Integral Design Methodology of Photocatalytic Reactors for Air Pollution Remediation
Abstract
:1. Introduction and Scope
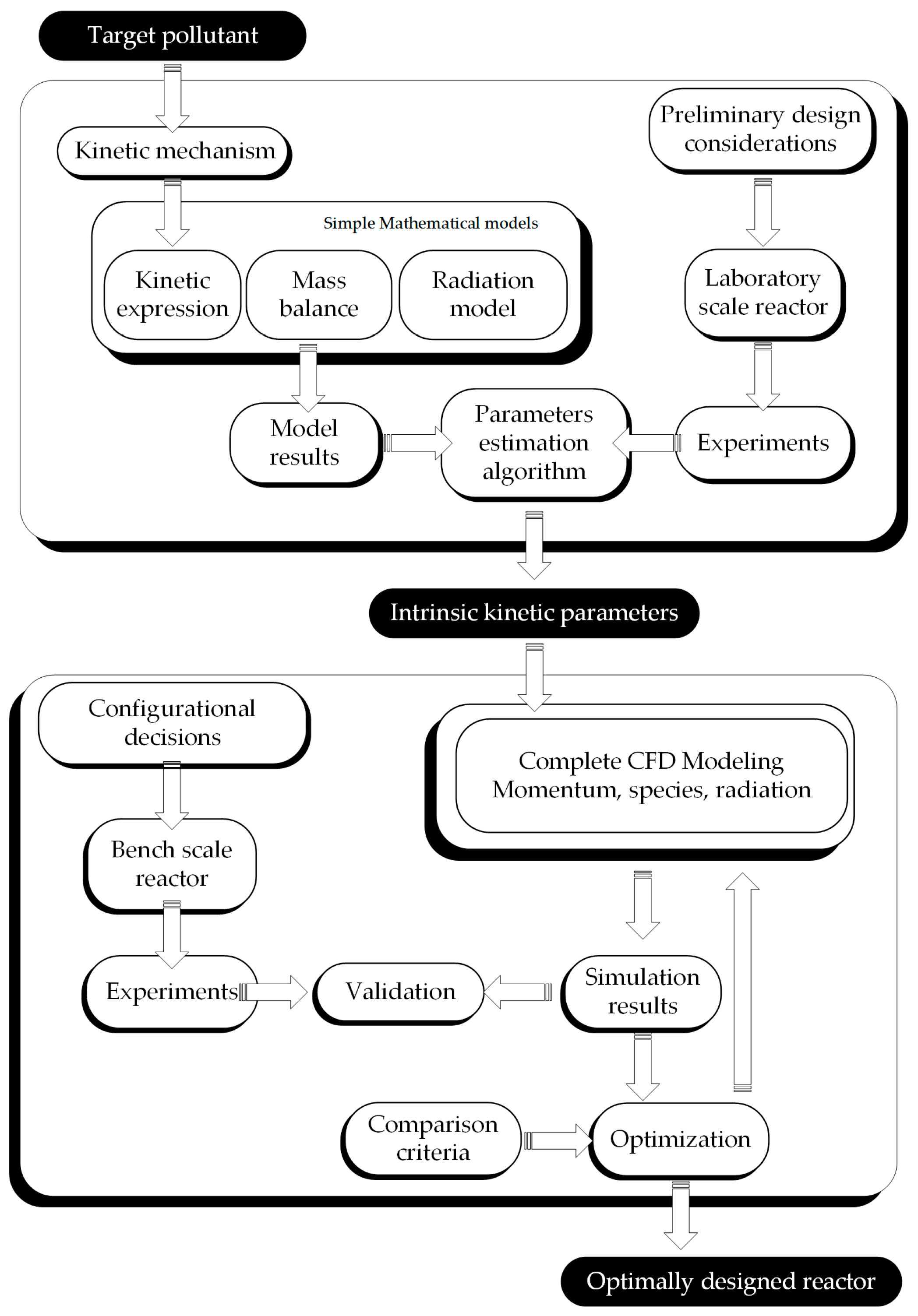
2. Integral Design Methodology
2.1. Kinetic Study
2.2. Scaling and Optimization Methodology
3. Application: Step-by-Step Design and Optimization of a Photocatalytic Reactor
3.1. Laboratory Scale Reactor
3.1.1. Experimental
3.1.2. Procedures
3.1.3. Kinetics
3.1.4. Modeling
3.1.5. Kinetic Parameters Estimation
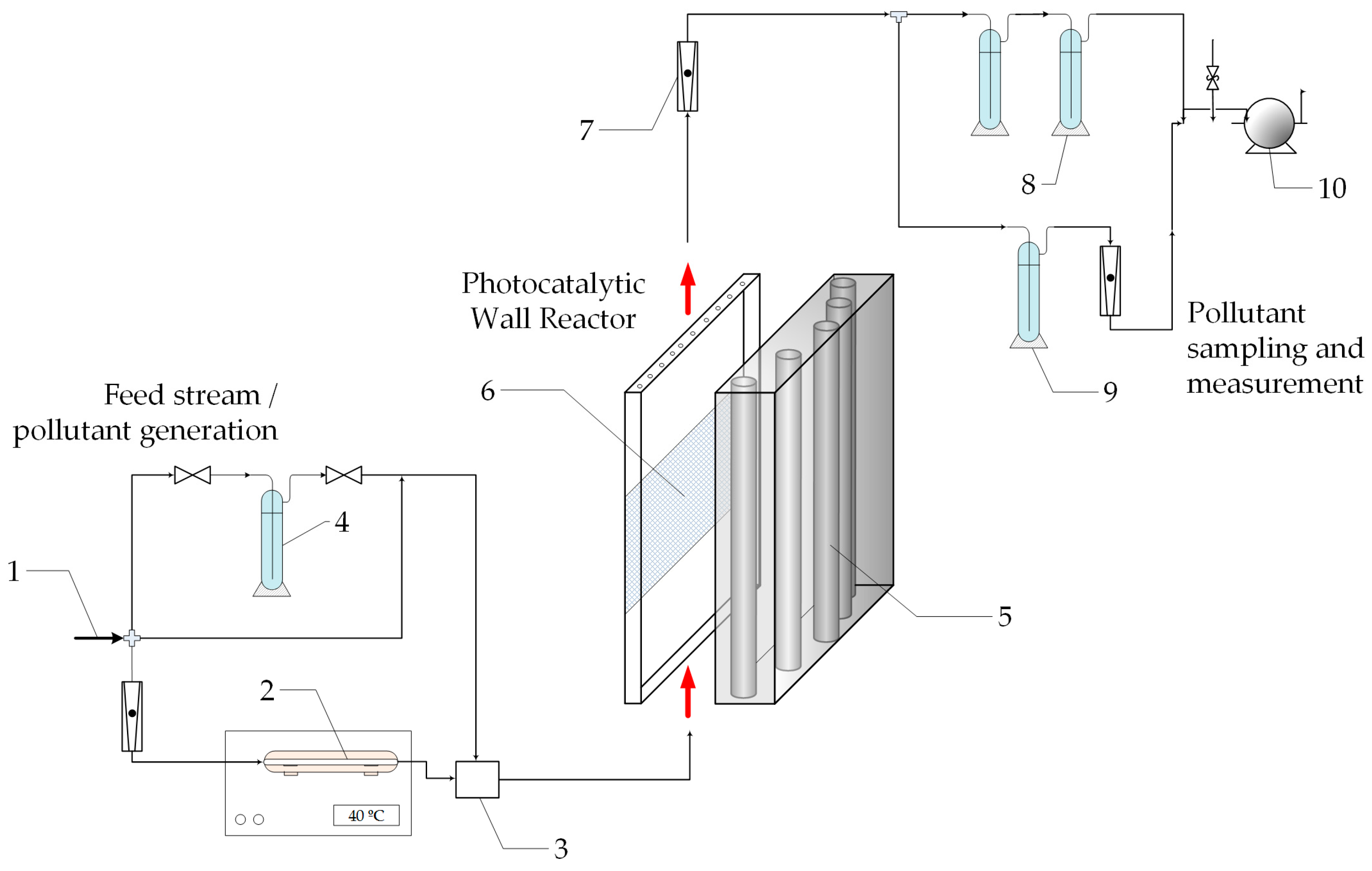
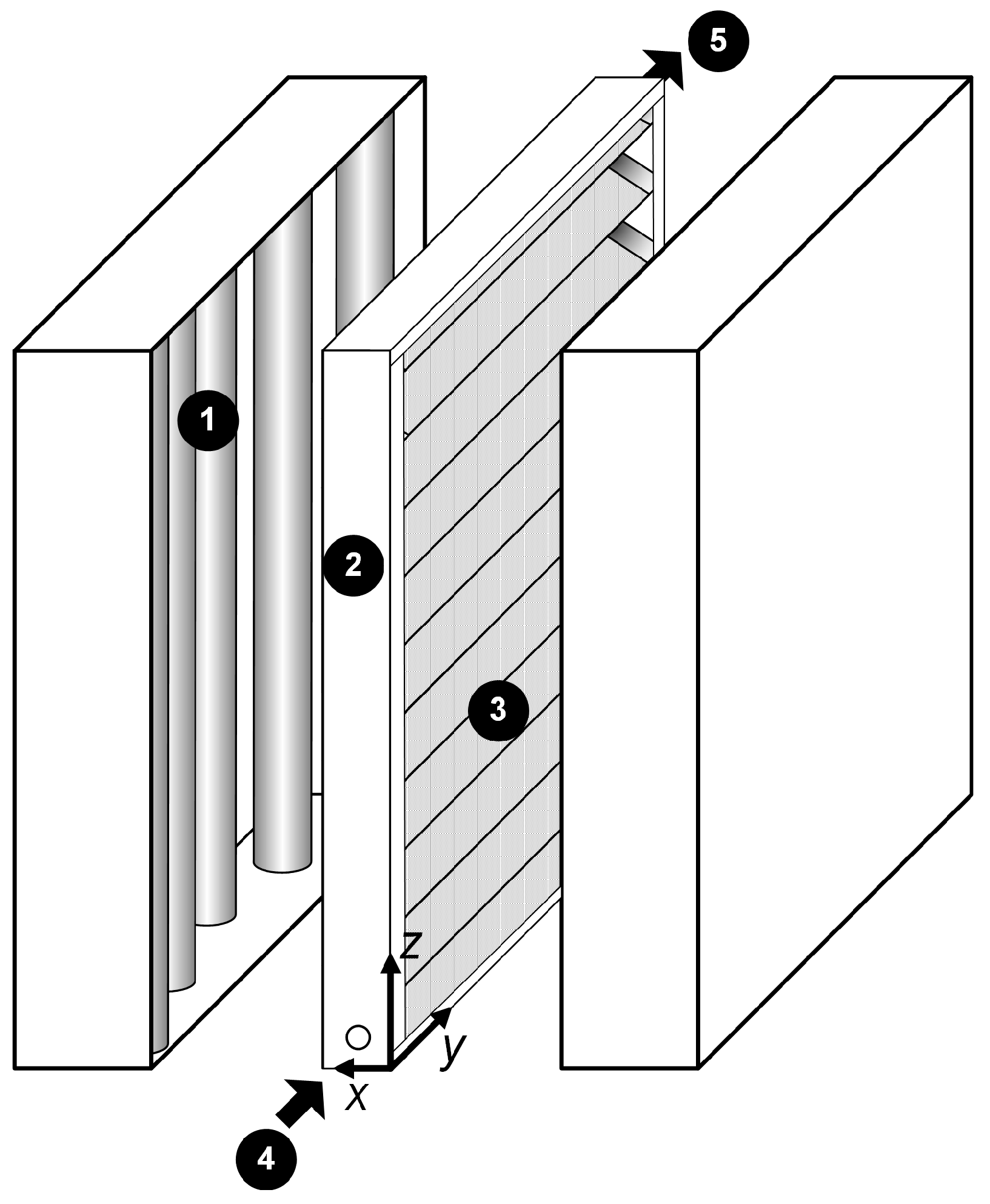
3.2. Bench Scale Reactor: Corrugated Plate Reactor
3.2.1. Experimental Set-Up and Procedures
3.2.2. CFD Modeling
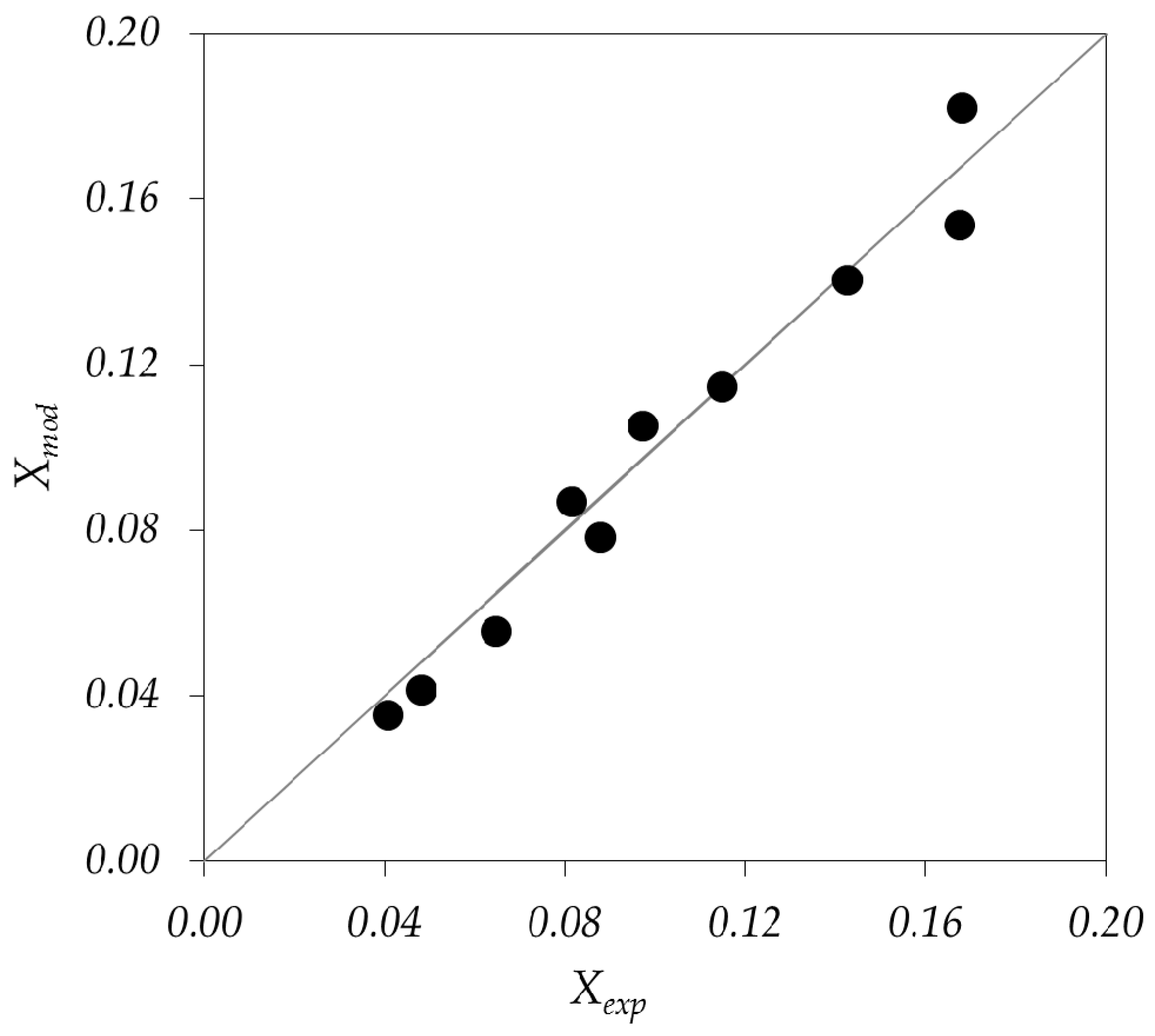
3.2.3. Simulation Results and Validation
3.3. Bench Scale Reactor Optimization
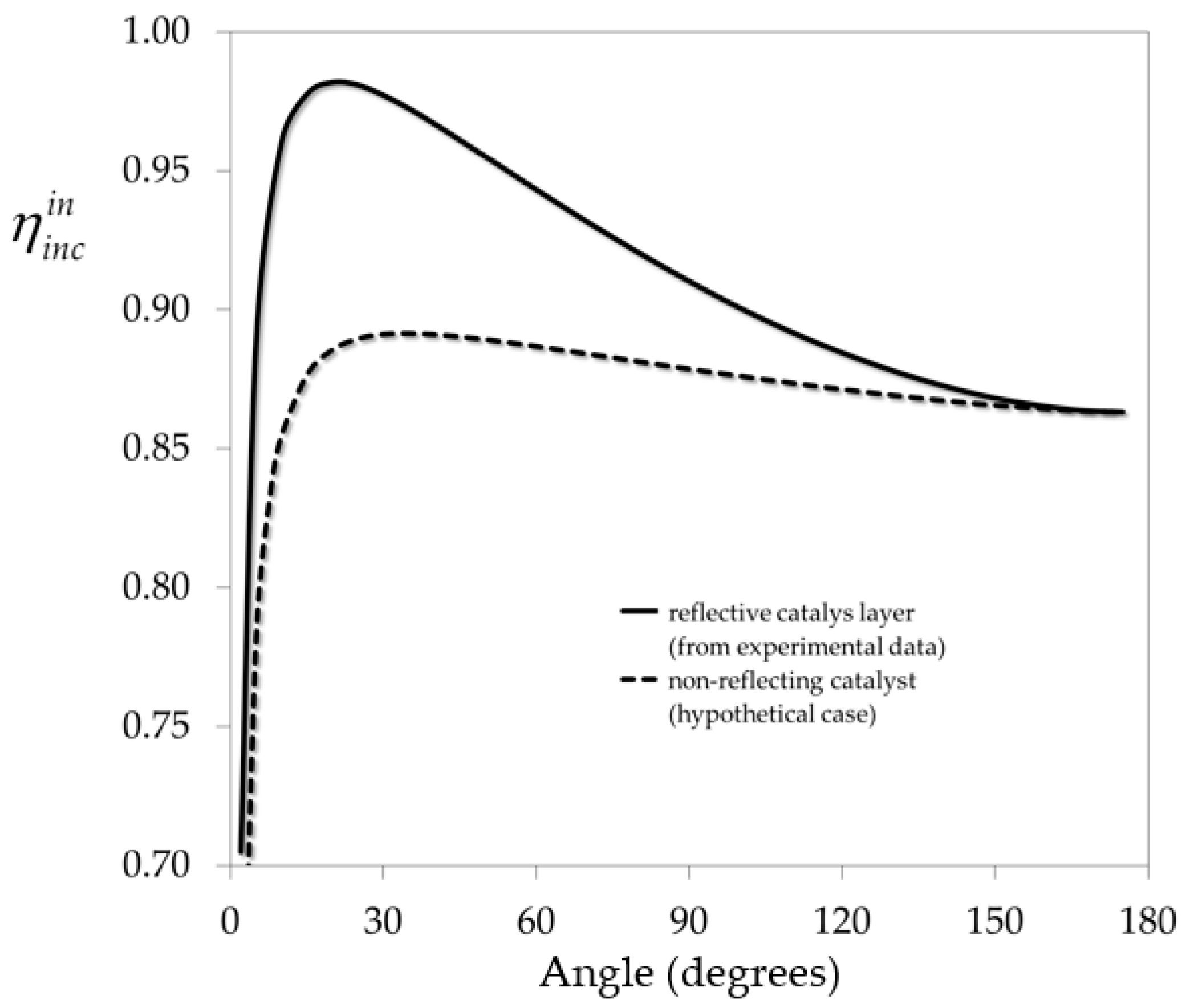
Optimal Folding Angle of the Corrugated Reactor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| external catalytic surface area per unit volume (cm−1) | |
| cross sectional area (cm2) | |
| photocatalytic area (cm2) | |
| molar concentration (mol cm−3) | |
| local superficial rate of photon absorption, LSRPA (einstein cm−2 s−1) | |
| H | height (cm) |
| I | specific radiation intensity (einstein cm−2 s−1 sr−1) |
| specific radiation intensity on the lamp surface (einstein cm−2 s−1 sr−1) | |
| gas phase mass transfer coefficient (cm s−1) | |
| L | length (cm) |
| P | emission power (einstein s−1) |
| q | radiation flux (einstein cm−2 s−1) |
| Q | volumetric gas flow rate (cm3 s−1) |
| R | homogeneous reaction rate (mol cm−3 s−1) |
| r | heterogeneous reaction rate (mol cm−2 s−1) |
| local superficial rate of electron-hole pair generation (mol cm−2 s−1) | |
| RLi | lamp radius (cm) |
| T | reactor thickness (cm) |
| spectral transmittance (dimensionless) | |
| axial velocity (cm s−1) | |
| W | reactor width (cm) |
| rectangular coordinate system (cm) | |
| position vector (cm) | |
| X | conversion (dimensionless) |
| Greek letters | |
| kinetic parameter (cm3 einstein−1) | |
| efficiency (dimensionless) | |
| adsorption constant (cm3 mol−1) | |
| wavelength (nm) | |
| gas density (g cm−3) | |
| viscous stress tensor | |
| spherical coordinates (rad) | |
| primary quantum yield (mol einstein−1) | |
| wavelength averaged primary quantum yield (mol einstein−1) | |
| Subscripts | |
| indicates adsorbed species | |
| relative to radiation absorption | |
| F | relative to formaldehyde |
| Fi | relative to radiation neutral filter |
| Li | relative to lamp |
| W | relative to water |
| Wi | relative to reactor window |
| indicates wavelength dependence | |
| exp | relative to experimental data |
| mod | relative to model |
| Superscripts | |
| in | relative to reactor inlet |
| out | relative to reactor outlet |
| Special symbols | |
| denotes an averaged property | |
| denotes a vector |
References
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull. World Health Organ. 2000, 78, 1078–1092. [Google Scholar] [PubMed]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: state of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, X.; Wang, X.; Qian, K.; Zhao, R. Influence of temperature on formaldehyde emission parameters of dry building materials. Atmos. Environ. 2007, 41, 3203–3216. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.; Ma, C.; Yang, H. The effect of activated carbon adsorption on the photocatalytic removal of formaldehyde. Build. Environ. 2010, 45, 615–621. [Google Scholar] [CrossRef]
- Shiraishi, F.; Toyoda, K.; Miyakawa, H. Decomposition of gaseous formaldehyde in a photocatalytic reactor with a parallel array of light sources: 2. Reactor performance. Chem. Eng. J. 2005, 114, 145–151. [Google Scholar] [CrossRef]
- Hanoune, B.; Lebris, T.; Allou, L.; Marchand, C.; Lecalve, S. Formaldehyde measurements in libraries: Comparison between infrared diode laser spectroscopy and a DNPH-derivatization method. Atmos. Environ. 2006, 40, 5768–5775. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef]
- Dumont, É.; Héquet, V. Determination of the Clean Air Delivery Rate (CADR) of photocatalytic oxidation (PCO) purifiers for indoor air pollutants using a closed-loop reactor. Part I: Theoretical considerations. Molecules 2017, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Héquet, V.; Batault, F.; Raillard, C.; Thévenet, F.; Le Coq, L.; Dumont, É. Determination of the clean air delivery rate (CADR) of photocatalytic oxidation (PCO) purifiers for indoor air pollutants using a closed-loop reactor. Part II: Experimental results. Molecules 2017, 22, 408. [Google Scholar]
- Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: Conceptualization of humidity effects, active oxygen species, problem of C1-C3 carbonyl pollutants. Appl. Catal. B Environ. 2010, 99, 428–434. [Google Scholar] [CrossRef]
- Passalía, C.; Martínez Retamar, M.E.; Alfano, O.M.; Brandi, R.J. Photocatalytic Degradation of Formaldehyde in Gas Phase on TiO2 Films: A Kinetic Study. Int. J. Chem. React. Eng. 2010, 8, A161. [Google Scholar] [CrossRef]
- Passalía, C.; Alfano, O.M.; Brandi, R.J. A methodology for modeling photocatalytic reactors for indoor pollution control using previously estimated kinetic parameters. J. Hazard. Mater. 2012, 211–212, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Passalía, C.; Alfano, O.M.; Brandi, R.J. Optimal design of a corrugated-wall photocatalytic reactor using efficiencies in series and computational fluid dynamics (CFD) modeling. Ind. Eng. Chem. Res. 2013, 52, 6916–6922. [Google Scholar] [CrossRef]
- Cassano, A.E.; Martin, C.A.; Brandi, R.J.; Alfano, O.M. Photoreactor analysis and design: Fundamentals and applications. Ind. Eng. Chem. Res. 1995, 34, 2155–2201. [Google Scholar] [CrossRef]
- Schneider, J.; Bahnemann, D.W.; Ye, J.; Puma, G.L.; Dionysiou, D.D. Photocatalysis: Fundamentals and Perspectives; RSC: London, UK, 2016. [Google Scholar]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Pichat, P. Photocatalysis: Fundamentals, Materials and Potential; MDPI AG: Basel, Switzerland, 2016. [Google Scholar]
- Hernandez-Alonso, M.D.; Fresno, F.; Suarez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 1, 3–16. [Google Scholar]
- Salvadó-Estivill, I.; Brucato, A.; Puma, G.L. Two-Dimensional Modeling of a Flat-Plate Photocatalytic Reactor for Oxidation of Indoor Air Pollutants. Ind. Eng. Chem. Res. 2007, 46, 7489–7496. [Google Scholar] [CrossRef]
- Zazueta, A.L.L.; Destaillats, H.; Puma, G.L. Radiation field modeling and optimization of a compact and modular multi-plate photocatalytic reactor (MPPR) for air/water purification by Monte Carlo method. Chem. Eng. J. 2013, 217, 475–485. [Google Scholar] [CrossRef]
- Singh, M.; Salvado-Estivill, I.; Li Puma, G. Radiation Field Optimization in Photocatalytic Monolith Reactors for Air Treatment. AIChE J. 2007, 53, 678–686. [Google Scholar] [CrossRef]
- Taranto, J.; Frochot, D.; Pichat, P. Photocatalytic air purification: Comparative efficacy and pressure drop of a TiO2-coated thin mesh and a honeycomb monolith at high air velocities using a 0.4 m3 close-loop reactor. Sep. Purif. Technol. 2009, 67, 187–193. [Google Scholar] [CrossRef]
- Ibrahim, H.; de Lasa, H. Novel photocatalytic reactor for the destruction of airborne pollutants reaction kinetics and quantum yields. Ind. Eng. Chem. Res. 1999, 38, 3211–3217. [Google Scholar] [CrossRef]
- Esterkin, C.R.; Negro, A.C.; Alfano, O.M.; Cassano, A.E. Air pollution remediation in a fixed bed photocatalytic reactor coated with TiO2. AIChE J. 2005, 51, 2298–2310. [Google Scholar] [CrossRef]
- Mohseni, M.; David, A. Gas phase vinyl chloride (VC) oxidation using TiO2-based photocatalysis. Appl. Catal. B Environ. 2003, 46, 219–228. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Cassano, A.E.; Irazoqui, H.A.; Alfano, O.M. Simulation of a multi-annular photocatalytic reactor for degradation of perchloroethylene in air: Parametric analysis of radiative energy efficiencies. Chem. Eng. Sci. 2007, 62, 1138–1154. [Google Scholar] [CrossRef]
- Ma, C.; Ku, Y.; Chou, Y.; Jeng, F. Performance of Tubular-Type Optical Fiber Reactor for Decomposition of Vocs in Gaseous Phase. J. Environ. Eng. Manag. 2008, 18, 363–369. [Google Scholar]
- Shang, H.; Zhang, Z.; Anderson, W.A. Nonuniform radiation modeling of a corrugated plate photocatalytic reactor. AIChE J. 2005, 51, 2024–2033. [Google Scholar] [CrossRef]
- Passalia, C.; Alfano, O.M.; Brandi, R.J. Modeling and Experimental Verification of a Corrugated Plate Photocatalytic Reactor Using CFD. Ind. Eng. Chem. Res. 2011, 50, 9077–9086. [Google Scholar] [CrossRef]
- Mohseni, M.; Taghipour, F. Experimental and CFD analysis of photocatalytic gas phase vinyl chloride (VC) oxidation. Chem. Eng. Sci. 2004, 59, 1601–1609. [Google Scholar] [CrossRef]
- Queffeulou, A.; Geron, L.; Schaer, E. Prediction of photocatalytic air purifier apparatus performances with a CFD approach using experimentally determined kinetic parameters. Chem. Eng. Sci. 2010, 65, 5067–5074. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Zhang, Z.; Li, Q.; Wang, H. A study of the photocatalytic oxidation of formaldehyde on Pt/Fe2O3/TiO2. J. Photochem. Photobiol. A Chem. 2000, 137, 197–202. [Google Scholar] [CrossRef]
- Walter, S.; Malmberg, S.; Schmidt, B.; Liauw, M.A. Mass transfer limitations in microchannel reactors. Catal. Today 2005, 110, 15–25. [Google Scholar] [CrossRef]
- Siegel, R.; Howell, J. Thermal Radiation Heat Transfer, 4th ed.; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Imoberdorf, G.E.; Irazoqui, H.A.; Cassano, A.E.; Alfano, O.M. Quantum efficiencies in a multi-annular photocatalytic reactor. Water Sci. Technol. 2007, 55, 161. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 104, 1–12. [Google Scholar] [CrossRef]
- Sun, L.; Bolton, J.R. Determination of the Quantum Yield for the Photochemical Generation of Hydroxyl Radicals in TiO2 Suspensions. J. Phys. Chem. 1996, 4127–4134. [Google Scholar] [CrossRef]
- Hernandez, J.M.G.; Rosales, B.S.; de Lasa, H. The photochemical thermodynamic efficiency factor (PTEF) in photocatalytic reactors for air treatment. Chem. Eng. J. 2010, 165, 891–901. [Google Scholar] [CrossRef]
- Cerdá, J.; Marchetti, J.L.; Cassano, A.E. Radiation efficiencies in elliptical photoreactors. Lat. Am. J. Heat Mass Transf. 1977, 1, 33–63. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Description | Laboratory Scale Reactor | Bench Scale Reactor |
|---|---|---|
| Configuration | Flat plate | Corrugated plate |
| Main dimensions | 8 (W) × 8 (L) × 0.4 (T) cm | 20 (W) × 30 (L) × 3 (T) cm |
| Reactor volume | 25.6 cm3 | 1800 cm3 |
| Catalytic surface area | 64 cm2 | 1843.5 cm2 |
| Number of lamps | 5 | 10 |
| Flow Rate | 1–3.5 L/min | |
| Inlet HCHO concentration | 5–35 ppmv | |
| Relative Humidity | 10–75% | |
| Maximum radiation | 8.94 × 10−5 einstein m−2 s−1 | |
| Radiation levels | 16%, 26%, 60%, 100% | |
| Step | Reaction | Reaction |
|---|---|---|
| Initiation | 1 | |
| Recombination | 2 | |
| generation | 3 | |
| Electron trapping | 4 | |
| HCHO Oxidation | 5 | |
| 6 | ||
| Ending reactions | 7 | |
| 8 | ||
| Adsorption equilibria | 9 | |
| 10 | ||
| Site balance | 11 |
| Angle | ||||
|---|---|---|---|---|
| (degrees) | - | (mol cm−2 s−1) | (mol Einstein−1) | - |
| 15.2 | 0.078 | 1.04 | 9.24 | 0.978 |
| 31.6 | 0.155 | 2.09 | 9.29 | 0.976 |
| 59.1 | 0.271 | 3.50 | 8.93 | 0.944 |
| 97.2 | 0.395 | 4.74 | 8.28 | 0.903 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passalía, C.; Alfano, O.M.; Brandi, R.J. Integral Design Methodology of Photocatalytic Reactors for Air Pollution Remediation. Molecules 2017, 22, 945. https://doi.org/10.3390/molecules22060945
Passalía C, Alfano OM, Brandi RJ. Integral Design Methodology of Photocatalytic Reactors for Air Pollution Remediation. Molecules. 2017; 22(6):945. https://doi.org/10.3390/molecules22060945
Chicago/Turabian StylePassalía, Claudio, Orlando M. Alfano, and Rodolfo J. Brandi. 2017. "Integral Design Methodology of Photocatalytic Reactors for Air Pollution Remediation" Molecules 22, no. 6: 945. https://doi.org/10.3390/molecules22060945





