Coumarin–Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications
Abstract
:1. Introduction
2. Coumarin–Tetrapyrrolic Macrocycle Conjugates
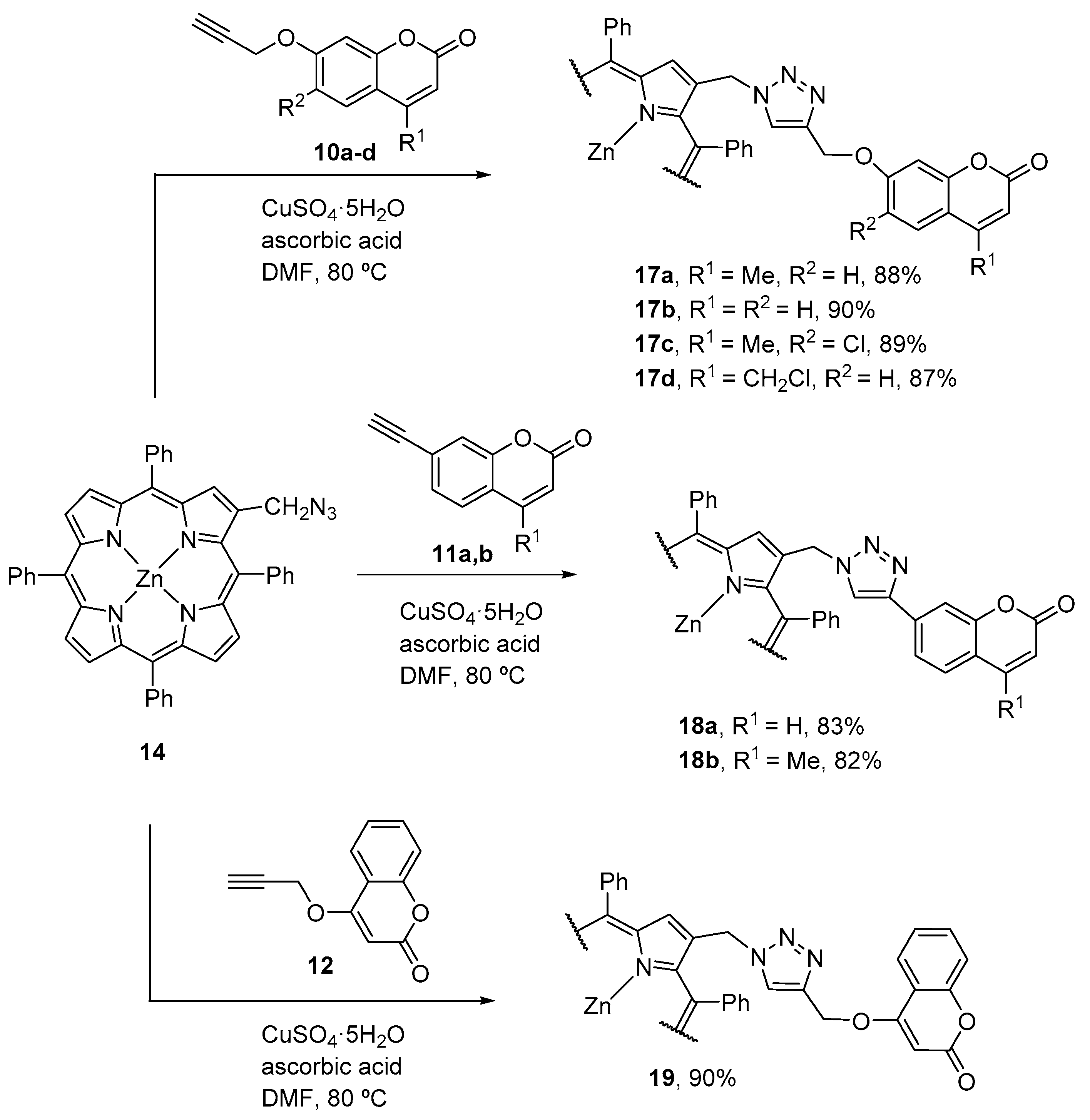
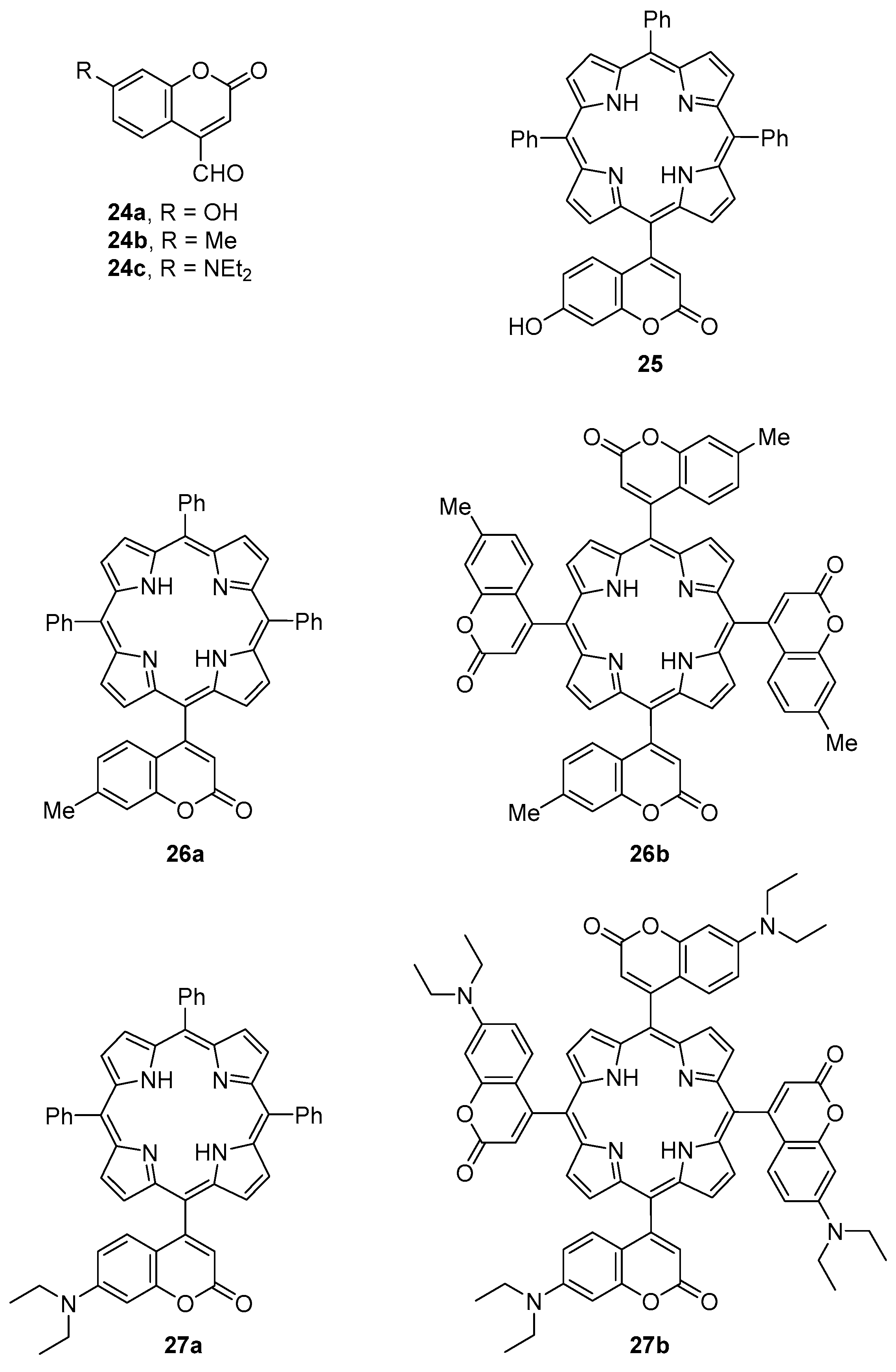
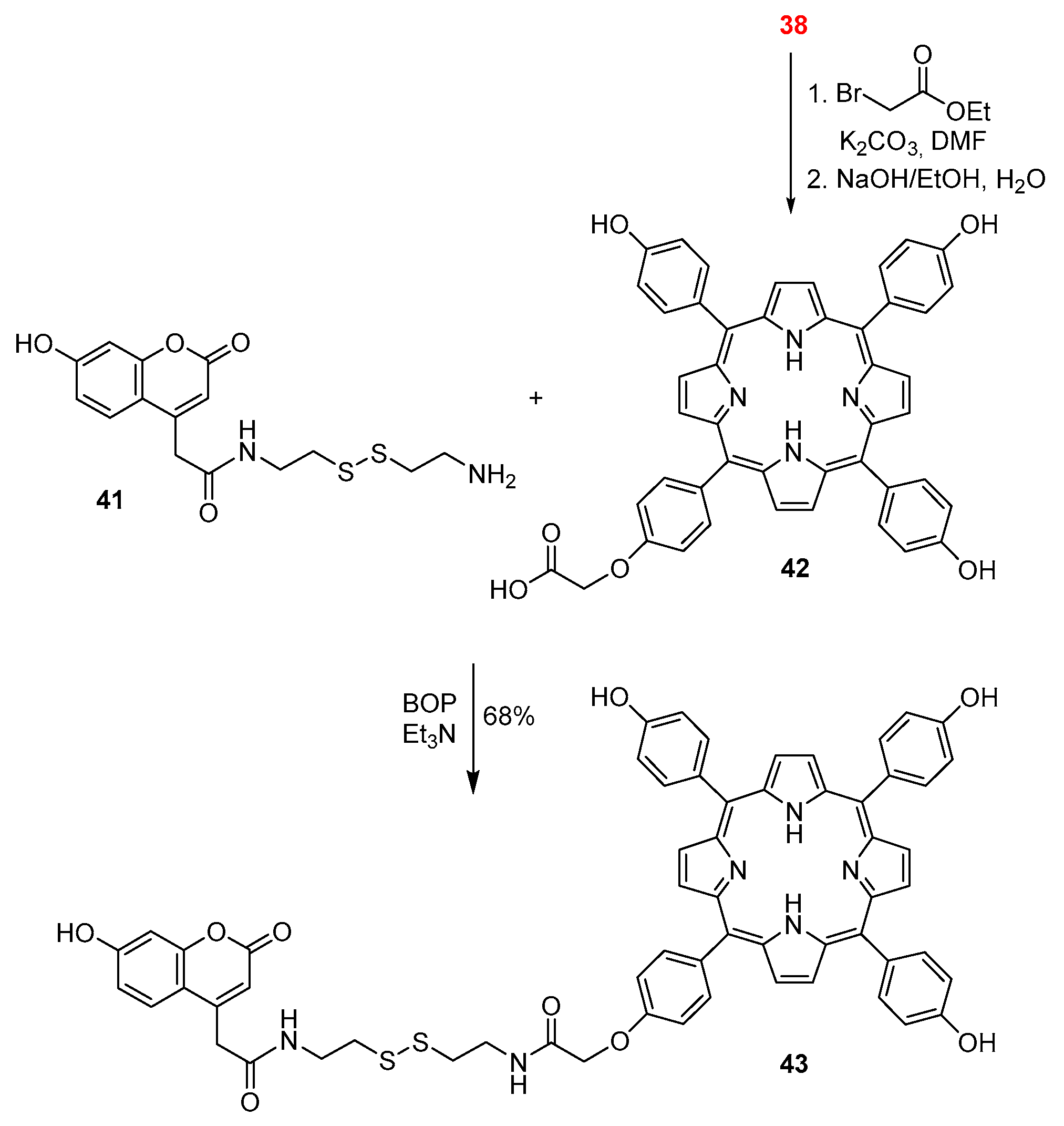
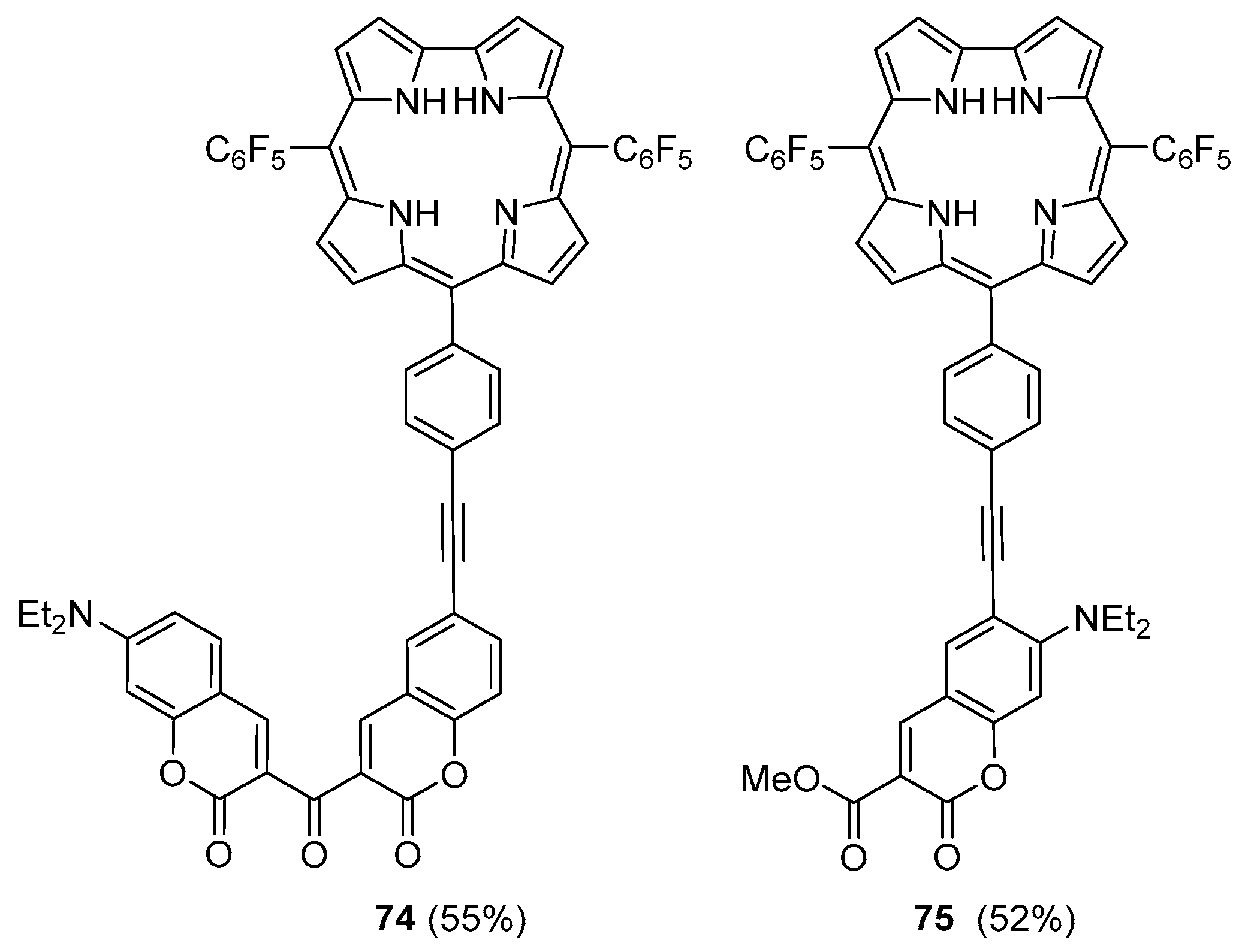
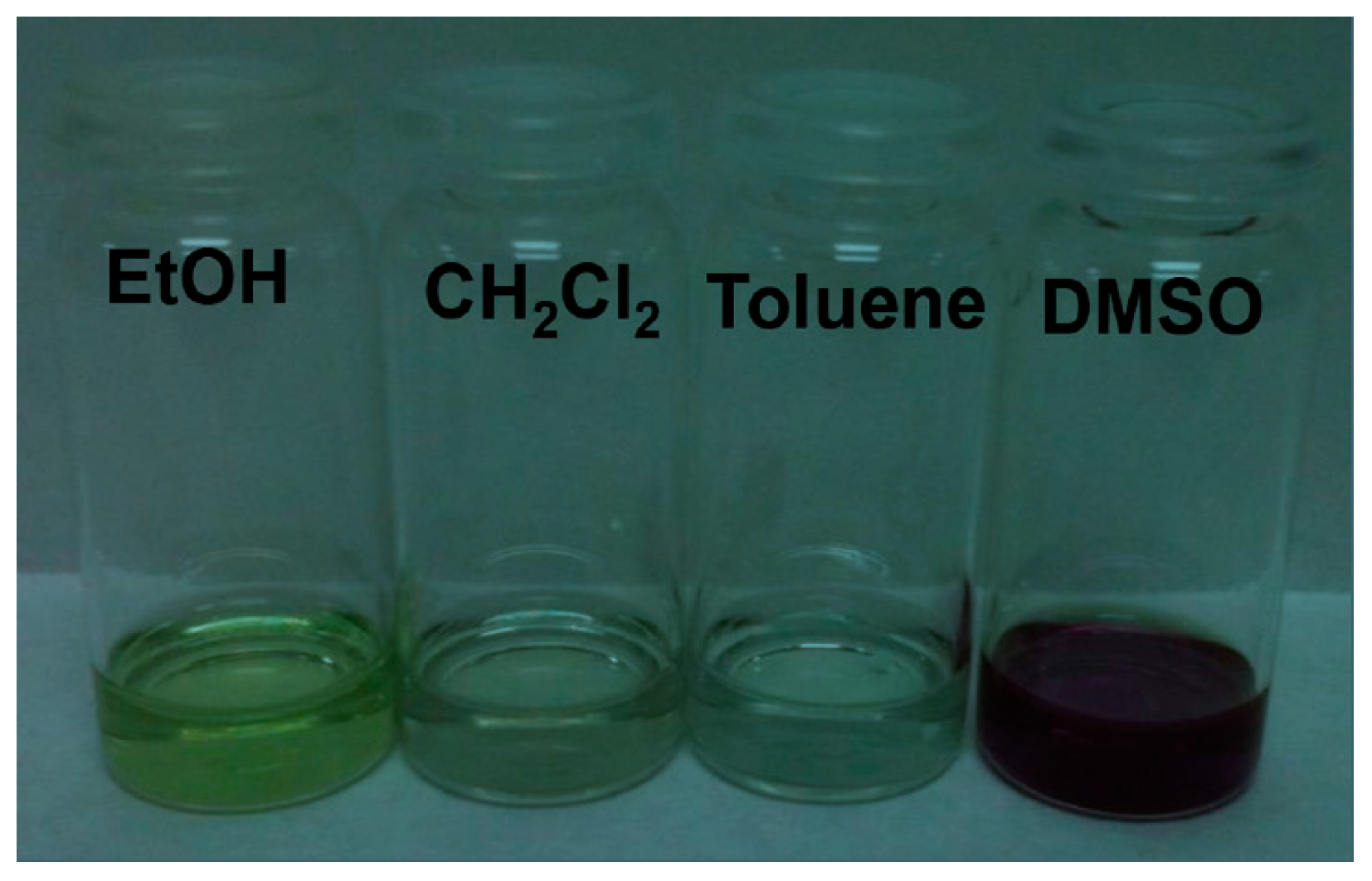
2.1. Coumarin–Porphyrin Dyads
2.2. Coumarin–Phthalocyanine Dyads
2.3. Coumarin–Corrole Conjugates
3. Final Remarks
Acknowledgments
Conflicts of Interest
References
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Shikishima, Y.; Takaishi, Y.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; et al. Coumarins and γ-pyrone derivatives from Prangos pabularia: Antibacterial activity and inhibition of cytokine release. Phytochemistry 2002, 59, 649–654. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules 2013, 18, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Olomola, T.O.; Mosebi, S.; Traut-Johnstone, T.; Coates, J.; Hewer, R.; Kaye, P.T. Novel furocoumarins as potential HIV-1 integrase inhibitors. Bioorg. Chem. 2014, 57, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Jambagi, V.B.; Barigidad, N.N.; Laxmeshwar, S.S.; Narayanachar, V.D. Synthesis, structure–activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and antimycobacterial agentes. Eur. J. Med. Chem. 2014, 74, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kohli, S.; Sandhu, S.; Bansal, Y.; Bansal, G. Coumarin: A promising scaffold for anticancer agents. Anti-Cancer Agents Med. Chem. 2015, 15, 1032–1048. [Google Scholar] [CrossRef]
- Zou, Q.; Fang, Y.; Zhao, Y.; Zhao, H.; Wang, Y.; Gu, Y.; Wu, F. Synthesis and in vitro photocytotoxicity of coumarin derivatives for one- and two-photon excited photodynamic therapy. J. Med. Chem. 2013, 56, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, A.E.; Hefti, H.; Meyer, H.R.; Schmidt, E. Fluorescent whitening agents 1973–1985. Rev. Prog. Coloration 1987, 17, 39–55. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Guo, M.; Du, L.; Liu, G.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener and surface sizing agent. Appl. Surface Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Caltagirone, C.; Cao, Z.; Chen, Q.; Natale, C.D.; Garau, A.; Lippolis, V.; Lvova, L.; Liu, H.; Lundström, I.; et al. Multimodal use of new coumarin-based fluorescent chemosensors: Towards highly selective optical sensors for Hg2+ probing. Chem. Eur. J. 2013, 19, 14639–14653. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Kondakova, M.E.; Giesen, D.J.; Rajeswaran, M.; Madaras, M.; Lenhart, W.C. Coumarin-based, electron-trapping iridium complexes as highly efficient and stable phosphorescent emitters for organic light-emitting diodes. Inorg. Chem. 2010, 49, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, K.J.; Kim, B.; Lee, J.; Kay, K.-Y.; Park, J. New ambipolar blue emitting materials based on amino coumarin derivatives with high efficiency for organic light emitting diodes. J. Nanosci. Nanotechnol. 2013, 13, 8020–8024. [Google Scholar] [CrossRef] [PubMed]
- Kotchapradist, P.; Prachumrak, N.; Sunonnam, T.; Tarsang, R.; Namuangruk, S.; Sudyoadsuk, T.; Keawin, T.; Jungsuttiwong, S.; Promarak, V. N-coumarin derivatives as hole-transporting emitters for high efficiency solution-processed pure green electroluminescent devices. Dyes Pigment. 2015, 112, 227–235. [Google Scholar] [CrossRef]
- Kumar, S.; Puttaraju, B.; Patil, S. A deep-blue electroluminescent device based on a coumarin derivative. ChemPlusChem 2016, 81, 384–390. [Google Scholar] [CrossRef]
- Esnal, I.; Duran-Sampedro, G.; Agarrabeitia, A.R.; Bañuelos, J.; García-Moreno, I.; Macías, M.A.; Peña-Cabrera, E.; López-Arbeloa, I.; de la Moya, S.; Ortiz, M.J. Coumarin–BODIPY hybrids by heteroatom linkage: Versatile, tunable and photostable dye lasers for UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 8239–8247. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, R.; Bunning, T.J.; Vaia, R.A.; Natarajan, L.V.; Tondiglia, V.P. Electrically switchable, one-dimensional polymeric resonators from holographic photopolymerization: A new approach for active photonic bandgap materials. Adv. Mater. 2003, 15, 241–244. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers: From light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-S.; Hara, K.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Suga, S.; Arakawa, H.; Sugihara, H. Photophysical and (photo)electrochemical properties of a coumarin dye. J. Phys. Chem. B 2005, 109, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Oltra, N.S.; Browne, W.R.; Roelfes, G. Hierarchical self-assembly of a biomimetic light-harvesting antenna based on DNA G-quadruplexes. Chem. Eur. J. 2013, 19, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Fang, X.Z.; Yang, P.R.; Yi, J.-S.; Ko, Y.-G.; Piao, M.J.; Chung, Y.D.; Park, Y.W.; Jeon, S.-J.; Cho, B.R. Design of molecular two-photon probes for in vivo imaging. 2H-Benzo[h]chromene-2-one derivatives. Tetrahedron Lett. 2007, 48, 2791–2795. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Schill, H.; Nizamov, S.; Bottanelli, F.; Bierwagen, J.; Belov, V.N.; Hell, S.W. 4-Trifluoromethyl-substituted coumarins with large stokes shifts: Synthesis, bioconjugates, and their use in super-resolution fluorescence microscopy. Chem. Eur. J. 2013, 19, 16556–16565. [Google Scholar] [CrossRef] [PubMed]
- Meimetis, L.G.; Carlson, J.C.T.; Giedt, R.J.; Kohler, R.H.; Weissleder, R. Ultrafluorogenic coumarin–tetrazine probes for real-time biological imaging. Angew. Chem. Int. Ed. 2014, 53, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Yanga, M.; Wang, H.; Huang, J.; Fang, M.; Mei, B.; Zhou, H.; Wu, J.; Tian, Y. Highly sensitive and selective colorimetric and fluorescent off–on probe for copper(II) based on unique addition reaction and its imaging in living cells. Sens. Actuators B 2014, 204, 710–715. [Google Scholar] [CrossRef]
- Rong, L.; Liu, L.-H.; Chen, S.; Cheng, H.; Chen, C.-S.; Li, Z.-Y.; Qin, S.-Y.; Zhang, X.-Z. A coumarin derivative as a fluorogenic glycoproteomic probe for biological imaging. Chem. Commun. 2014, 50, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Listunov, D.; Mazères, S.; Volovenko, Y.; Joly, E.; Génisson, Y.; Maraval, V.; Chauvin, R. Fluorophore-tagged pharmacophores for antitumor cytotoxicity: Modified chiral lipidic dialkynylcarbinols for cell imaging. Bioorg. Med. Chem. Lett. 2015, 25, 4652–4656. [Google Scholar] [CrossRef] [PubMed]
- Sednev, M.V.; Belov, V.N.; Hell, S.W. Fluorescent dyes with large Stokes shifts for super-resolution optical microscopy of biological objects: A review. Method. Appl. Fluoresc. 2015, 3, 042004. [Google Scholar] [CrossRef]
- Bochkov, A.Y.; Akchurin, I.O.; Dyachenko, O.A.; Traven, V.F. NIR-fluorescent coumarin-fused BODIPY dyes with large Stokes shifts. Chem. Commun. 2013, 49, 11653–11655. [Google Scholar] [CrossRef] [PubMed]
- Brites, M.J.; Santos, C.; Nascimento, S.; Gigante, B.; Luftmann, H.; Fedorov, A.; Berberan-Santos, M.N. Synthesis and fluorescence properties of [60] and [70]fullerene–coumarin dyads: Efficient dipole–dipole resonance energy transfer from coumarin to fullerene. New J. Chem. 2006, 30, 1036–1045. [Google Scholar] [CrossRef]
- Abellán-Flos, M.; Tanç, M.; Supuran, C.T.; Vincent, S.P. Exploring carbonic anhydrase inhibition with multimeric coumarins displayed on a fullerene scaffold. Org. Biomol. Chem. 2015, 13, 7445–7451. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.; Fedorov, A.; Brites, M.J.; Berberan-Santos, M.N. New coumarin–[60]fullerene dyads connected by an alkynyl linkage: Synthesis and fluorescence studies. Evidence for efficient singlet–singlet energy transfer. Dyes Pigment. 2015, 114, 158–165. [Google Scholar] [CrossRef]
- Hurenkamp, J.H.; Browne, W.R.; Augulis, R.; Pugzlys, A.; van Loosdrecht, P.H.M.; van Esch, J.H.; Feringa, B.L. Intramolecular energy transfer in a tetra-coumarin perylene system: Influence of solvent and bridging unit on electronic properties. Org. Biomol. Chem. 2007, 5, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Augulis, R.; Pugzlys, A.; Hurenkamp, J.H.; Feringa, B.L.; van Esch, J.H.; van Loosdrecht, P.H.M. Optical energy transport and interactions between the excitations in a coumarin–perylene bisimide dendrimer. J. Phys. Chem. A 2007, 111, 12944–12953. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Nisanci, B.; Acar, M.; Dastan, A.; Bozdemir, O.A. Synthesis and use of ”clickable” bay-region tetrasubstituted perylene tetracarboxylic tetraesters and a perylene monoimide diester as energy acceptors. New J. Chem. 2015, 39, 548–554. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Gomes, A.T.P.C.; Silva, A.M.S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; da Silva, F.C.; Ferreira, V.F.; Cavaleiro, J.A.S. Reaction of β-vinyl-meso-tetraphenylporphyrin with o-quinone methines. Synlett 2011, 13, 1841–1844. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Menezes, J.C.J.M.D.S.; Barata, J.F.B.; Faustino, M.A.F.; Ferreira, V.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. New coumarin–corrole and –porphyrin conjugate multifunctional probes for anionic or cationic interactions: Synthesis, spectroscopy, and solid supported studies. Tetrahedron 2014, 70, 3361–3370. [Google Scholar] [CrossRef]
- Callot, H.J. Nouvelles voies d’accès aux vinylporphyrins. Tetrahedron 1973, 29, 899–901. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Santos, H.M.; Menezes, J.C.J.M.D.S.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Capelo, J.L.; Neves, M.G.P.M.S.; Lodeiro, C. Untangling interactions of a zinc(II) complex containing a coumarin–porphyrin unit with alkaloids in water solutions: A photophysical study. Photochem. Photobiol. Sci. 2015, 14, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Barros, C.; Santos, S.M.; da Silva, F.C.; Ferreira, V.F.; Domingues, M.R.M. Gas phase reactions of β-substituted hetero-Diels–Alder adducts of meso-tetraphenylporphyrin using tandem mass spectrometry. Int. J. Mass Spectrom. 2013, 343–344, 1–8. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Faustino, M.A.F.; de Oliveira, K.T.; Uliana, M.P.; Ferreira, V.F.; Hackbarth, S.; Röder, B.; Tasso, T.T.; Furuyama, T.; Kobayashi, N.; et al. Synthesis of new chlorin e6 trimethyl and protoporphyrin IX dimethyl ester derivatives and their photophysical and electrochemical characterizations. Chem. Eur. J. 2014, 20, 13644–13655. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.F.C.; Gomes, A.T.P.C.; Silva, V.L.M.; Silva, A.M.S.; Neves, M.G.P.M.S.; da Silva, F.C.; Ferreira, V.F.; Cavaleiro, J.A.S. Ohmic heating assisted synthesis of coumarinyl porphyrin derivatives. RSC Adv. 2015, 5, 66192–66199. [Google Scholar] [CrossRef]
- Shen, D.-M.; Liu, C.; Chen, Q.-Y. Synthesis and versatile reactions of β-azidotetraarylporphyrins. Eur. J. Org. Chem. 2007, 2007, 1419–1422. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. Synthesis and photophysical properties of β-triazole bridged porphyrincoumarin dyads. RSC Adv. 2015, 5, 68209–68217. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. Synthesis, characterization and photophysical studies of β-triazolomethyl-bridged porphyrin-benzo-α-pyrone dyads. J. Chem. Sci. 2016, 128, 545–554. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. meso-Phenyl-triazole bridged porphyrin-coumarin dyads: Synthesis, characterization and photophysical properties. Dyes Pigment. 2015, 121, 256–264. [Google Scholar] [CrossRef]
- Lin, W.; Long, L.; Feng, J.; Wang, B.; Guo, C. Synthesis of meso-coumarin-conjugated porphyrins and investigation of their luminescence properties. Eur. J. Org. Chem. 2007, 4301–4304. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Amaravathi, M.; Rajitha, B.; Rao, M.K.; Sitadevi, P. Synthesis of meso-tetrakis(4-chlorocoumarin-3-yl)porphyrins. J. Heterocycl. Chem. 2007, 44, 821–825. [Google Scholar] [CrossRef]
- Hecht, S.; Ihre, H.; Fréchet, J.M.J. Porphyrin core star polymers: Synthesis, modification, and implication for site isolation. J. Am. Chem. Soc. 1999, 121, 9239–9240. [Google Scholar] [CrossRef]
- Hecht, S.; Vladimirov, N.; Fréchet, J.M.J. Encapsulation of functional moieties within branched star polymers: Effect of chain length and solvent on site isolation. J. Am. Chem. Soc. 2001, 123, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, W.R.; Hecht, S.; Fréchet, J.M.J. Functionally layered dendrimers: A new building block and its application to the synthesis of multichromophoric light-harvesting systems. Org. Lett. 2005, 7, 4451–4454. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Song, Q.-H. Non-conjugated dendrimers with a porphyrin core and coumarin chromophores as peripheral units: Synthesis and photophysical properties. Dyes Pigment. 2012, 92, 975–981. [Google Scholar] [CrossRef]
- Concellón, A.; Marcos, M.; Romero, P.; Serrano, J.L.; Termine, R.; Golemme, A. Not only columns: High hole mobility in a discotic nematic mesophase formed by metal-containing porphyrin-core dendrimers. Angew. Chem. Int. Ed. 2017, 56, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lin, W.; Yu, Q. A ratiometric fluorescent probe for thiols based on a tetrakis(4-hydroxyphenyl)porphyrin-coumarin scaffold. J. Org. Chem. 2011, 76, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.Y.; Troxler, T.; Vinogradov, S.A. Design of metalloporphyrin-based dendritic nanoprobes for two-photon microscopy of oxygen. J. Porphyr. Phthalocyanines 2008, 12, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hania, P.R.; Heijs, D.J.; Bowden, T.; Pugžlys, A.; van Esch, J.; Knoester, J.; Duppen, K. Ultrafast energy transport in a first-generation coumarin-tetraphenylporphyrin dendrimer. J. Phys. Chem. B 2004, 108, 71–81. [Google Scholar] [CrossRef]
- Concellón, A.; Bucos, M.; Serrano, J.L.; Romero, P.; Marcos, M. Supramolecular liquid crystalline dendrimers with a porphyrin core and functional carboxylic acid dendrons. RSC Adv. 2016, 6, 65179–65185. [Google Scholar] [CrossRef]
- Garg, V.; Kodis, G.; Liddell, P.A.; Terazono, Y.; Moore, T.A.; Moore, A.L.; Gust, D. Artificial photosynthetic reaction center with a coumarin-based antenna system. J. Phys. Chem. B 2013, 117, 11299–11308. [Google Scholar] [CrossRef] [PubMed]
- Medyouni, R.; Mtibaa, A.C.; Mellouli, L.; Romerosa, A.; Hamdi, N. Convenient synthesis of novel unmetalled and metallophthalocyanines bearing coumarin derivatives: Synthesis, characterization, aggregation behaviors and antimicrobial activity. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 201–210. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Bulut, M. Synthesis and characterization of metal-free and metallo-phthalocyanines with four pendant coumarinthio/oxy-substituents. Dyes Pigment. 2008, 76, 249–255. [Google Scholar] [CrossRef]
- Guo, J.-J.; Wang, S.-R.; Li, X.-G.; Zhang, F.; Shao, X.-N.; Lian, X.-J. The synthesis, molecular structure and photophysical properties of 2,9,16,23-tetrakis(7-coumarinoxy-4-methyl)phthalocyanine sensitizer. J. Mol. Struct. 2014, 1060, 17–23. [Google Scholar] [CrossRef]
- Guo, J.-J.; Wang, S.-R.; Li, X.-G.; Zhang, F.; Xiao, Y.; Teng, C. The synthesis, characterisation, photophysical and thermal properties, and photovoltaic performance of 7-coumarinoxy-4-methyltetrasubstituted metallophthalocyanines. Aust. J. Chem. 2015, 68, 1025–1034. [Google Scholar] [CrossRef]
- Han, J.L.; You, J.; Yonemura, H.; Yamada, S.; Wang, S.R.; Li, X.G. Metallophthalocyanines as triplet sensitizers for highly efficient photon upconversion based on sensitized triplet–triplet annihilation. Photochem. Photobiol. Sci. 2016, 15, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Chohan, S.; Booysen, I.N.; Mambanda, A.; Akerman, M.P. Synthesis, characterization and electrocatalytic behavior of cobalt and iron phthalocyanines bearing chromone or coumarin substituents. J. Coord. Chem. 2015, 68, 1829–1846. [Google Scholar] [CrossRef]
- Altun, S.; Özkaya, A.R.; Bulut, M. Peripheral octa-substituted metal-free, cobalt(II) and zinc(II) phthalocyanines bearing coumarin and chloro groups: Synthesis, characterization, spectral and electrochemical properties. Polyhedron 2012, 48, 31–42. [Google Scholar] [CrossRef]
- Odabas, Z.; Orman, E.B.; Durmus, M.; Dumludag, F.; Özkaya, A.R.; Bulut, M. Novel alpha-7-oxy-4-(4-methoxyphenyl)-8-methylcoumarin substituted metal-free, Co(II) and Zn(II) phthalocyanines: Photochemistry, photophysics, conductance and electrochemistry. Dyes Pigment. 2012, 95, 540–552. [Google Scholar] [CrossRef]
- Altun, S.; Odabaş, Z.; Altındal, A.; Özkaya, A.R. Coumarin-substituted manganese phthalocyanines: Synthesis, characterization, photovoltaic behaviour, spectral and electrochemical properties. Dalton Trans. 2014, 43, 7987–7997. [Google Scholar] [CrossRef] [PubMed]
- Altun, S.; Orman, E.B.; Odabaş, Z.; Altındal, A.; Özkaya, A.R. Gas sensing and electrochemical properties of tetra and octa 2H-chromen-2-one substituted iron(II) phthalocyanines. Dalton Trans. 2015, 44, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Çalik, A.E.; Köksoy, B.; Orman, E.B.; Durmus, M.; Özkaya, A.R.; Bulut, M. 4-Carboxymethyl-8-methyl-7-oxycoumarin substituted zinc, cobalt and indium phthalocyanines: Electrochemical and photochemical properties. J. Porphyr. Phthalocyanines 2013, 17, 1046–1054. [Google Scholar] [CrossRef]
- Alemdar, A.; Özkaya, A.R.; Bulut, M. Synthesis, spectroscopy, electrochemistry and in situ spectroelectrochemistry of partly halogenated coumarin phthalonitrile and corresponding metal-free, cobalt and zinc phthalocyanines. Polyhedron 2009, 28, 3788–3796. [Google Scholar] [CrossRef]
- Alemdar, A.; Özkaya, A.R.; Bulut, M. Preparation, characterization, electrochemistry and in situ spectroelectrochemistry of novel α-tetra[7-oxo-3-(2-chloro-4-fluorophenyl)coumarin]-substituted metal-free, cobalt and zinc phthalocyanines. Synth. Met. 2010, 160, 1556–1565. [Google Scholar] [CrossRef]
- Piskin, M.; Durmus, M.; Bulut, M. Highly soluble 7-oxy-3-(4-methoxyphenyl)coumarin bearing zinc phthalocyanines: Synthesis and investigation of photophysical and photochemical properties. J. Photochem. Photobiol. A 2011, 223, 37–49. [Google Scholar] [CrossRef]
- Odabas, Z.; Kara, H.; Ozkaya, A.R.; Bulut, M. Synthesis, characterization and electrochemical properties of novel β 7-oxy-4-(4-methoxyphenyl)-8-methylcoumarin substituted metal-free, Zn(II) and Co(II) phthalocyanines. Polyhedron 2012, 39, 38–47. [Google Scholar] [CrossRef]
- Kaya, E.N.; Durmus, M.; Bulut, M. The effects of substituents’ positions and variety of axial groups on the photochemical properties of coumarin substituted indium(III) phthalocyanines. J. Organomet. Chem. 2014, 774, 94–100. [Google Scholar] [CrossRef]
- Kaya, E.N.; Durmus, M.; Bulut, M. Novel 7-oxy-3-(3′,4′,5′-trimethoxyphenyl)coumarin substituted zinc(II) phthalocyanines: Synthesis, characterization, photophysical and photochemical properties. J. Porphyr. Phthalocyanines 2015, 19, 1114–1122. [Google Scholar] [CrossRef]
- Tastemel, A.; Karaca, B.Y.; Durmus, M.; Bulut, M. Photophysical and photochemical properties of novel metallophthalocyanines bearing 7-oxy-3-(m-methoxyphenyl)coumarin groups. J. Lumin. 2015, 168, 163–171. [Google Scholar] [CrossRef]
- Erdogan, T.; Bulut, M.; Çamur, M. Novel phthalocyanines bearing 7-oxy-3-(3,5-difluorophenyl)coumarin moieties: Synthesis, characterization, photophysical and photochemical properties. J. Photochem. Photobiol. A 2015, 300, 6–14. [Google Scholar] [CrossRef]
- Gok, A.; Orman, E.B.; Salan, U.; Özkaya, A.R.; Bulut, M. Synthesis, characterization and electrochemical properties of tetra 7-oxy-3-biphenylcoumarin substituted metal-free, zinc(II), cobalt(II) and indium(III) phthalocyanines. Dyes Pigment. 2016, 133, 311–323. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Durmaz, E.; Karaca, F.; Bulut, M. Synthesis and characterization of metallo phthalocyanines bearing 7-oxy-3-(4-pyridyl)coumarin substituents and their supramolecular structures with vanadyl bis(acetylacetonate). Polyhedron 2012, 38, 267–274. [Google Scholar] [CrossRef]
- Çamur, M.; Esenpinar, A.A.; Özkaya, A.R.; Bulut, M. Synthesis, characterization, spectroscopic and electrochemical properties of phthalocyanines substituted with four 3-ferrocenyl-7-oxycoumarin moieties. J. Organomet. Chem. 2011, 696, 1868–1873. [Google Scholar] [CrossRef]
- Piskin, M.; Durmus, M.; Bulut, M. Synthesis, characterization, photophysical and photochemical properties of 7-oxy-3-methyl-4-phenylcoumarin-substituted indium phthalocyanines. Inorg. Chim. Acta 2011, 373, 107–116. [Google Scholar] [CrossRef]
- Piskin, M.; Durmus, M.; Bulut, M. Synthesis and investigation on photophysical and photochemical properties of 7-oxy-3-methyl-4-phenylcoumarin bearing zinc phthalocyanines. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Dur, E.; Bulut, M. Derivatizable novel β-tetra 7-oxycoumarin-3-carboxylate substituted metallophthalocyanines: Synthesis and characterization. Polyhedron 2010, 29, 2689–2695. [Google Scholar] [CrossRef]
- Dur, E.; Ozkaya, A.R.; Bulut, M. Synthesis, characterisation and electrochemistry of derivatisable novel α-tetra 7-oxycoumarin-3-carboxylate-substituted metallophthalocyanines. Supramol. Chem. 2011, 23, 379–388. [Google Scholar] [CrossRef]
- Cakici, H.; Esenpinar, A.A.; Bulut, M. Synthesis and characterization of novel phthalocyanines bearing quaternizable coumarin. Polyhedron 2008, 27, 3625–3630. [Google Scholar] [CrossRef]
- Esenpınar, A.A.; Durmus, M.; Bulut, M. Tetra-3-[(2-diethylamino)ethyl]-7-oxo-4-methylcoumarin- substituted zinc phthalocyanines: Synthesis, characterization and aggregation effects on photophysical/photochemical properties. J. Photochem. Photobiol. A 2010, 213, 171–179. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Durmus, M.; Bulut, M. Photophysical, photochemical and BSA binding/BQ quenching properties of quaternizable coumarin containing water soluble zinc phthalocyanine complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kartaloglu, N.; Esenpinar, A.A.; Bulut, M. Synthesis, characterization, and photophysical and photochemical properties of 3-(4-phenyloxy)coumarin containing metallo- and metal-free phthalocyanines. Turk. J. Chem. 2014, 38, 1102–1117. [Google Scholar] [CrossRef]
- Çamur, M.; Bulut, M.; Kandaz, M.; Guney, O. Effects of coumarin substituents on the photophysical properties of newly synthesized phthalocyanine derivatives. Supramol. Chem. 2009, 21, 624–631. [Google Scholar] [CrossRef]
- Çamur, M.; Bulut, M. The synthesis and characterization of novel soluble phthalocyanines substituted with 7-octyloxy-3-(4-oxyphenyl)coumarin moieties. Dyes Pigment. 2008, 77, 165–170. [Google Scholar] [CrossRef]
- Camur, M.; Bulut, M.; Kandaz, M.; Guney, O. Synthesis, characterization and fluorescence behavior of new fluorescent probe phthalocyanines bearing coumarin substituents. Polyhedron 2009, 28, 233–238. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Bulut, M. Synthesis and characterization of novel α- or β-tetra[6,7-dihexyloxy-3-(4-oxyphenyl)coumarin]-substituted metal-free and metallo phthalocyanines. Polyhedron 2009, 28, 3129–3137. [Google Scholar] [CrossRef]
- Çamur, M.; Özkaya, A.R.; Bulut, M. Synthesis, characterization and spectroscopic properties of new fluorescent 7,8-dihexyloxy-3-(4-oxyphenyl)coumarin substituted phthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 691–701. [Google Scholar] [CrossRef]
- Çamur, M.; Durmus, M.; Bulut, M. Highly singlet oxygen generative water-soluble coumarin substituted zinc(II) phthalocyanine photosensitizers for photodynamic therapy. Polyhedron 2012, 41, 92–103. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Durmus, M.; Bulut, M. Synthesis and properties of crown ether functionalized coumarin substituted zinc phthalocyanine. Polyhedron 2011, 30, 2067–2074. [Google Scholar] [CrossRef]
- Esenpinar, A.A.; Özkaya, A.R.; Bulut, M. Synthesis and electrochemical properties of crown ether functionalized coumarin substituted cobalt and copper phthalocyanines. J. Organomet. Chem. 2011, 696, 3873–3881. [Google Scholar] [CrossRef]
- Camur, M.; Durmus, M.; Ozkaya, A.R.; Bulut, M. Synthesis, photophysical, photochemical and electrochemical properties of crown ether bearing coumarin substituted phthalocyanines. Inorg. Chim. Acta 2012, 383, 287–299. [Google Scholar] [CrossRef]
- Çamur, M.; Durmus, M.; Bulut, M. Coumarino-12-crown-4 bearing phthalocyanine photosensitizers for singlet oxygen production. J. Photochem. Photobiol. A 2011, 222, 266–275. [Google Scholar] [CrossRef]
- Koksoy, M.A.; Koksoy, B.; Durmus, M.; Bulut, M. Preparation, characterization and photophysicochemical properties of novel tetra 7-(diethyl 2-methylmalonatoxy)-3-(p-oxyphenyl)coumarin-substituted zinc(II) and indium(III)chloride phthalocyanines. J. Organomet. Chem. 2016, 822, 125–134. [Google Scholar] [CrossRef]
- Can, O.S.; Kaya, E.N.; Durmus, M.; Bulut, M. High photosensitized singlet oxygen generating zinc(II) and indium(III) acetate phthalocyanines containing 6,8-di-tert-butyl-3-(p-oxyphenyl)coumarin groups. J. Photochem. Photobiol. A 2016, 317, 56–67. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Meng, L.-B.; Huang, Q.; Li, J.; Zheng, K.; Zhang, F.-L.; Liu, J.-Y.; Xue, J.-P. Synthesis and in vitro anticancer activity of zinc(II) phthalocyanines conjugated with coumarin derivatives for dual photodynamic and chemotherapy. Chem. Med. Chem. 2015, 10, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Altun, S.; Altindal, A.; Bulut, M. Synthesis, characterization and dielectric properties of novel axial coumarin-substituted titanium(IV) phthalocyanines. Polyhedron 2013, 49, 41–49. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S. Strategies for corrole functionalization. Chem. Rev. 2017, 117, 3192–3253. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Gryko, D.T.; Pielacińska, D.J.; Zanelli, A.; Flamigni, L. Trans-A2B-corroles bearing a coumarin moiety—From synthesis to photophysics. Chem. Asian J. 2010, 5, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Dhanalekshmi, S.; Taniguchi, M.; Ambroise, A.; Lindsey, J.S. A scalable synthesis of meso-substituted dipyrromethanes. Org. Process Res. Dev. 2003, 7, 799–812. [Google Scholar] [CrossRef]
- Gryko, D.T.; Tasior, M.; Peterle, T.; Bröring, M. Meso-substituted corroles bearing peripheral donor sites. J. Porphyr. Phthalocyanines 2006, 10, 1360–1370. [Google Scholar] [CrossRef]
- Rao, P.D.; Dhanalekshmi, S.; Littler, B.J.; Lindsey, J.S. Rational syntheses of porphyrins bearing up to four different meso substituents. J. Org. Chem. 2000, 65, 7323–7344. [Google Scholar] [CrossRef]
- Koszarna, B.; Gryko, D.T. Efficient synthesis of meso-substituted corroles in a H2O–MeOH mixture. J. Org. Chem. 2006, 71, 3707–3717. [Google Scholar] [CrossRef]
- Tasior, M.; Voloshchuk, R.; Poronik, Y.M.; Rowicki, T.; Gryko, D.T. Corroles bearing diverse coumarin units—Synthesis and optical properties. J. Porphyr. Phthalocyanines 2011, 15, 1011–1023. [Google Scholar] [CrossRef]
- Bursa, B.; Barszcz, B.; Bednarski, W.; Lewtak, J.P.; Koszelewski, D.; Vakuliuk, O.; Gryko, D.T.; Wróbel, D. New meso-substituted corroles possessing pentafluorophenyl groups—Synthesis and spectroscopic characterization. Phys. Chem. Chem. Phys. 2015, 17, 7411–7423. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Oliveira, E.; Barata, J.F.B.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Lodeiro, C. Corroles as anion chemosensors: Exploiting their fluorescence behaviour from solution to solid-supported devices. J. Mater. Chem. 2012, 22, 13811–13819. [Google Scholar] [CrossRef]
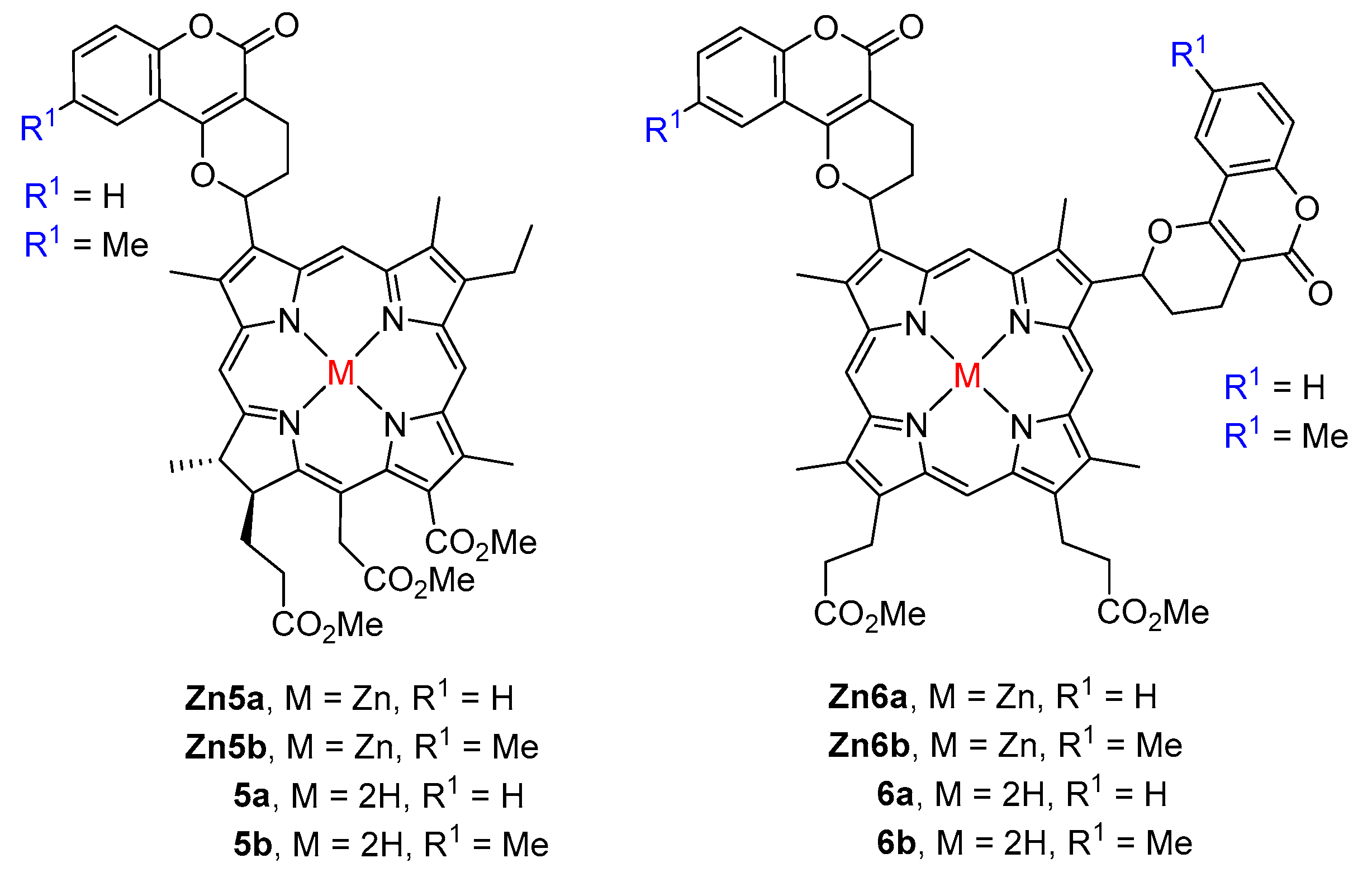




















| R1 | R2 | R3 | X | M | 57 | Ref. |
|---|---|---|---|---|---|---|
| Yield (%) | ||||||
| H | H | H, Me | 4-NO2 | 2H, Zn, Co, Ni, Cu | 50–96 | [63,64] |
| H | Me | H | - | 2H, Zn, Co, | - | [65,66,67] |
| H | H, CF3 | H | 4-NO2 | Zn, Co | 51–58 | [68] |
| H | 4-MeOC6H4 | Me | 3-NO2, 4-NO2, | 2H, Co, Zn, Fe | 35–74 | [69,70,71,72] |
| 4,5-dichloro | ||||||
| H | CH2CO2H | Me | 3-NO2 | Zn, Co, In(OAc) | 14–30 | [73] |
| 2-Cl-4-FC6H3 | H | H | 3-NO2, 4-NO2 | 2H, Co, Zn | 16–36 | [74,75] |
| 3-MeOC6H4, | H | H | 3-NO2, 4-NO2, | Zn, In, Mg, Mn | 9–36 | [76,77,78,79,80] |
| 4-MeOC6H4, | 4,5-dichloro | |||||
| 3,4,5-(MeO)3C6H2 | ||||||
| 3,5-F2C6H3 | H | H | 3-NO2, 4-NO2 | Zn, In | 38–39 | [81] |
| biphenyl | H | H | 3-NO2, 4-NO2 | 2H, In, Co, Zn | 19–48 | [82] |
| 4-pyridyl | H | H | 3-NO2, 4-NO2 | Zn, Co | 55–80 | [83] |
| ferrocenyl | H | H | 4-NO2 | Zn, Co | 45, 51 | [84] |
| Me | Ph | H | 3-NO2, 4-NO2, | In | 26–31 | [85,86] |
| 4,5-dichloro | ||||||
| CO2Et | H | H | 3-NO2, 4-NO2 | Zn, Co, Ni, Cu | 18–36 | [87,88] |
| CH2CH2NEt2 | Me | H | 3-NO2, 4-NO2 | 2H, Zn | 43, 77 | [89,90,91] |
| Ar | Position of the ArO Group at the Phthalonitrile | M | 61 | Ref. |
|---|---|---|---|---|
| Yield (%) | ||||
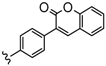 | 3 or 4 | 2H, Zn, Co, In(OAc) | 15–50 | [92] |
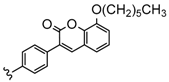 | 4 | 2H, Zn, Co | 33–73 | [93] |
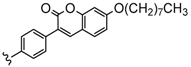 | 4 | 2H, Zn, Co, Ni, Cu | 40–89 | [94] |
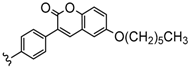 | 4 | 2H, Zn, Co | 25–30 | [95] |
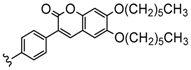 | 3 or 4 | 2H, Zn, Co, Cu | 42–80 | [96,97] |
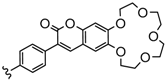 | 3 or 4 | Zn, Co, Cu | 34–53 | [99,100,101] |
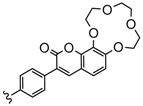 | 3 or 4 | 2H, Zn | 23–68 | [102] |
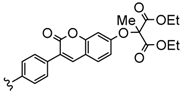 | 3 or 4 | Zn, InCl | 30–33 | [103] |
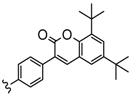 | 3 or 4 or 4,5 | Zn, InOAc | 40–57 | [104] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, A.F.R.; Almodôvar, V.A.S.; Neves, M.G.P.M.S.; Tomé, A.C. Coumarin–Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications. Molecules 2017, 22, 994. https://doi.org/10.3390/molecules22060994
Cerqueira AFR, Almodôvar VAS, Neves MGPMS, Tomé AC. Coumarin–Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications. Molecules. 2017; 22(6):994. https://doi.org/10.3390/molecules22060994
Chicago/Turabian StyleCerqueira, Ana F. R., Vítor A. S. Almodôvar, Maria G. P. M. S. Neves, and Augusto C. Tomé. 2017. "Coumarin–Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications" Molecules 22, no. 6: 994. https://doi.org/10.3390/molecules22060994







