Enhanced Antibacterial Activity of Ent-Labdane Derivatives of Salvic Acid (7α-Hydroxy-8(17)-ent-Labden-15-Oic Acid): Effect of Lipophilicity and the Hydrogen Bonding Role in Bacterial Membrane Interaction
Abstract
:1. Introduction
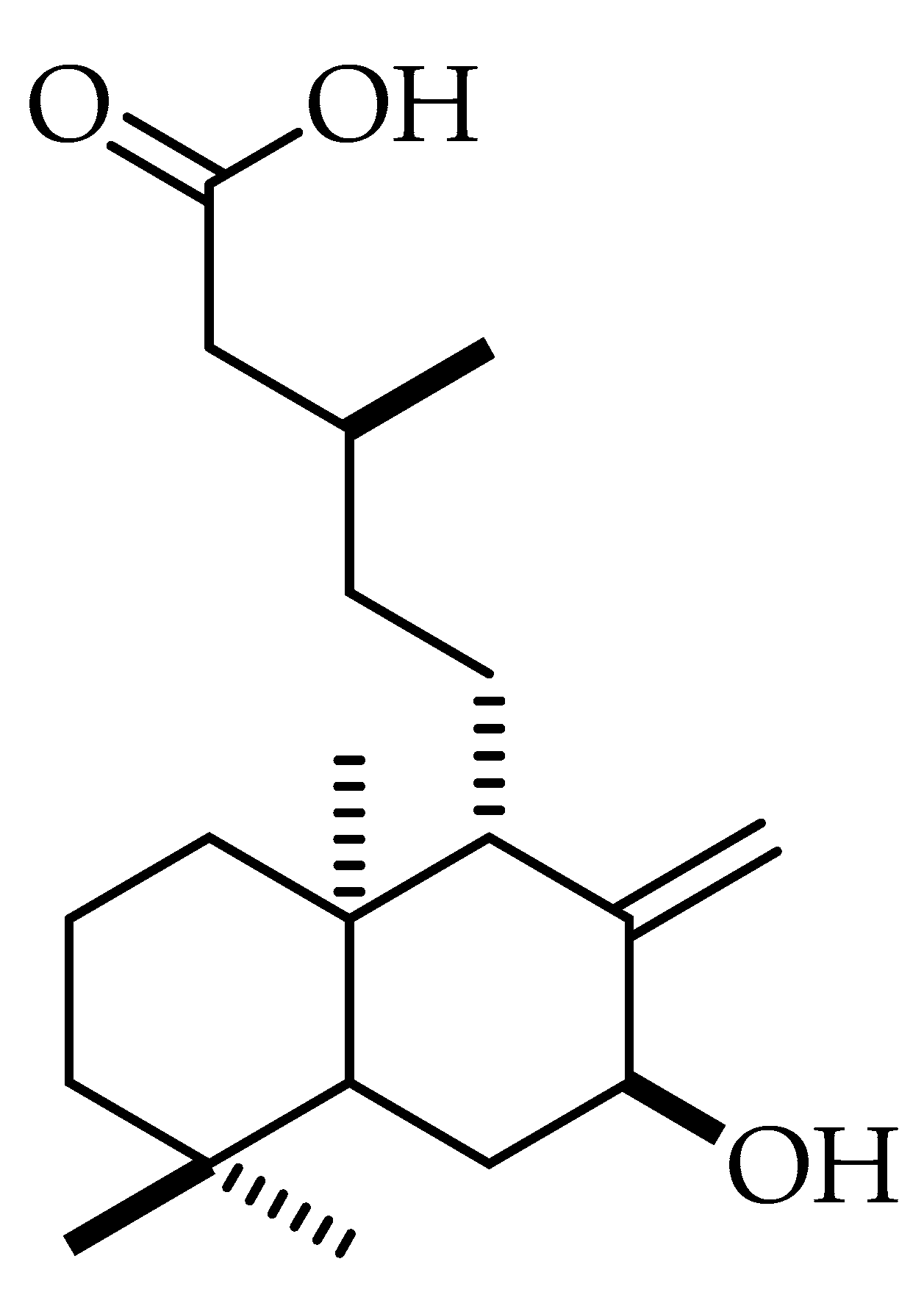
2. Results and Discussion
2.1. Antibacterial Activity of the Salvic Acid Derivatives
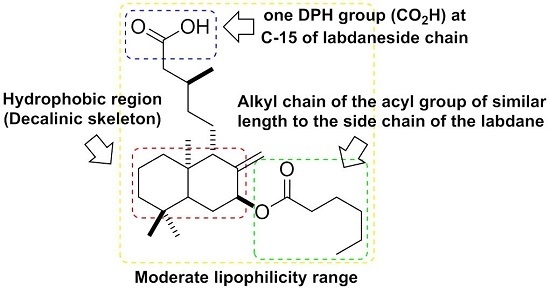
2.2. Lipophilicity and Structure-Activity Relationship of Antibacterial of Salvic Acid and Its Derivatives
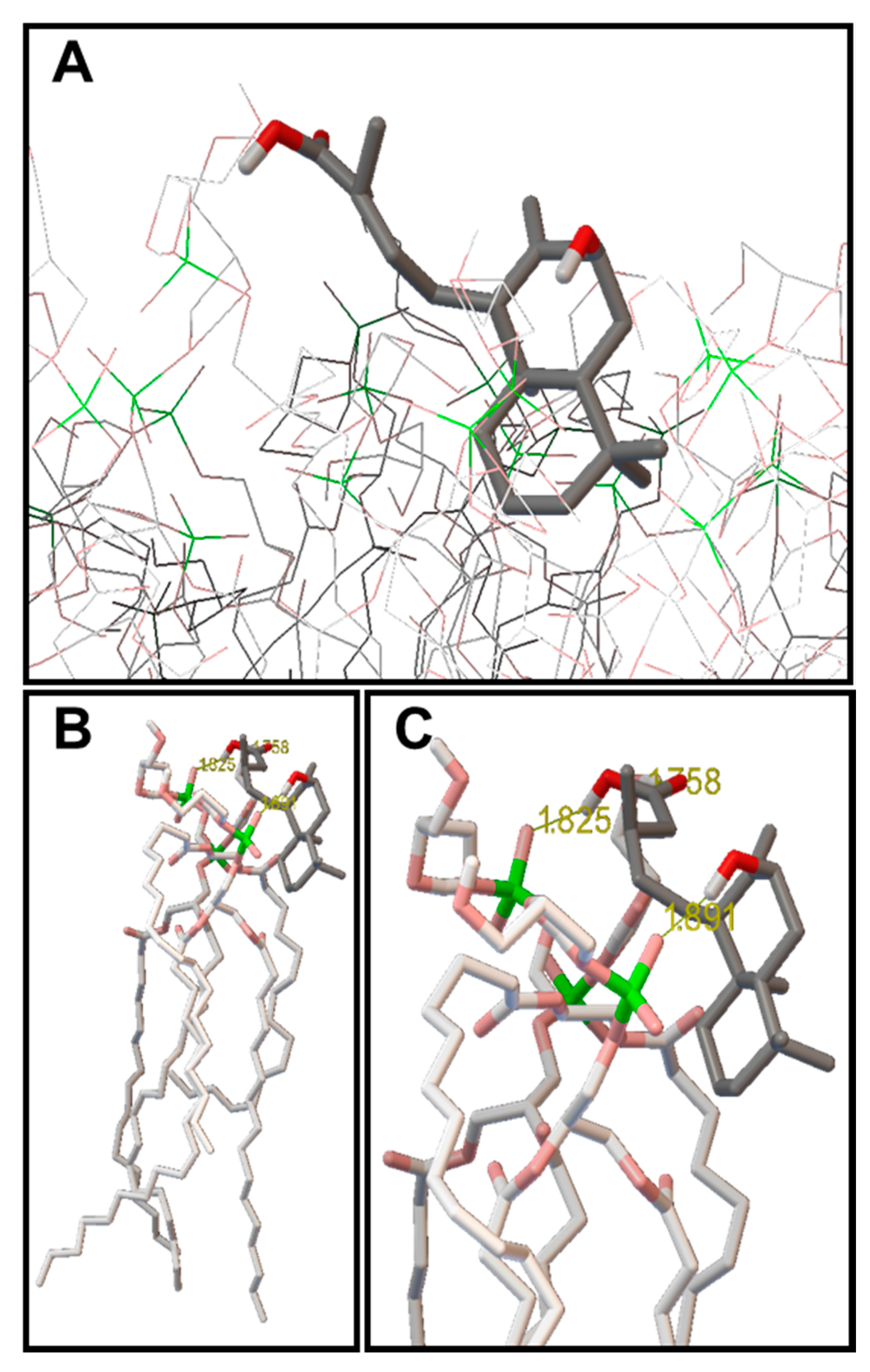
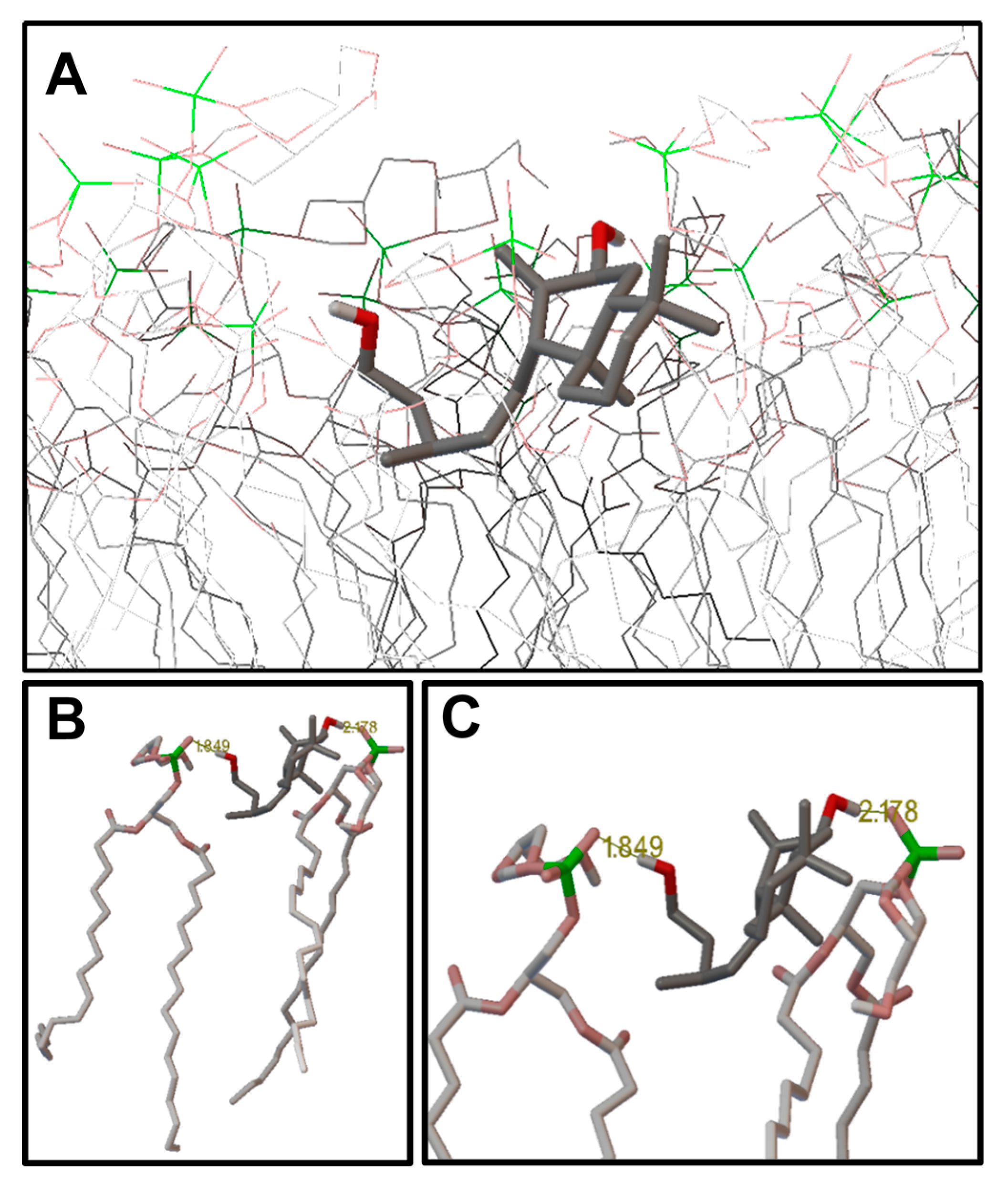
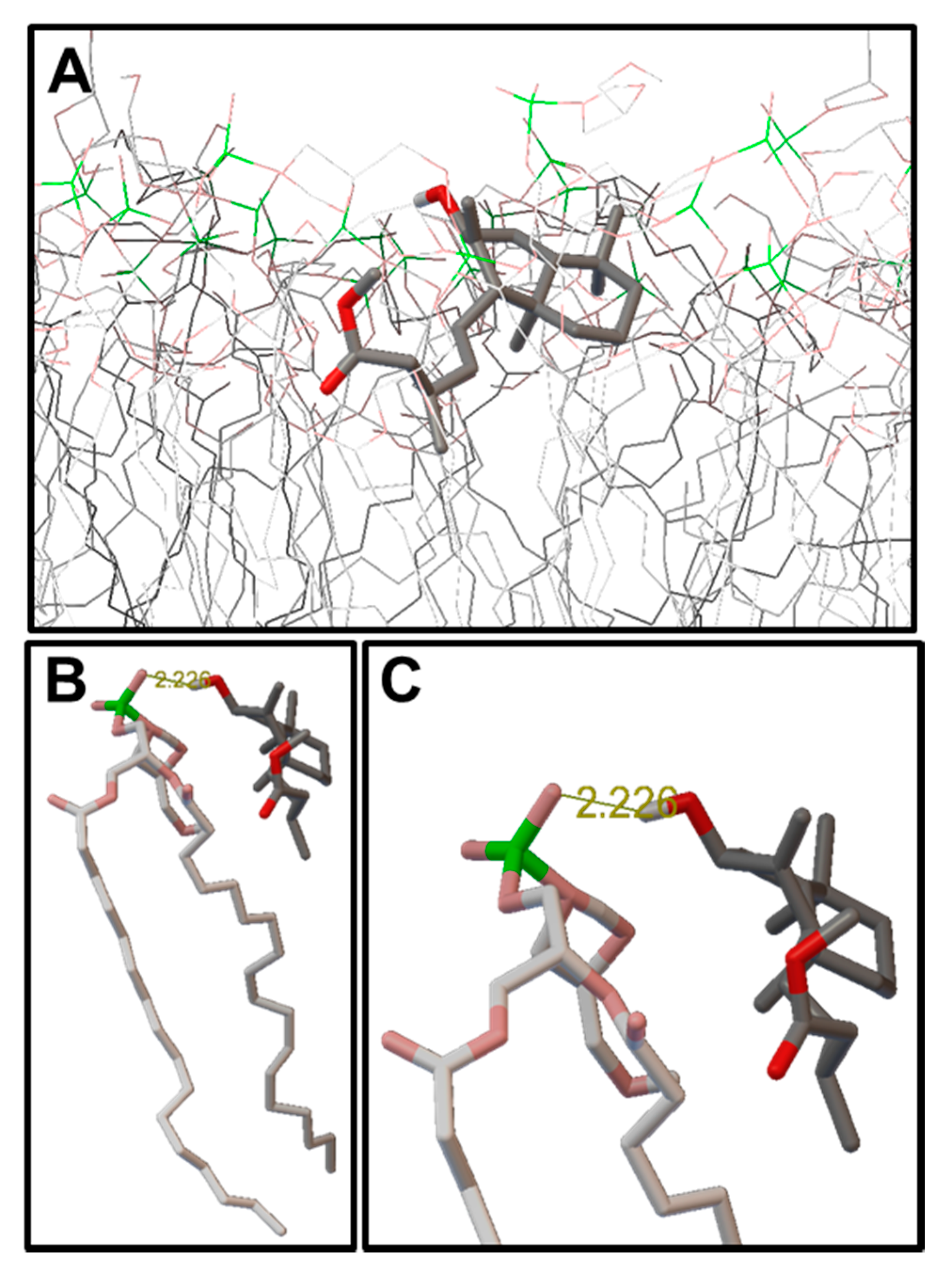
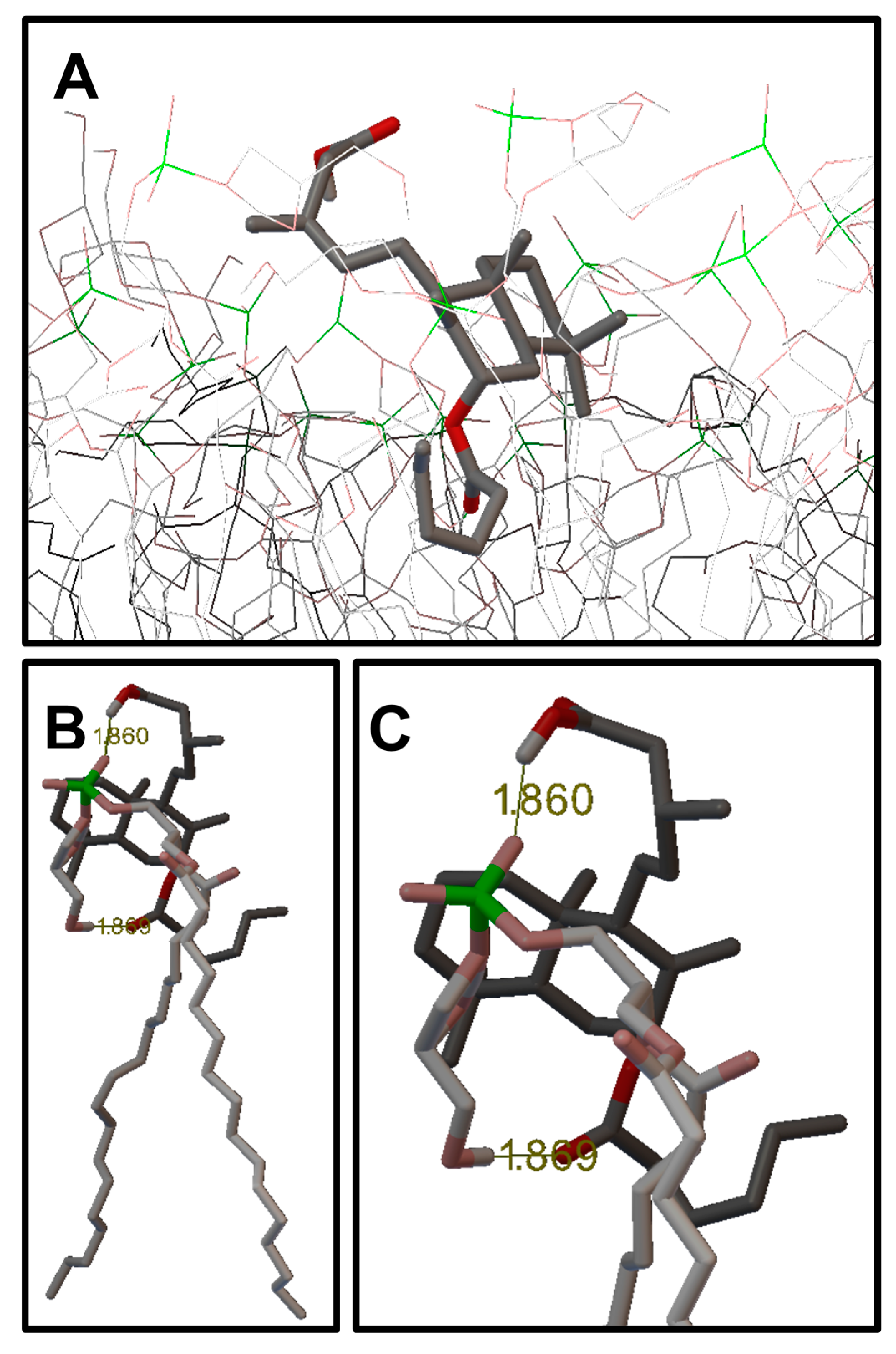
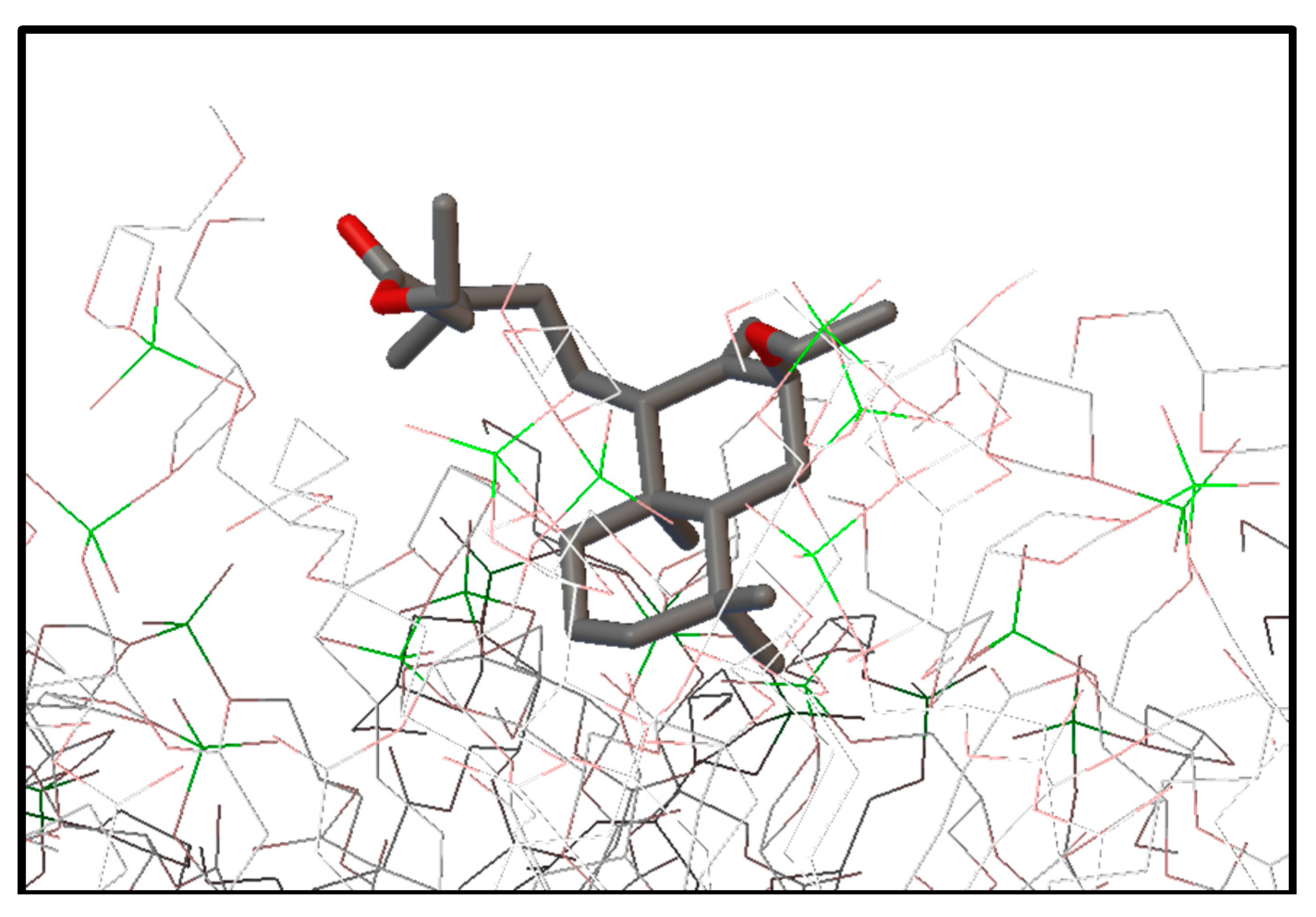
2.3. Molecular Modeling: Docking Studies of Labdane-Membrane Interactions
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation of Salvic Acid (1)
3.4. Reduction of Salvic Acid: Diol (2)
3.5. Methylation of Salvic Acid: Methylsalvate (3)
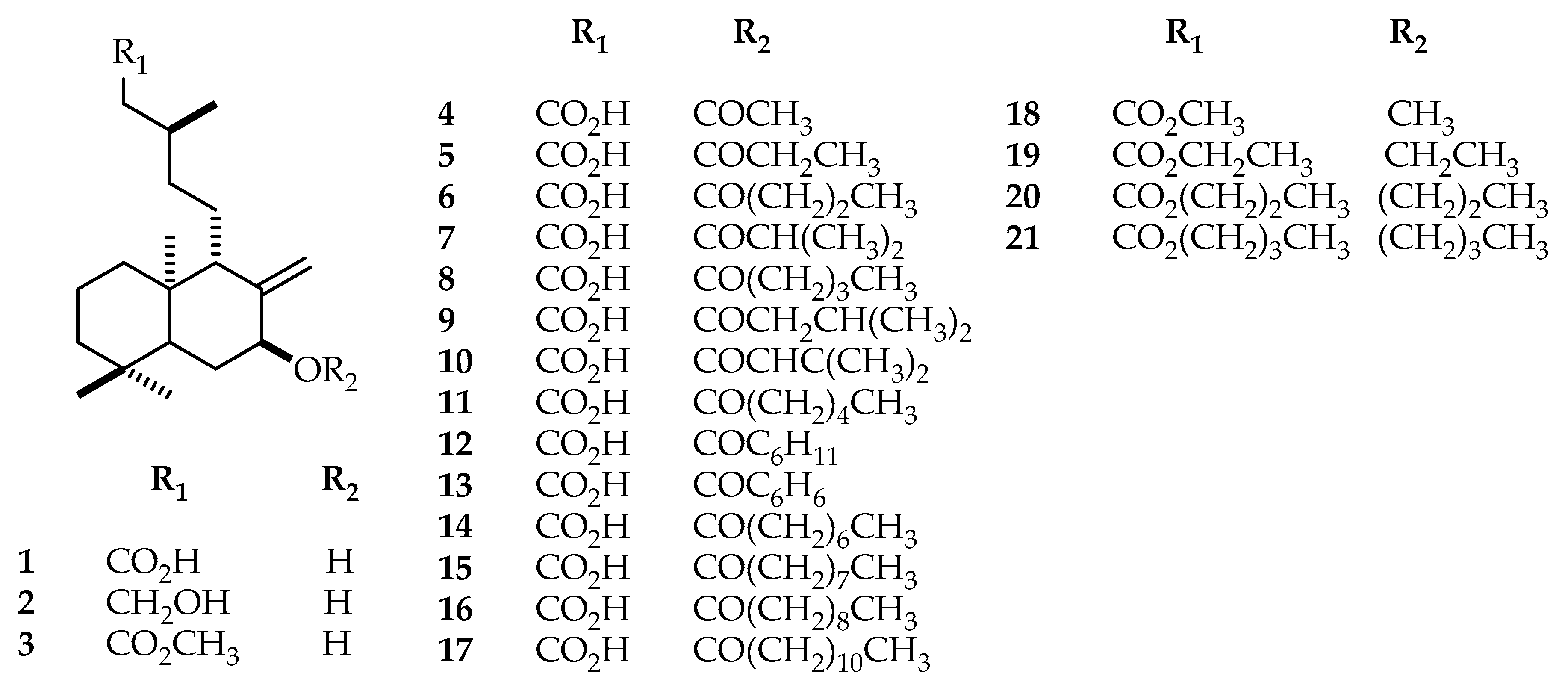
3.6. Acylation of Salvic Acid: 7-O-Acyl Derivatives (4–17)
3.7. Esterification and Etherification of Salvic Acid: 7-Alkoxy, 15 Ester Derivatives (18–21)
3.8. Purity of the Evaluated Diterpenes
3.9. Antimicrobial Assays
3.9.1. Solid Media Bioassays
3.9.2. Liquid Media Bioassays
3.10. Docking Study
3.11. Estimated Lipophilicity Values
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATCC | American type culture collection. |
| B3LYP | Becke three-parameter, Lee-Yang-Parr |
| CC | Column chromatography |
| CFU | Colony forming units |
| CHelpG | CHarges from ELectrostatic Potentials using a Grid |
| CLSI | Clinical Laboratory Standards |
| DFT | Density functional theory |
| GC | growth control |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| IC | inhibition control |
| logPow | Octanol–water partition coefficient |
| MHB | Mueller-Hinton broth |
| MIA | Minimum inhibitory amount |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear magnetic resonance |
| OD | optical density |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] |
| SAR | Structure-activity relationships |
| SolC | Solvent control |
| SteC | Sterility control |
| TSA | Tryptic soy agar media |
| TSB | Tryptic soy broth media |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Wencewicz, T.A. Prospects for new antibiotics: A molecule-centered perspective. J. Antibiot. (Tokyo) 2014, 67, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pal, M.; Sharma, R.P. Biological activity of the labdane diterpenes. Planta Med. 1999, 65, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem. 2001, 25, 235–292. [Google Scholar]
- Chinou, I. Labdanes of natural origin-biological activities (1981–2004). Curr. Med. Chem. 2005, 12, 1295–1317. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Zengin, G.; Fernández-de Córdova, M.L.; Bender, O.; Atalay, A.; Ceylan, R.; Mollica, A.; Mocan, A.; Uysal, S.; Guler, G.O.; et al. Traditionally Used Lathyrus Species: Phytochemical Composition, Antioxidant Activity, Enzyme Inhibitory Properties, Cytotoxic Effects, and in silico Studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vásquez, L. A structure-activity study of antibacterial diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A.; Echeverría, J.; Rezende, M.C.; Wilkens, M. Antibacterial Properties of 3 H-Spiro [1-benzofuran-2, 1′-cyclohexane] Derivatives from Heliotropium filifolium. Molecules 2008, 13, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Urzúa, A.; Echeverría, J.; Bucio, M.A.; Hernández-Barragán, A.; Joseph-Nathan, P. Determination of absolute configuration of salvic acid, an ent-labdane from Eupatorium salvia, by vibrational circular dichroism. Phytochemisty 2012, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.J. Side Effects and Drug Design; Marcel Dekker: New York, NY, USA, 1987; p. 94. [Google Scholar]
- Mallavadhani, U.V.; Mahapatra, A.; Jamil, K.; Reddy, P.S. Antimicrobial activity of some pentacyclic triterpenes and their synthesized 3-O-lipophilic chains. Biol. Pharm. Bull. 2004, 27, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.B.; De Souza, M.G.; Moreira, M.A.; Moreira, M.R.; Furtado, N.A.; Martins, C.H.; Bastos, J.K.; dos Santos, R.A.; Heleno, V.C.G.; Ambrosio, S.R.; et al. Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. Molecules 2011, 16, 9611–9619. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.B.; Martins, C.H.; Souza, M.G.; Furtado, N.A.; Heleno, V.C.; de Sousa, J.P.B.; Rocha, E.M.P.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.S.; et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother. Res. 2011, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Furtado, N.A.; Heleno, V.C.; Martins, C.H.; Da Costa, F.B.; Severiano, M.E.; Silva, A.N.; Veneziani, R.C.S.; Ambrosio, S.R. Antimicrobial ent-pimarane diterpenes from Viguiera arenaria against Gram-positive bacteria. Fitoterapia 2009, 80, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Rangel, R.; Furtado, N.A.; De Carvalho, T.C.; Martins, C.H.; Veneziani, R.; Da costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; et al. Pimarane-type diterpenes: Antimicrobial activity against oral pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Severiano, M.E.; Simao, M.R.; Porto, T.S.; Martins, C.H.; Veneziani, R.; Furtado, N.A.; Arakawa, N.S.; Said, S.; de Oliveira, D.C.R.; Cunha, W.R.; et al. Anticariogenic properties of ent-pimarane diterpenes obtained by microbial transformation. Molecules 2010, 15, 8553–8566. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Simão, M.R.; Ambrósio, S.R.; Furtado, N.A.; Veneziani, R.; Heleno, V.C.G.; Da Costa, F.B.; Gomes, B.P.F.A.; Souza, M.G.M.; Borges dos Reis, E.; et al. Antimicrobial activity of diterpenes from Viguiera arenaria against endodontic bacteria. Molecules 2011, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.; Denić, M.; Stojanović-Radić, Z. Antimicrobial phenolic abietane diterpene from Lycopus europaeus L. (Lamiaceae). Bioorg. Med. Chem. Lett. 2010, 20, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Moujir, L.M.; Seca, A.M.; Araujo, L.; Silva, A.M.; Barreto, M.C. A new natural spiro heterocyclic compound and the cytotoxic activity of the secondary metabolites from Juniperus brevifolia leaves. Fitoterapia 2011, 82, 225–229. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Doens, D.; Santamaría, R.; Ramos, M.; Restrepo, C.M.; de Arruda, L.B.; Lleonart, R.; Gutiérrez, M.; Fernández, P.L.A. Pseudopterane diterpene isolated from the octocoral Pseudopterogorgia acerosa inhibits the inflammatory response mediated by TLR-ligands and TNF-alpha in macrophages. PLoS ONE 2013, 8, e841107. [Google Scholar] [CrossRef] [PubMed]
- Kyrikou, I.; Georgopoulos, A.; Hatziantoniou, S.; Mavromoustakos, T.; Demetzos, C. A comparative study of the effects of cholesterol and sclareol, a bioactive labdane type diterpene, on phospholipid bilayers. Chem. Phys. Lipids 2005, 133, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Matsingou, C.; Demetzos, C. Effect of the nature of the 3β-substitution in manoyl oxides on the thermotropic behavior of DPPC lipid bilayer and on DPPC liposomes. J. Liposome Res. 2007, 17, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Hatziantoniou, S.; Mourelatou, E.A.; Amaro-Luis, J.M.; Villalobos-Osorio, D.; Demetzos, C. Preparation and thermal behavior of liposomal nanoparticles incorporating bioactive labdane epimers. Adv. Sci. Lett. 2012, 16, 336–341. [Google Scholar] [CrossRef]
- Souza-Fagundes, E.M.; Brumatti, G.; Martins-Filho, O.A.; Corrêa-Oliveira, R.; Zani, C.L.; Amarante-Mendes, G.P. Myriadenolide, a labdane diterpene isolated from Alomia myriadenia (asteraceae) induces depolarization of mitochondrial membranes and apoptosis associated with activation of caspases-8,-9, and-3 in Jurkat and THP-1 cells. Exp. Cell Res. 2003, 290, 420–426. [Google Scholar] [CrossRef]
- Haraguchi, H.; Oike, S.; Muroi, H.; Kubo, I. Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med. 1996, 62, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Micol, V.; Mateo, C.R.; Shapiro, S.; Aranda, F.J.; Villalaín, J. Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. BBA Biomembr. 2001, 1511, 281–290. [Google Scholar] [CrossRef]
- Aranda, F.J.; Villalaín, J. The interaction of abietic acid with phospholipid membranes. BBA Biomembr. 1997, 1327, 171–180. [Google Scholar] [CrossRef]
- Villalaín, J. Location of the toxic molecule abietic acid in model membranes by MAS–NMR. BBA Biomembr. 1997, 1328, 281–289. [Google Scholar] [CrossRef]
- Rodríguez, S.; Garda, H.A.; Heinzen, H.; Moyna, P. Effect of plant monofunctional pentacyclic triterpenes on the dynamic and structural properties of dipalmitoylphosphatidylcholine bilayers. Chem. Phys. Lipids 1997, 89, 119–130. [Google Scholar] [CrossRef]
- Broniatowski, M.; Flasiński, M.; Wydro, P. Investigation of the interactions of lupane type pentacyclic triterpenes with outer leaflet membrane phospholipids–Langmuir monolayer and synchrotron X-ray scattering study. J. Colloid Interface Sci. 2012, 381, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Flasiński, M.; Wydro, P. Lupane-type pentacyclic triterpenes in Langmuir monolayers: A synchrotron radiation scattering study. Langmuir 2012, 28, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.; Vögler, O.; Alemany, R.; Gomez-Florit, M.; Funari, S.S.; Ruiz-Gutiérrez, V.; Barceló, F. Plant pentacyclic triterpenic acids as modulators of lipid membrane physical properties. BBA Biomembr. 2011, 1808, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Ko, Y.I.; Park, S.J.; Jin, I.J.; Kim, Y.M. Oleanolic acid and ursolic acid stabilize liposomal membranes. Lipids 1997, 32, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Flasiński, M.; Zięba, K.; Miśkowiec, P. Langmuir monolayer studies of the interaction of monoamphiphilic pentacyclic triterpenes with anionic mitochondrial and bacterial membrane phospholipids—Searching for the most active terpene. BBA Biomembr. 2014, 1838, 2460–2472. [Google Scholar] [CrossRef] [PubMed]
- Matsingou, C.; Dimas, K.; Demetzos, C. Design and development of liposomes incorporating a bioactive labdane-type diterpene. In Vitro growth inhibiting and cytotoxic activity against human cancer cell lines. Biomed. Pharmacother. 2006, 60, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Espinoza, P.; Urzúa, A.; Vivanco, M.; Cotoras, M. In Vitro antifungal activity of the diterpenoid 7 alpha-hydroxy-8(17)-labden-15-oic acid and its derivatives against Botrytis cinerea. Molecules 2009, 14, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Hoeneisen, M.; Sammes, P.; Silva, M.; Watson, W.H. A new diterpenic acid and other constituents from Eupatorium salvia. Rev. Latinoam. Quim. 1979, 10, 37–40. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |
| Compound | S. aureus | B. cereus | E. coli | logP 3 | |||
|---|---|---|---|---|---|---|---|
| Salvic acid (1) | 50.00 1 | 20.00 2 | 50.00 1 | 22.50 2 | >100.00 1 | >10.00 2 | 4.90 |
| 2 | >100.00 1 | >25.00 2 | >100.00 1 | >25.00 2 | >100.00 1 | >10.00 2 | 5.08 |
| 3 | 25.00 1 | 3.00 2 | 50.00 1 | 2.00 2 | >100.00 1 | >10.00 2 | 5.23 |
| 4 | 50.00 1 | 4.50 2 | 25.00 1 | 4.00 2 | >100.00 1 | >10.00 2 | 5.47 |
| 5 | 25.00 1 | 2.50 2 | 12.50 1 | 1.50 2 | >100.00 1 | >10.00 2 | 5.94 |
| 6 | 6.25 1 | 1.50 2 | 3.13 1 | 1.25 2 | >100.00 1 | >10.00 2 | 6.30 |
| 7 | 6.25 1 | 1.25 2 | 6.25 1 | 1.00 2 | >100.00 1 | >10.00 2 | 6.52 |
| 8 | 3.13 1 | 0.75 2 | 3.13 1 | 0.50 2 | >100.00 1 | >10.00 2 | 6.84 |
| 9 | 3.13 1 | 0.50 2 | 3.13 1 | 0.30 2 | >100.00 1 | >10.00 2 | 6.74 |
| 10 | 3.13 1 | 0.75 2 | 3.13 1 | 0.40 2 | >100.00 1 | >10.00 2 | 6.92 |
| 11 | 3.13 1 | 0.35 2 | 3.13 1 | 0.25 2 | >100.00 1 | >10.00 2 | 7.38 |
| 12 | 3.13 1 | 1.00 2 | 3.13 1 | 0.75 2 | >100.00 1 | >10.00 2 | 7.55 |
| 13 | 6.25 1 | 1.25 2 | 6.25 1 | 1.25 2 | >100.00 1 | >10.00 2 | 7.13 |
| 14 | 3.13 1 | 0.40 2 | 3.13 1 | 0.45 2 | >100.00 1 | >10.00 2 | 8.47 |
| 15 | 12.50 1 | 3.00 2 | 6.25 1 | 2.50 2 | >100.00 1 | >10.00 2 | 9.01 |
| 16 | >100.00 1 | >10.00 2 | 25.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 9.55 |
| 17 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 10.63 |
| 18 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 5.76 |
| 19 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 6.50 |
| 20 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 7.55 |
| 21 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | >100.00 1 | >10.00 2 | 8.26 |
| Penicillin | 0.03 1 | 1.23 2 | 2.50 1 | 5.00 2 | n/t | n/t | - |
| Ciprofloxacin | 0.25 1 | 2.50 2 | 0.13 1 | 2.50 2 | n/t | n/t | - |
| Kanamycin | 1.00 1 | 2.50 2 | 1.00 1 | 5.00 2 | n/t | n/t | - |
| Tetracycline | 1.00 1 | 1.23 2 | 5.00 1 | 5.00 2 | n/t | n/t | - |
| Chloramphenicol | 2.00 1 | 1.50 2 | 0.25 1 | 0.25 2 | n/t | n/t | - |
| Methanol | i 1 | i 2 | i 1 | i 2 | i 1 | i 2 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría, J.; Urzúa, A.; Sanhueza, L.; Wilkens, M. Enhanced Antibacterial Activity of Ent-Labdane Derivatives of Salvic Acid (7α-Hydroxy-8(17)-ent-Labden-15-Oic Acid): Effect of Lipophilicity and the Hydrogen Bonding Role in Bacterial Membrane Interaction. Molecules 2017, 22, 1039. https://doi.org/10.3390/molecules22071039
Echeverría J, Urzúa A, Sanhueza L, Wilkens M. Enhanced Antibacterial Activity of Ent-Labdane Derivatives of Salvic Acid (7α-Hydroxy-8(17)-ent-Labden-15-Oic Acid): Effect of Lipophilicity and the Hydrogen Bonding Role in Bacterial Membrane Interaction. Molecules. 2017; 22(7):1039. https://doi.org/10.3390/molecules22071039
Chicago/Turabian StyleEcheverría, Javier, Alejandro Urzúa, Loreto Sanhueza, and Marcela Wilkens. 2017. "Enhanced Antibacterial Activity of Ent-Labdane Derivatives of Salvic Acid (7α-Hydroxy-8(17)-ent-Labden-15-Oic Acid): Effect of Lipophilicity and the Hydrogen Bonding Role in Bacterial Membrane Interaction" Molecules 22, no. 7: 1039. https://doi.org/10.3390/molecules22071039