Impact of Maturity of Malay Cherry (Lepisanthes alata) Leaves on the Inhibitory Activity of Starch Hydrolases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Total Proanthocyanidins
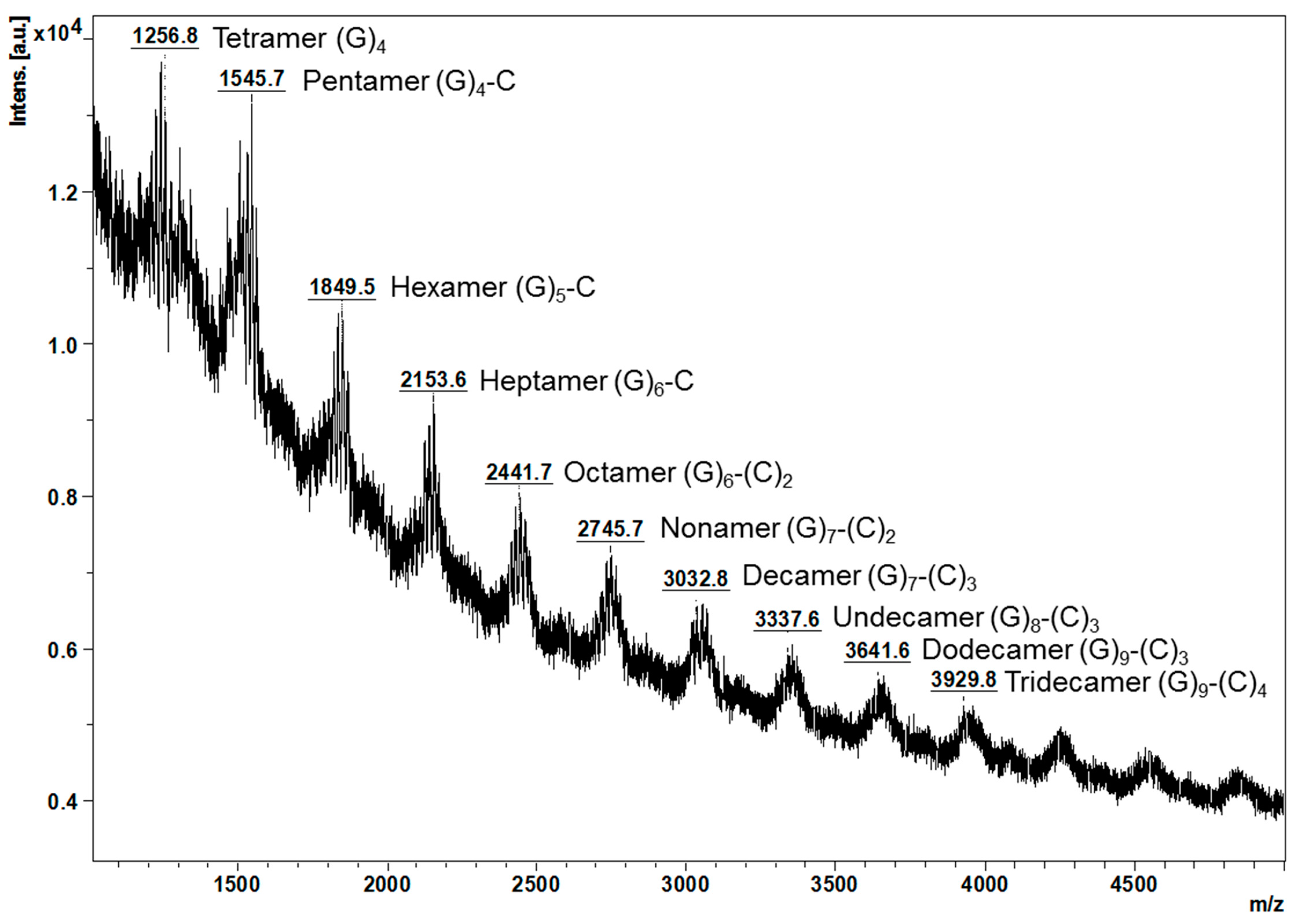
2.2. Characterisation of Proanthocyanidins Using HPLC-ESI/MS2 and MALDI-TOF/MS
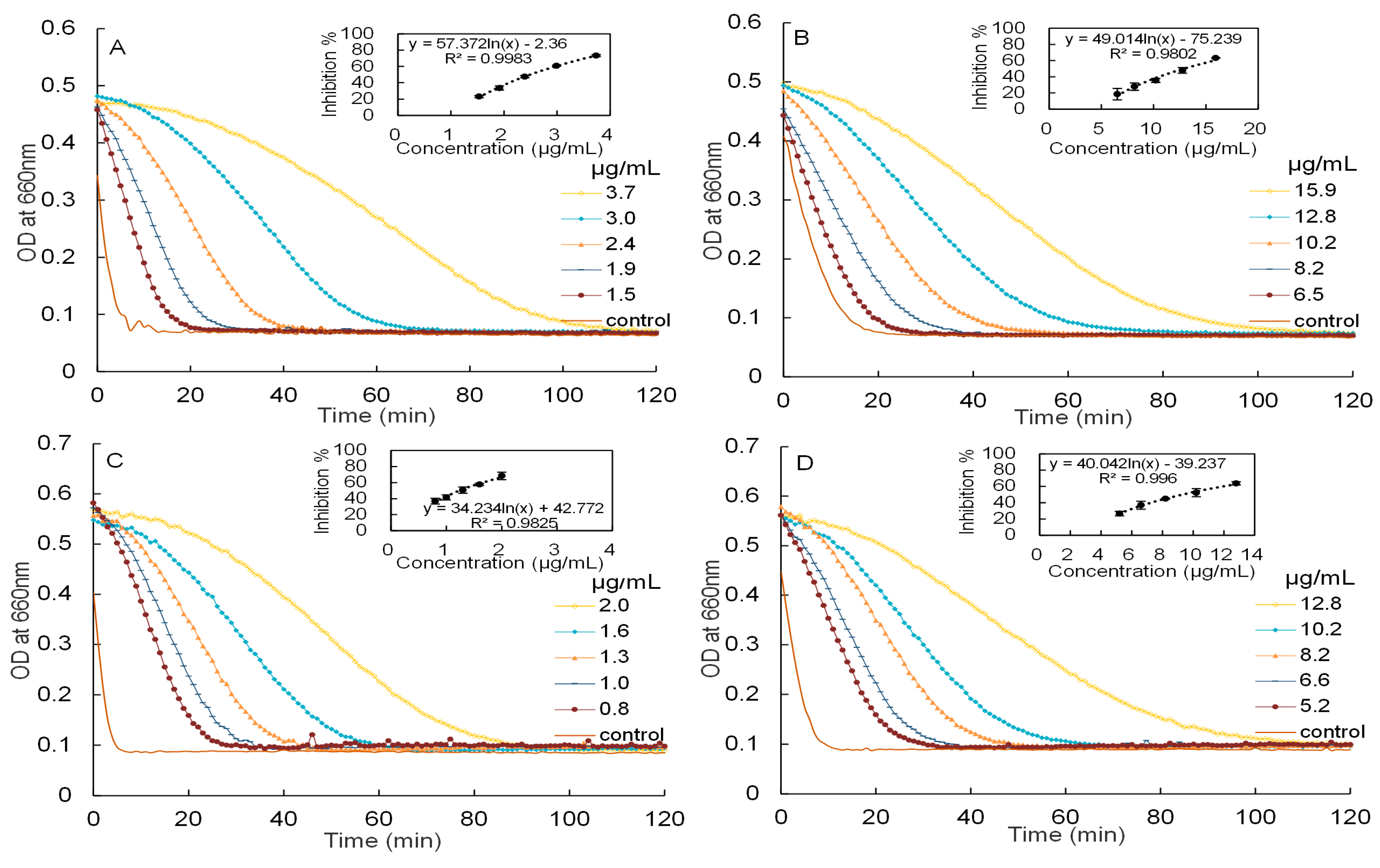
2.3. Inhibitory Activities against α-Amylase and α-Glucosidase
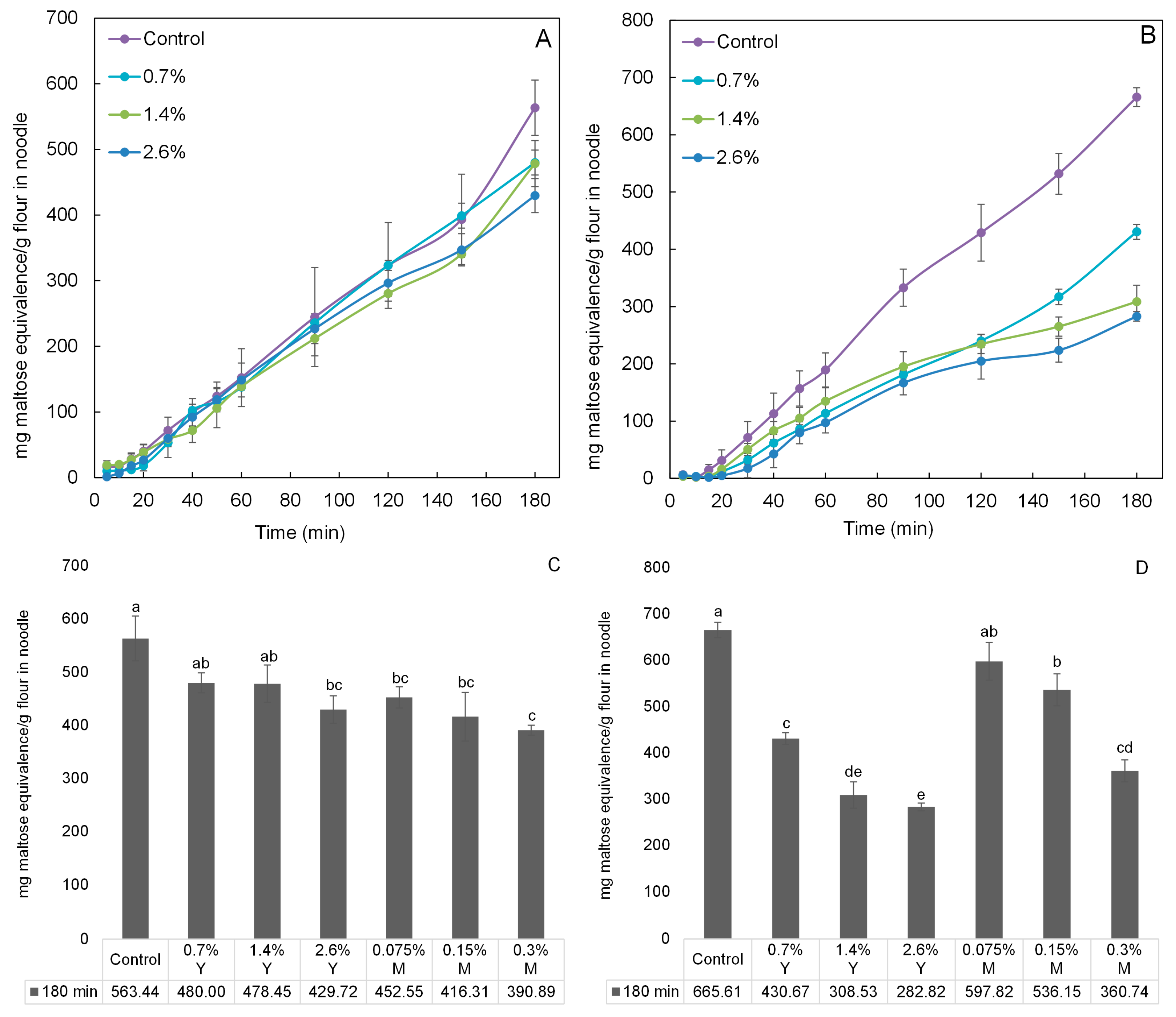
2.4. In Vitro Digestibility Analysis of Noodles
3. Materials and Methods
3.1. Materials
3.2. Extraction, Purification and Characterisation of Proanthocyanidins from Young Leaves of Malay Cherry
3.3. Quantification of Total Proanthocyanidins
3.4. Inhibitory Activities against α-Amylase and α-Glucosidase
3.5. Preparation of Wheat and Rice Noodles with Reduced Digestibility
3.6. In Vitro Digestibility Analysis of Noodles
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Jabir, N.R.; Manjunath, N.C.; Khan, M.S.; Ashraf, G.M.; Kamal, M.A.; Tabrez, S. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J. Biol. Sci. 2015, 22, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Ismail, T.S.E.S.; Deshmukh, S.A. Comparative study of effect of α glucosidase inhibitors-Miglitol, acarbose and voglibose on postprandial hyperglycemia and glycosylated hemoglobin in type-2 diabetes mellitus. Int. J. Pharm. Biol. Sci. 2012, 3, 337–343. [Google Scholar]
- Liu, T.; Yip, Y.M.; Song, L.; Feng, S.; Liu, Y.; Lai, F.; Zhang, D.; Huang, D. Inhibiting enzymatic starch digestion by the phenolic compound diboside A: A mechanistic and in silico study. Food Res. Int. 2013, 54, 595–600. [Google Scholar] [CrossRef]
- Hou, G.G. Preface. In Asian Noodles: Science, Technology, and Processing, 1st ed.; Hou, G.G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hormdok, R.; Noomhorm, A. Hydrothermal treatments of rice starch for improvement of rice noodle quality. LWT-Food Sci. Technol. 2007, 40, 1723–1731. [Google Scholar] [CrossRef]
- Zhang, Y.; Wong, A.I.C.; Wu, J.; Karim, N.B.A.; Huang, D. Lepisanthes alata (Malay cherry) leaves are potent inhibitors of starch hydrolases due to proanthocyanidins with high degree of polymerization. J. Funct. Foods 2016, 25, 568–578. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Koupai-Abyazani, M.R.; McCallum, J.; Muir, A.D.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. Developmental changes in the composition of proanthocyanidins from leaves of sainfoin (Onobrychis viciifolia Scop.) as determined by HPLC analysis. J. Agric. Food Chem. 1993, 41, 1066–1070. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Dawra, R.K.; Singh, B. Changes in tannin content, polymerization and protein precipitation capacity in oak (Quercus incana) leaves with maturity. J. Sci. Food Agric. 1988, 44, 301–307. [Google Scholar] [CrossRef]
- Fujii, H.; Sun, B.; Nishioka, H.; Hirose, A.; Aruoma, O.I. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem. Toxicol. 2007, 45, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hümmer, W.; Schreier, P. Analysis of proanthocyanidins. Mol. Nutr. Food Res. 2008, 52, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Liu, J.W.; Xiang, P.; Lin, P.; Ding, Z.H.; Sternberg, L.D.L. Tannins and nitrogen dynamics in mangrove leaves at different age and decay stages (Jiulong River Estuary, China). Hydrobiologia 2007, 583, 285–295. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Hartmann, T. Diversity and variability of plant secondary metabolism: A mechanistic view. In Proceedings of the 9th International Symposium on Insect-Plant Relationships, 1st ed.; Städler, E., Rowell-Rahier, M., Baur, R., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1996; pp. 177–188. [Google Scholar]
- Wang, H.; Liu, T.; Song, L.; Huang, D. Profiles and α-amylase inhibition activity of proanthocyanidins in unripe Manilkara zapota (chiku). J. Agric. Food Chem. 2012, 60, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Schreckinger, M.; Lila, M.A.; Yousef, G.; González de Mejía, E. Inhibition of α-Glucosidase and α-Amylase by Vaccinium floribundum and Aristotelia chilensis Proanthocyanidins. In Hispanic Foods: Chemistry and Bioactive Compounds, 1st ed.; Tunick, M.H., González de Mejía, E., Eds.; ACS Press: Washington, DC, USA, 2012; Chapter 6; pp. 71–82. [Google Scholar]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Zhai, Y.; McClements, D.J.; Liu, J. Separation and characterization of polyphenolics from underutilized byproducts of fruit production (Choerospondias axillaris peels): Inhibitory activity of proanthocyanidins against glycolysis enzymes. Food Funct. 2015, 6, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sui, X.; Huang, D. Mitigating the in vitro enzymatic digestibility of noodles by aqueous extracts of Malay cherry leaves. Food Chem. 2017, 232, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-Laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, L.; Wang, H.; Huang, D. A high-throughput assay for quantification of starch hydrolase inhibition based on turbidity measurement. J. Agric. Food Chem. 2011, 59, 9756–9762. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
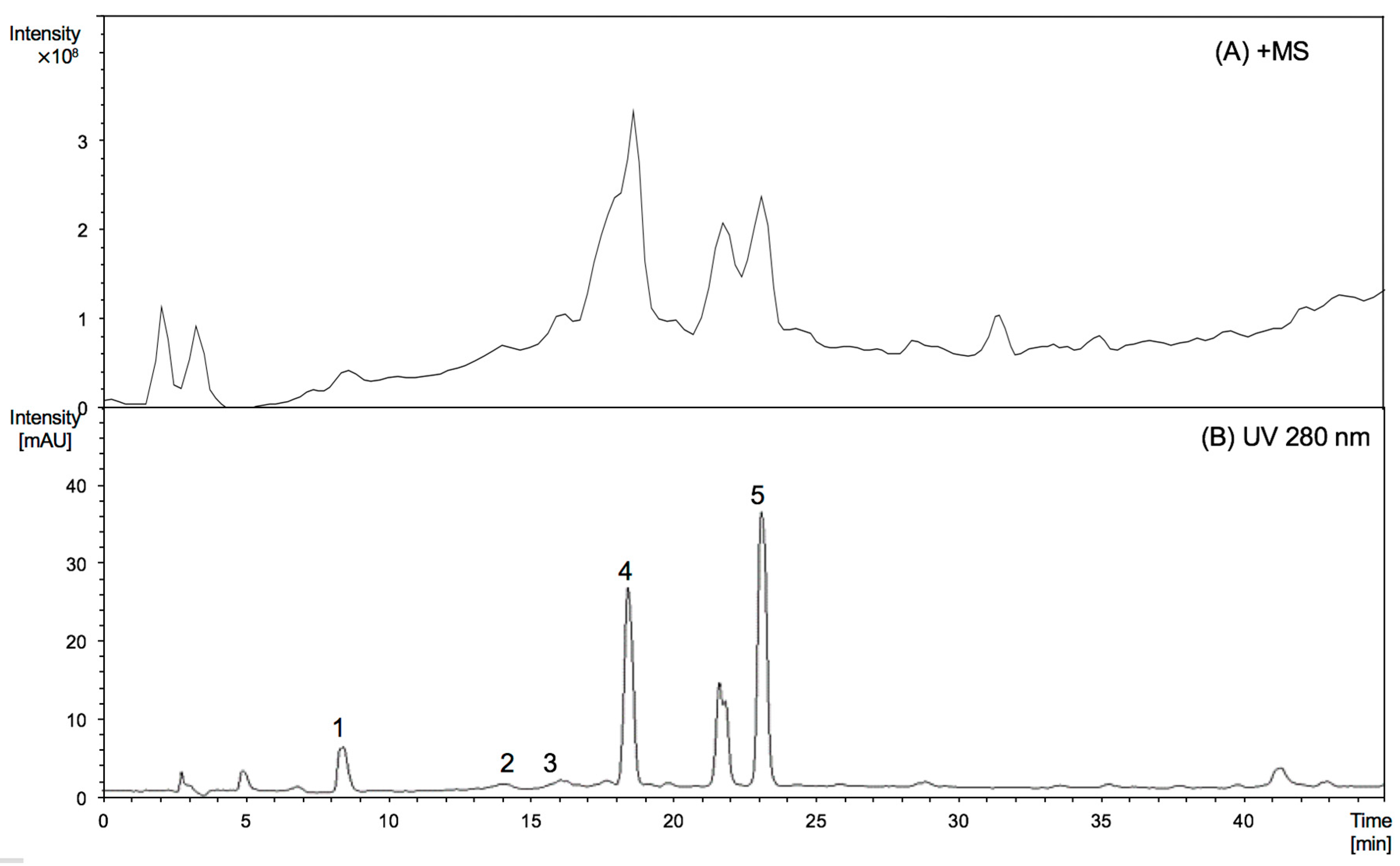
| RT (min) | Peak No. | Compound | MW | [M − H]− (m/z) | MS2 Main Fragments (m/z) | [M + H]+ (m/z) | MS2 Main Fragments (m/z) |
|---|---|---|---|---|---|---|---|
| 9.3 | 1 * | Epigallocatechin | 306 | 305 | 179 | 307 | 139 |
| 14.0 | 2 | (Epi)gallocatechin thioether | 410 | 409 | 303 | 411 | 305, 287 |
| 14.7 | 3 * | Epicatechin | 290 | 289 | 245, 205 | 291 | 271 |
| 18.7 | 4 | (Epi)gallocatechin thioether | 410 | 409 | 303 | 411 | 305, 287 |
| 23.1 | 5 | (Epi)catechin thioether | 394 | 393 | 287 | 395 | 289, 271 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gui, Y.; Huang, D. Impact of Maturity of Malay Cherry (Lepisanthes alata) Leaves on the Inhibitory Activity of Starch Hydrolases. Molecules 2017, 22, 873. https://doi.org/10.3390/molecules22060873
Zhang Y, Gui Y, Huang D. Impact of Maturity of Malay Cherry (Lepisanthes alata) Leaves on the Inhibitory Activity of Starch Hydrolases. Molecules. 2017; 22(6):873. https://doi.org/10.3390/molecules22060873
Chicago/Turabian StyleZhang, Yan, Yuyang Gui, and Dejian Huang. 2017. "Impact of Maturity of Malay Cherry (Lepisanthes alata) Leaves on the Inhibitory Activity of Starch Hydrolases" Molecules 22, no. 6: 873. https://doi.org/10.3390/molecules22060873






