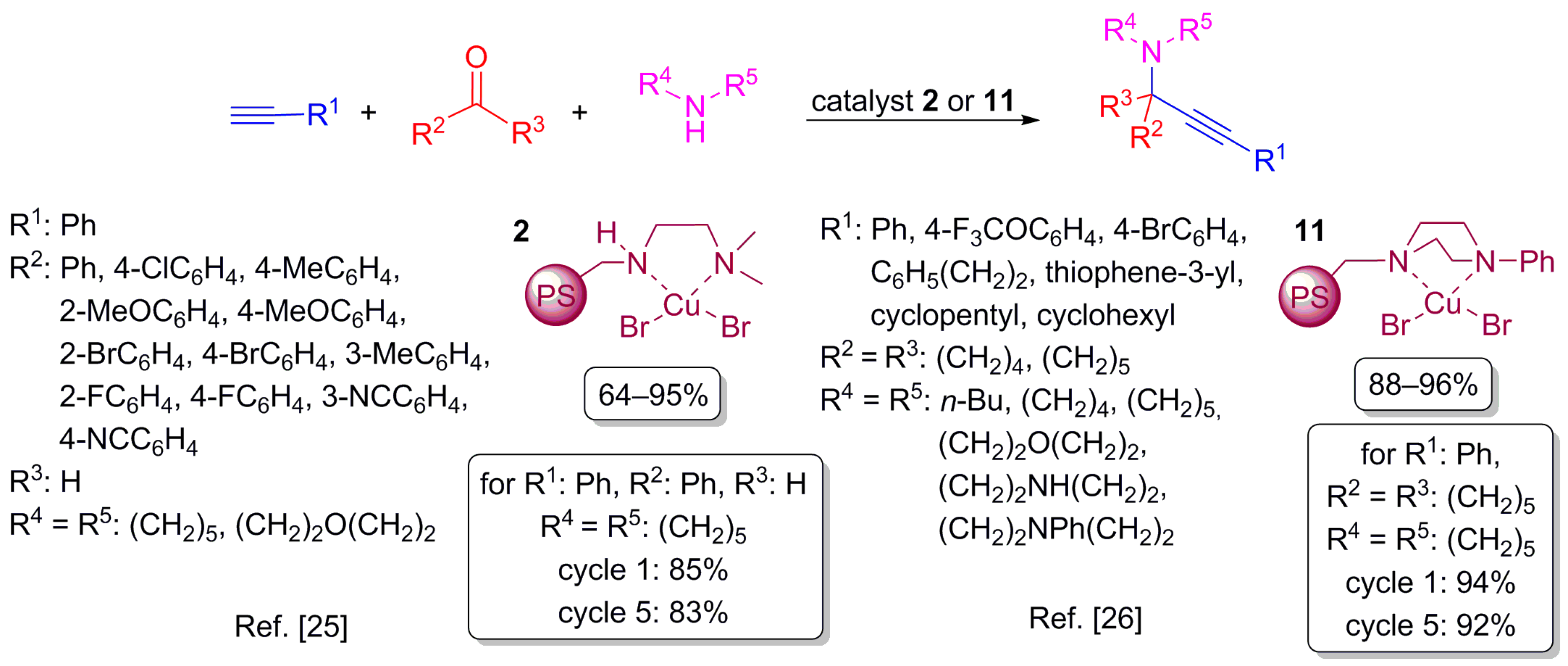
2.4. Addition of Alkynes on Double Bond Carbon–Nitrogen
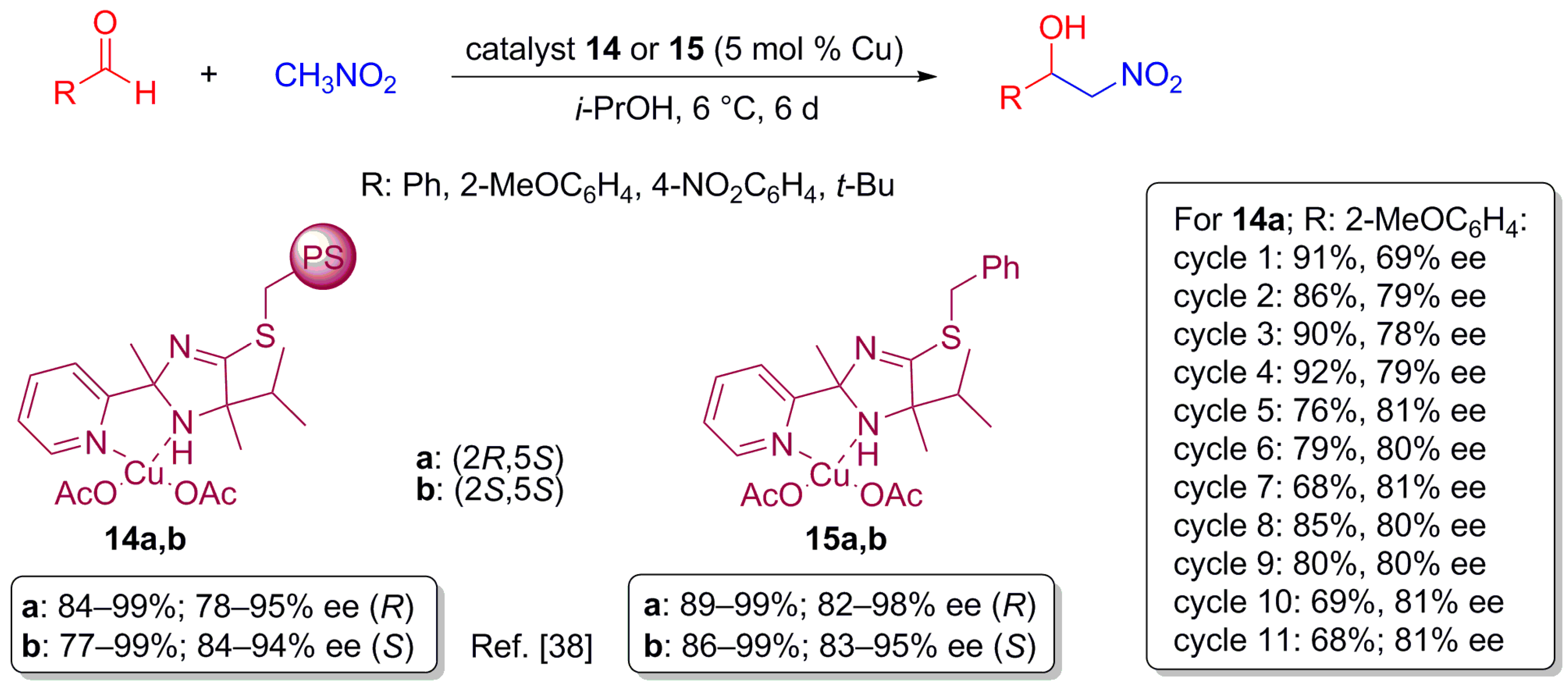
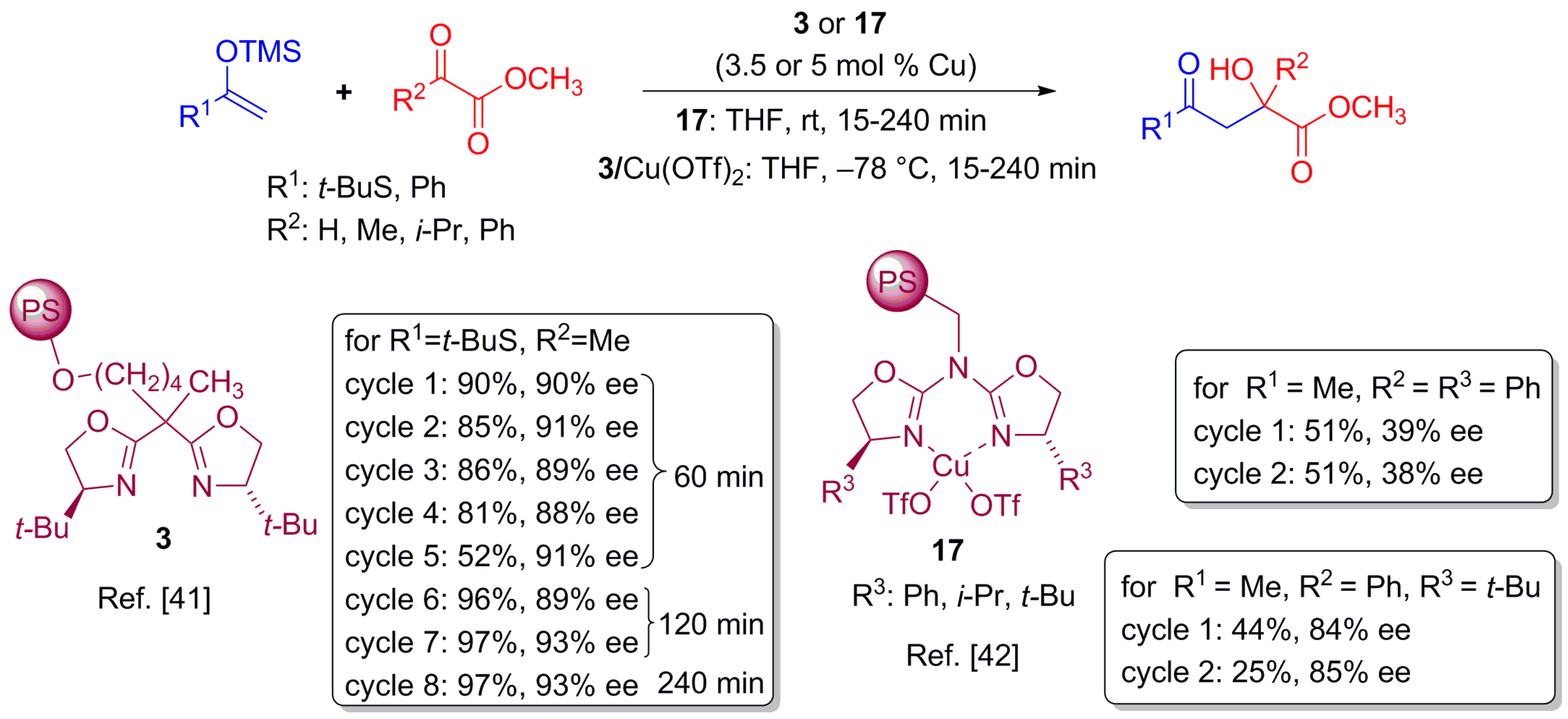
2.5. Nitroaldolization Reaction
2.6. Asymmetric Friedel–Crafts Reaction
2.7. Asymmetric Mukaiyama Aldol Reaction
3. Carbon–Nitrogen Forming Reactions
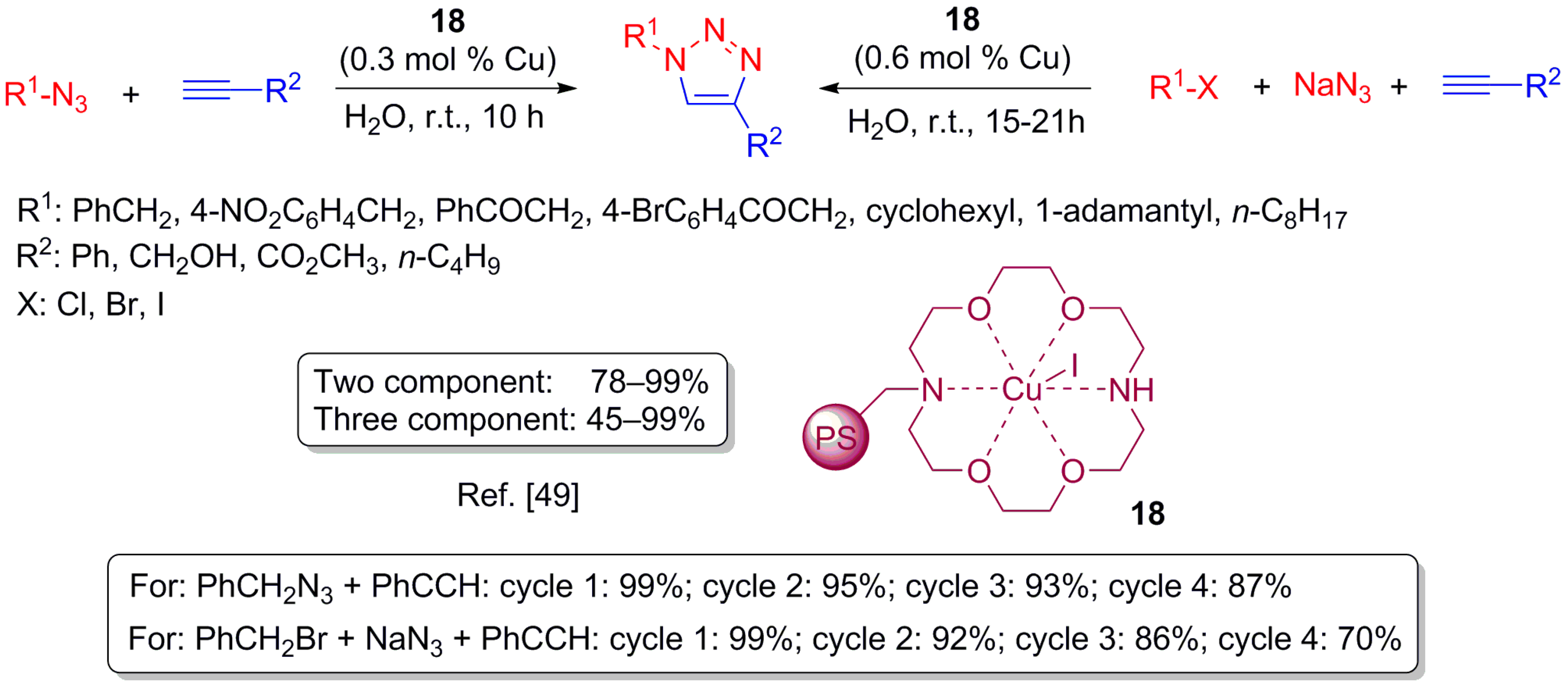
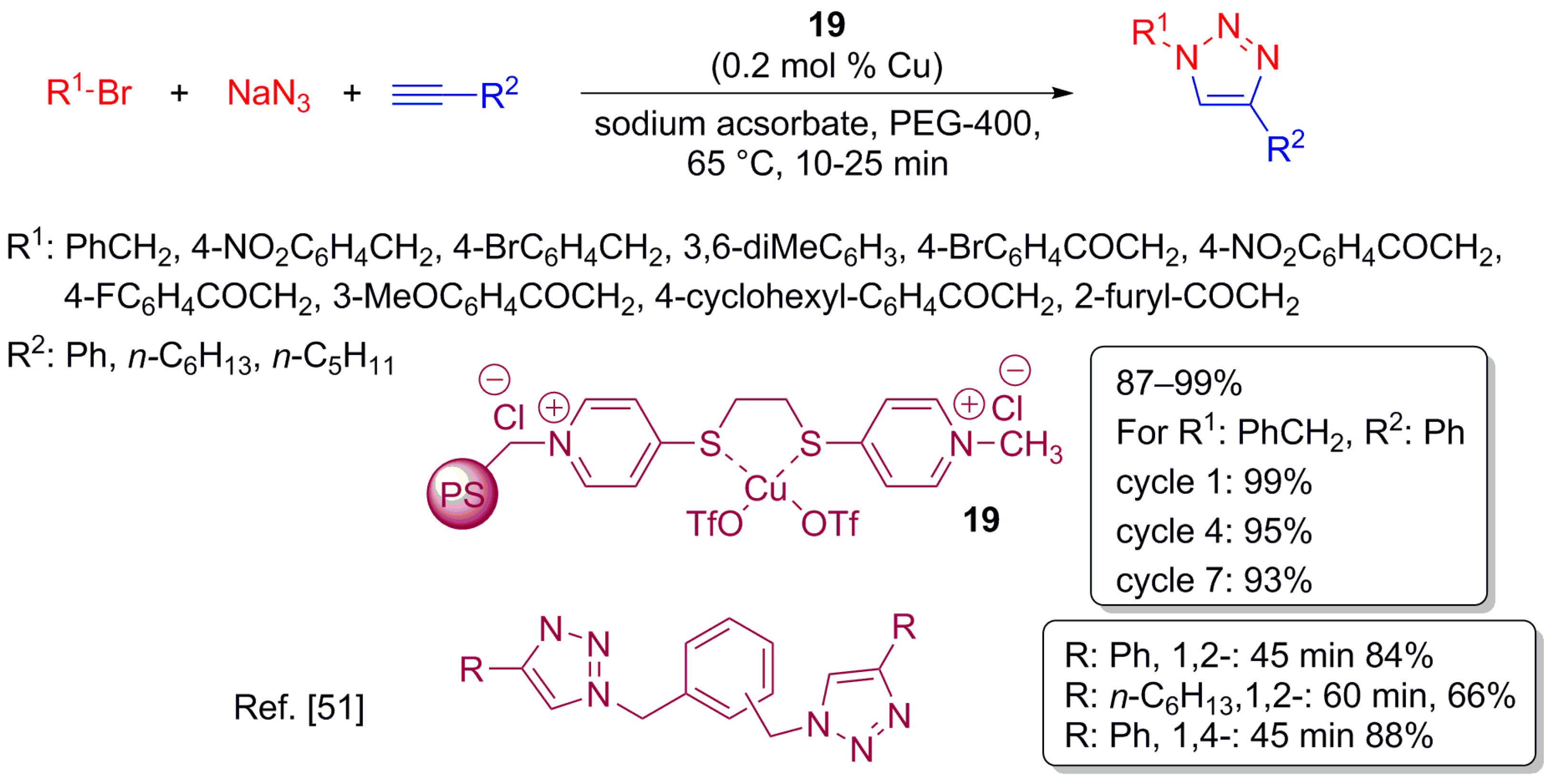
3.1. Copper(I)-Catalysed Azide Alkyne Cycloaddition
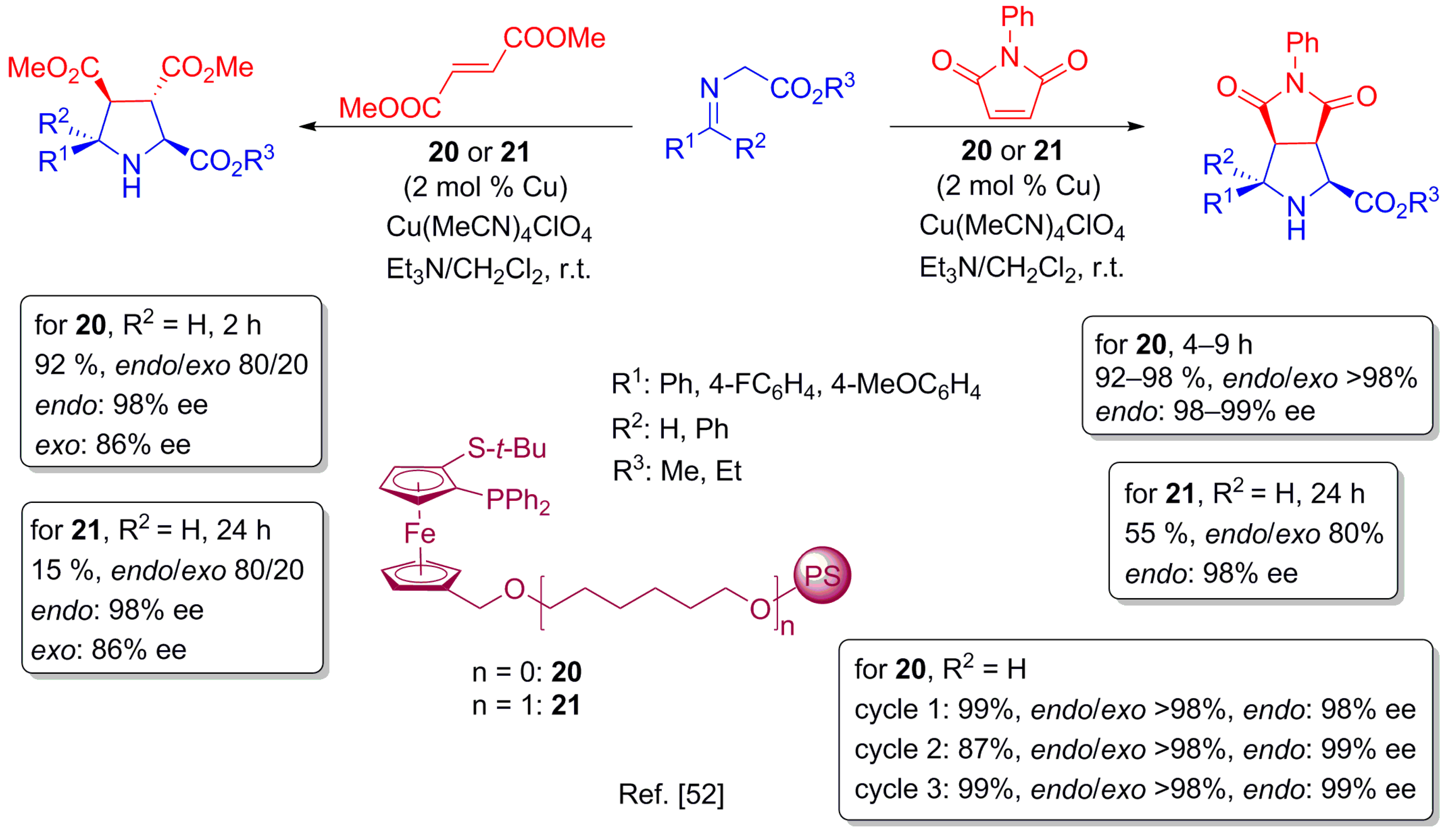
3.2. Other Ring-Formation Reactions
3.3. N-Arylation of Amines, Amides and Nitrogen Containing Heterocycles
3.4. Aza-Michael Addition
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van Santen, R.; Khoe, D.; Vermeer, B. Sustainable materials. In 2030 Technology that will Change the World; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Tools of Green Chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Cozzi, F. Immobilization of organic catalysts: When, why, and how. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Benaglia, M. Insoluble Resin-supported Catalysts. In Recoverable and Recyclable Catalysts; John Wiley & Sons: Wiltshire, UK, 2009. [Google Scholar]
- Jimeno, C.; Sayalero, S.; Pericàs, M.A. Covalent Heterogenization of Asymmetric Catalysts on Polymers and Nanoparticles. In Heterogenized Homogenous Catalysts for Fine Chemicals Production: Materials and Processes; Barbaro, P., Liguori, F., Eds.; Springer Science and Business Media: Dordrecht, the Netherlands, 2010. [Google Scholar]
- Merrifield, R.B.J. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Kristensen, T.E.; Hansen, T. Polymer-supported chiral organocatalysts: Synthetic strategies for the road towards affordable polymeric immobilization. Eur. J. Org. Chem. 2010, 3179–3204. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge zur Kenntniss des Acetenylbenzols. Ber. Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Anilkumar, G. Recent advances and applications of Glaser coupling employing greener protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Yan, S.; Pan, S.; Osako, T.; Uozumi, Y. Recyclable polystyrene-supported copper catalysts for the aerobic oxidative homocoupling of terminal alkynes. Synlett 2016, 27, 1232–1236. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, Z.; Du, Y.; Wu, Q.; Wang, L.; Zhu, L.; Meng, X.; Chen, W.; Xiao, F.-S. Recyclable porous polymer-supported copper catalysts for Glaser and Huisgen 1,3-dipolar cycloaddition reactions. Chem. Asian J. 2013, 8, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Barot, N.; Patel, S.B.; Kaur, H. Nitro resin supported copper nanoparticles: An effective heterogeneous catalyst for C–N cross coupling and oxidative C–C homocoupling. J. Mol. Catal. A Chem. 2016, 423, 77–84. [Google Scholar] [CrossRef]
- Kodicherla, B.; Perumgani, P.C.; Mandapati, M.R. A reusable polystyrene-supported copper(II) catalytic system for N-arylation of indoles and Sonogashira coupling reactions in water. Appl. Catal. A Gen. 2014, 483, 110–115. [Google Scholar] [CrossRef]
- Doyle, M.P.; McKervey, M.A.; Ye, T. Catalysts for Metal Carbene Transformations. In Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Mandoli, A.; Orlandi, S.; Pini, D.; Salvadori, P. A reusable, insoluble polymer-bound bis(oxazoline) (IPB-box) for highly enantioselective heterogeneous cyclopropanation reactions. Chem. Commun. 2003, 2466–2467. [Google Scholar] [CrossRef]
- Mandoli, A.; Garzelli, R.; Orlandi, S.; Pini, D.; Lessi, M.; Salvadori, P. Some factors affecting the catalytic efficiency in the enantioselective cyclopropanation of olefins by the use of insoluble polystyrene-bound bisoxazoline-copper(I) complex. Catal. Today 2009, 140, 51–57. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fraile, J.M.; García, J.I.; García-Verdugo, E.; Herrerías, C.I.; Luis, S.V.; Mayoral, J.A. Bis(oxazoline)copper complexes covalently bonded to insoluble support as catalysts in cyclopropanation reactions. J. Org. Chem. 2001, 66, 8893–8901. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Belcher, P.E. 2-Aryl-5,5-bisoxazolin-2-yl[1,3]dioxanes as solution phase and immobilised ligands for highly enantioselective cyclopropanations. Tetrahedron Asymmetry 2005, 16, 1415–1418. [Google Scholar] [CrossRef]
- Werner, H.; Herrerías, C.I.; Glos, M.; Gissibl, A.; Fraile, J.M.; Péres, I.; Mayoral, J.A.; Reiser, O. Synthesis of polymer bound azabis(oxazoline) ligands and their application in asymmetric cyclopropanations. Adv. Synth. Catal. 2006, 348, 125–132. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fraile, J.M.; García-Verdugo, E.; Luis, S.V.; Martínez-Merino, V.; Mayoral, J.A. Polymer-supported bis(oxazolines) and related systems: Toward new heterogeneous enantioselective catalysts. Ind. Eng. Chem. Res. 2006, 44, 8580–8587. [Google Scholar] [CrossRef]
- Maestre, L.; Ozkal, E.; Ayats, C.; Beltrán, A.; Díaz-Requejo, M.M.; Pérez, P.J.; Pericàs, M.A. A fully recyclable heterogenized Cu catalyst for the general carbene transfer reaction in batch and flow. Chem. Sci. 2015, 6, 1510–1515. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, C. Reusable polymer-anchored amino acid copper complex for the synthesis of propargylamines. J. Chem. Res. 2008, 32, 538–541. [Google Scholar] [CrossRef]
- Islam, M.; Roy, A.S.; Islam, S.M. Functionalized polystyrene supported copper(I) complex as an effective and reusable catalyst for propargylamines synthesis in aqueous medium. Catal. Lett. 2016, 146, 1128–1137. [Google Scholar] [CrossRef]
- Kodicherla, B.; Perumgani, P.C.; Mandapati, M.R. Polymer-anchored copper(II) complex: An efficient reusable catalyst for the synthesis of propargylamines. Appl. Organometal. Chem. 2014, 28, 756–759. [Google Scholar] [CrossRef]
- Perumgani, P.C.; Keesara, S.; Parvathaneni, S.; Mandapati, M.R. Polystyrene supported N-phenylpiperazine–Cu(II) complex: An efficient and reusable catalyst for KA2-coupling reactions under solvent-free conditions. New J. Chem. 2016, 40, 5113–5120. [Google Scholar] [CrossRef]
- Gommermann, N.; Knochel, P. Practical highly enantioselective synthesis of propargylamines through a copper-catalyzed one-pot three-component condensation reaction. Chem. Eur. J. 2006, 12, 4380–4392. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ohara, M.; Nakamura, Y.; Shibata, N.; Toru, T. Copper-catalyzed enantioselective three-component synthesis of optically active propargylamines from aldehydes, amines, and aliphatic alkynes. Chem. Eur. J. 2010, 16, 2360–2362. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, H.; Moradian, M. Thioether-based copper(I) Schiff base complex as a catalyst for a direct and asymmetric A3-coupling reaction. Tetrahedron Asymmetry 2014, 25, 427–434. [Google Scholar] [CrossRef]
- Drabina, P.; Funk, P.; Růžička, A.; Hanusek, J.; Sedlák, M. Synthesis, copper(II) complexes and catalytic activity of substituted 6-(1,3-oxazolin-2-yl)pyridine-2-carboxylates. Transit. Met. Chem. 2010, 35, 363–371. [Google Scholar] [CrossRef]
- Zeng, T.; Yang, L.; Hudson, R.; Song, G.; Moores, A.R.; Li, C.-J. Fe3O4 Nanoparticle-supported copper(I) pybox catalyst: Magnetically recoverable catalyst for enantioselective direct-addition of terminal alkynes to imines. Org. Lett. 2011, 13, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Sasai, H.; Suzuki, T.; Arai, S.; Arai, T.; Shibasaki, M. Basic character of rare earth metal alkoxides. Utilization in catalytic C–C bond-forming reactions and catalytic asymmetric nitroaldol reactions. J. Am. Chem. Soc. 1992, 114, 4418–4420. [Google Scholar] [CrossRef]
- Ananthi, N.; Velmathi, S. Asymmetric Henry reaction catalyzed by transition metal complexes: A short review. Ind. J. Chem. 2013, 52, 87–108. [Google Scholar]
- Corey, E.J.; Zhang, F. re- and si-Face-selective nitroaldol reactions catalyzed by a rigid chiral quaternary ammonium salt: A highly stereoselective synthesis of the HIV protease inhibitor Amprenavir (Vertex 478). Angew. Chem. Int. Ed. 1999, 38, 1931–1934. [Google Scholar] [CrossRef]
- Drabina, P.; Harmand, L.; Sedlák, M. Enantioselective Henry reaction catalyzed by supported transition metal complexes. Curr. Org. Synth. 2014, 11, 879–888. [Google Scholar] [CrossRef]
- Harmand, L.; Drabina, P.; Pejchal, V.; Husáková, L.; Sedlák, M. Recyclable catalyst for the asymmetric Henry reaction based on functionalized imidazolidine-4-one-copper(II) complexes supported by a polystyrene copolymer. Tetrahedron Lett. 2015, 56, 6240–6243. [Google Scholar] [CrossRef]
- Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. Highly enantioselective nitroaldol reactions catalyzed by copper(II) complexes derived from substituted 2-(pyridin-2-yl)imidazolidin-4-one ligands. J. Org. Chem. 2011, 76, 4787–4793. [Google Scholar] [CrossRef] [PubMed]
- Nováková, G.; Drabina, P.; Frumarová, B.; Sedlák, M. Recyclable enantioselective catalysts based on copper(II) complexes of 2-(pyridine-2-yl)imidazolidine-4-thione: Their application in asymmetric Henry reactions. Adv. Synth. Catal. 2016, 358, 2541–2552. [Google Scholar] [CrossRef]
- Arai, T.; Yokoyama, N.; Yanagisawa, A. A library of chiral imidazoline–aminophenol ligands: Discovery of an efficient reaction sphere. Chem. Eur. J. 2008, 14, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Desyatkin, V.G.; Anokhin, M.V.; Rodionov, V.O.; Beletskaya, I.P. Polystyrene-supported Cu(II)-R-Box as recyclable catalyst in asymmetric Friedel–Crafts reaction. Russ. J. Org. Chem. 2016, 52, 1717–1727. [Google Scholar] [CrossRef]
- Orlandi, S.; Mandoli, A.; Pini, D.; Salvadori, P. An insoluble polymer-bound bis-oxazoline copper(II) complex: A highly efficient heterogeneous catalyst for the enantioselective Mukaiyama aldol reaction. Angew. Chem. Int. Ed. 2001, 40, 2519–2521. [Google Scholar] [CrossRef]
- Fraile, J.M.; Pérez, I.; Mayoral, J.A. Comparison of immobilized Box and azaBox–Cu(II) complexes as catalysts for enantioselective Mukaiyama aldol reactions. J. Catal. 2007, 252, 303–311. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Proc. Chem. Soc. 1961, 357–396. [Google Scholar]
- Tornøe, C.W.; Christensen, C.; Medal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar]
- Rostovtset, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B.A. Stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B.A. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Girard, C.; Onen, E.; Aufort, M.; Beauviere, S.; Samson, E.; Herscovici, J. Reusable polymer-supported catalyst for the [3+2] Huisgen cycloaddition in automation protocols. Org. Lett. 2006, 8, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC Click Chemistry′ goes heterogenous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
- Movassagh, B.; Rezaei, N. Polystyrene resin-supported CuI-cryptand 22 complex: A highly efficient and reusable catalyst for three-component synthesis of 1,4-disubstituted 1,2,3-triazoles under aerobic conditions in water. Tetrahedron 2014, 70, 8885–8892. [Google Scholar] [CrossRef]
- Rezaei, N.; Movassagh, B. Polystyrene resin-supported CuI-cryptand 22 complex: A highly efficient and reusable catalyst for the formation of aryl-sulfur bonds in aqueous media. Tetrahedron Lett. 2016, 57, 1625–1628. [Google Scholar] [CrossRef]
- Tavassoli, M.; Landarani-Isfahani, A.; Moghadam, M.; Tangestaninejad, S.; Valliolah Mirkhani, V.; Mohammadpoor-Baltork, I. Polystyrene-supported ionic liquid copper complex: A reusable catalyst for one-pot three-component click reaction. Appl. Catal. A 2015, 503, 186–195. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Pereira, S.I.; Peña-Cabrera, E.; Adrio, J.; Silva, A.M.S.; Carretero, J.C. Synthesis of polymer-supported Fesulphos ligands and their application in asymmetric catalysis. Adv. Synth. Catal. 2007, 349, 1714–1724. [Google Scholar] [CrossRef]
- Maurya, M.R.; Sikarwar, S.; Joseph, T.; Halligudi, S.B. Bis(2-[α-hydroxyethyl]benzimidazolato)copper(II) anchored onto chloromethylated polystyrene for the biomimetic oxidative coupling of 2-aminophenol to 2-aminophenoxazine-3-one. J. Mol. Catal. A Chem. 2005, 236, 132–138. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, F.; Jean Bielecki, J. Ueber Synthesen in der Biphenylreihe. Chem. Ber. 1901, 34, 2174–2185. [Google Scholar] [CrossRef]
- Lan, J.-B.; Chen, L.; Yu, X.-Q.; You, J.-S.; Xie, R.-G. A simple copper salt catalyzed the coupling of imidazole with arylboronic acids in protic solvent. Chem. Commun. 2004, 188–189. [Google Scholar] [CrossRef] [PubMed]
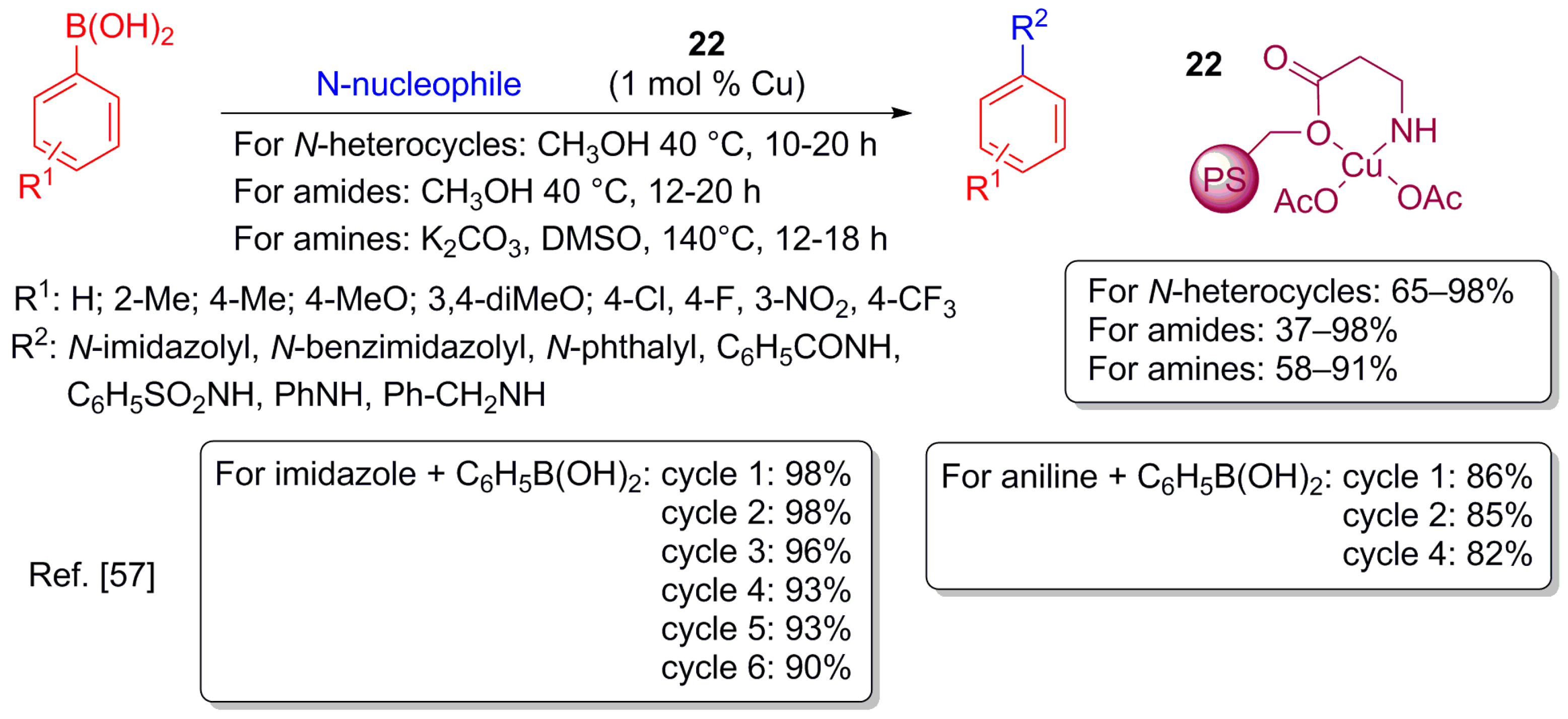
- Islam, S.M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K; Salam, N.; Mobarak, M. A reusable polymer supported copper catalyst for the C–N and C–O bond cross-coupling reaction of aryl halides as well as arylboronic acids. J. Organomet. Chem. 2012, 696, 4264–4274. [Google Scholar] [CrossRef]
- Islam, S.M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K.; Salam, N.; Sumantra, P.; Hossain, D.; Mobarok, M. An efficient polymer-supported copper(II) catalyst for the N-arylation reaction of N(H)-heterocycles with aryl halides as well as arylboronic acids. Transit. Met. Chem. 2011, 36, 447–458. [Google Scholar] [CrossRef]
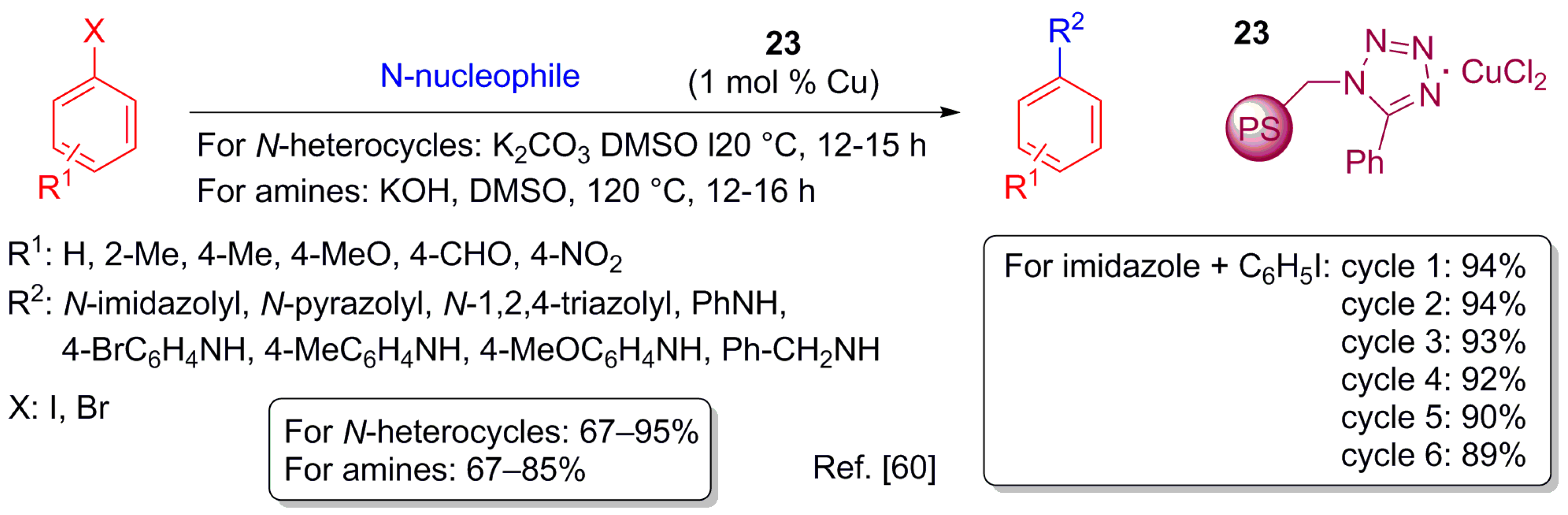
- Islam, M.; Salam, N.; Mondal, P.; Roy, A.S.; Ghosh, K.; Tuhina, K. A highly active reusable polymer anchored copper catalyst for C–O, C–N and C–S cross coupling reactions. J. Mol. Catal. A Chem. 2014, 387, 7–19. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Zahraei, A.; Pourbasheer, E. Catalytic activity and antibacterial properties of nanopolymer-supported copper complex for C–N coupling reactions of amines and nitrogen-containing heterocycles with aryl halides. Monatsh. Chem. 2015, 146, 1329–1334. [Google Scholar] [CrossRef]
- Islam, M.M.; Halder, H.; Roy, A.S.; Chatterjee, S.; Bhaumik, A.; Islam, S.M. Copper(II) incorporated functionalized polystyrene catalyzed N-arylation of amides under solvent free condition with broad substrate scope. RSC Adv. 2016, 6, 109692–109701. [Google Scholar] [CrossRef]
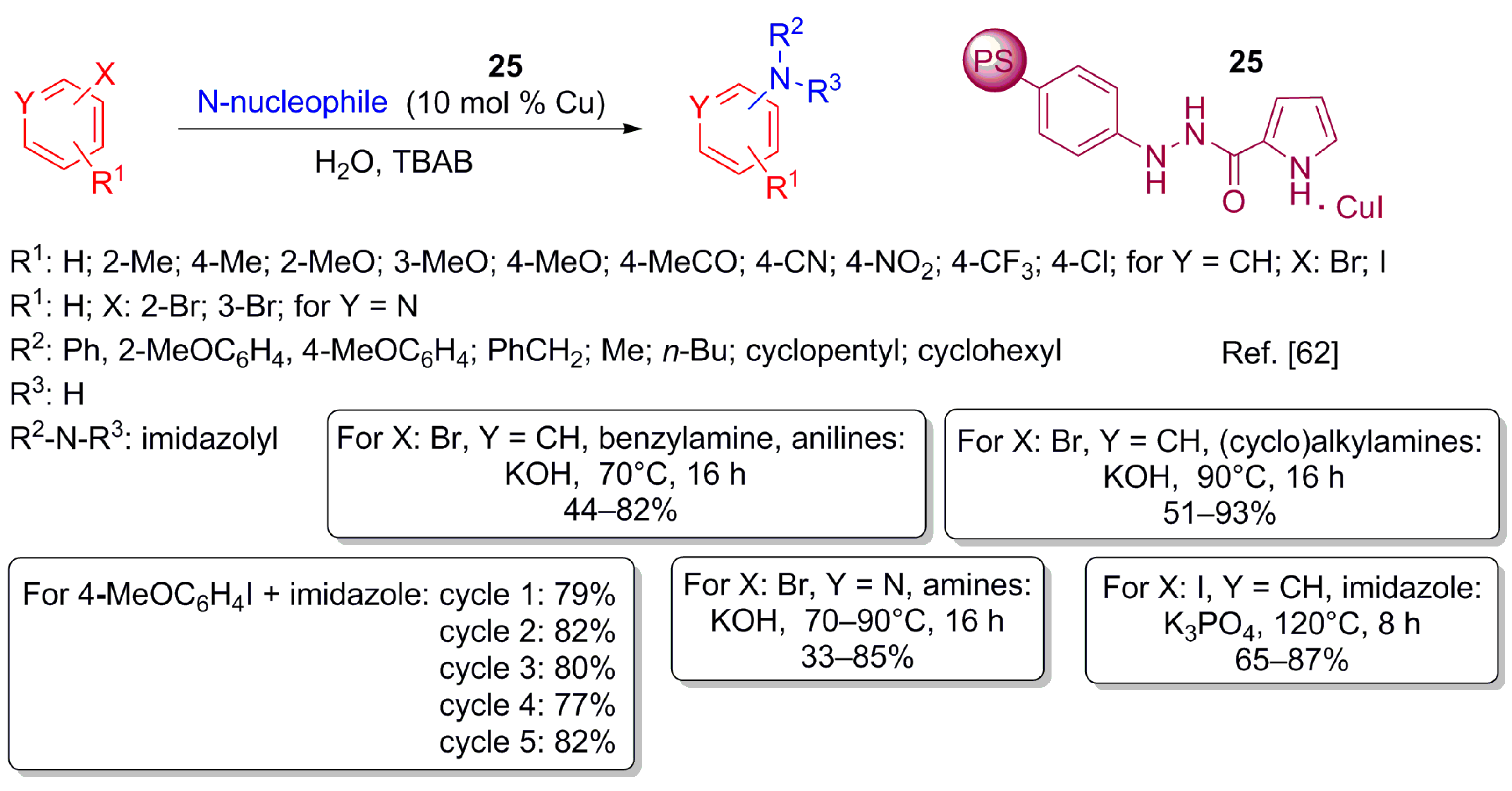
- Huang, L.; Yu, R.; Zhu, X.; Wan, Y. A recyclable Cu-catalyzed C–N coupling reaction in water and its application to synthesis of imidazo[1,2-a]quinoxaline. Tetrahedron 2013, 69, 8974–8977. [Google Scholar] [CrossRef]
- Cardillo, G.; Tomasini, C. Asymmetric synthesis of β-amino acids and α-substituted β-amino acids. Chem. Soc. Rev. 1996, 25, 117–128. [Google Scholar] [CrossRef]
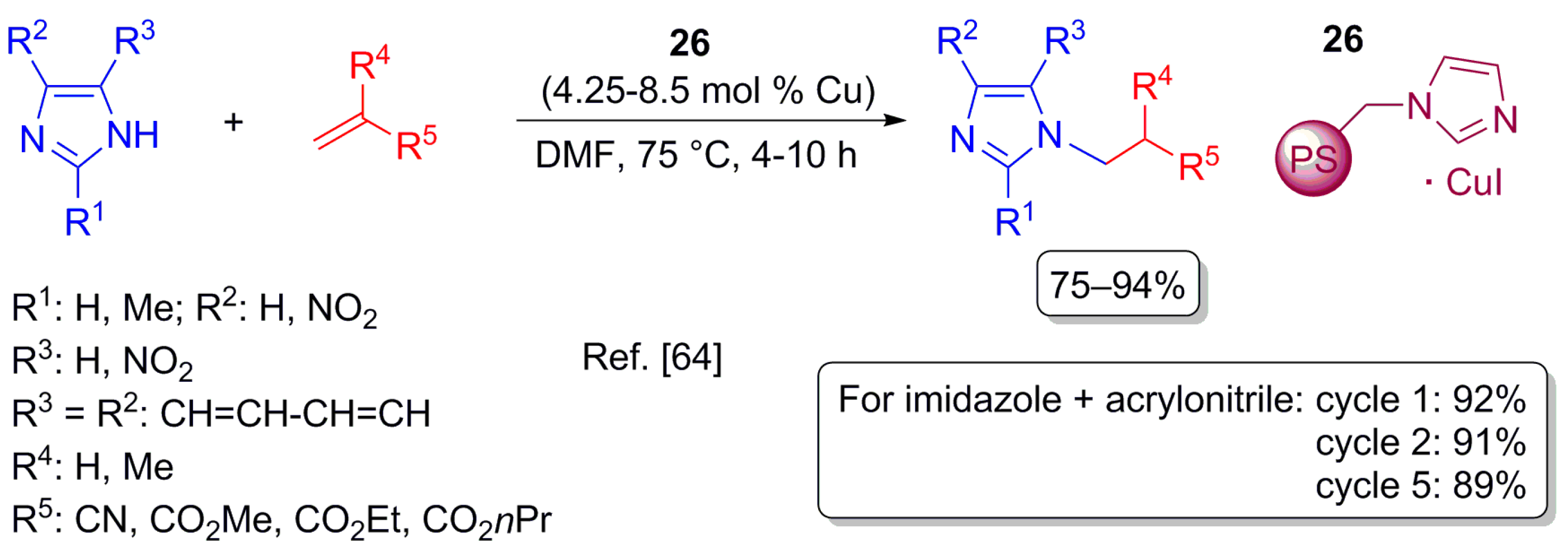
- Li, L.; Liu, Z.; Ling, Q.; Xing, X. Polystyrene-supported CuI–imidazole complex catalyst for aza-Michael reaction of imidazoles with α,β-unsaturated compounds. J. Mol. Catal. A Chem. 2012, 353, 178–184. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabina, P.; Svoboda, J.; Sedlák, M. Recent Advances in C–C and C–N Bond Forming Reactions Catalysed by Polystyrene-Supported Copper Complexes. Molecules 2017, 22, 865. https://doi.org/10.3390/molecules22060865
Drabina P, Svoboda J, Sedlák M. Recent Advances in C–C and C–N Bond Forming Reactions Catalysed by Polystyrene-Supported Copper Complexes. Molecules. 2017; 22(6):865. https://doi.org/10.3390/molecules22060865
Chicago/Turabian StyleDrabina, Pavel, Jan Svoboda, and Miloš Sedlák. 2017. "Recent Advances in C–C and C–N Bond Forming Reactions Catalysed by Polystyrene-Supported Copper Complexes" Molecules 22, no. 6: 865. https://doi.org/10.3390/molecules22060865