Calcineurin Antagonizes AMPK to Regulate Lipolysis in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
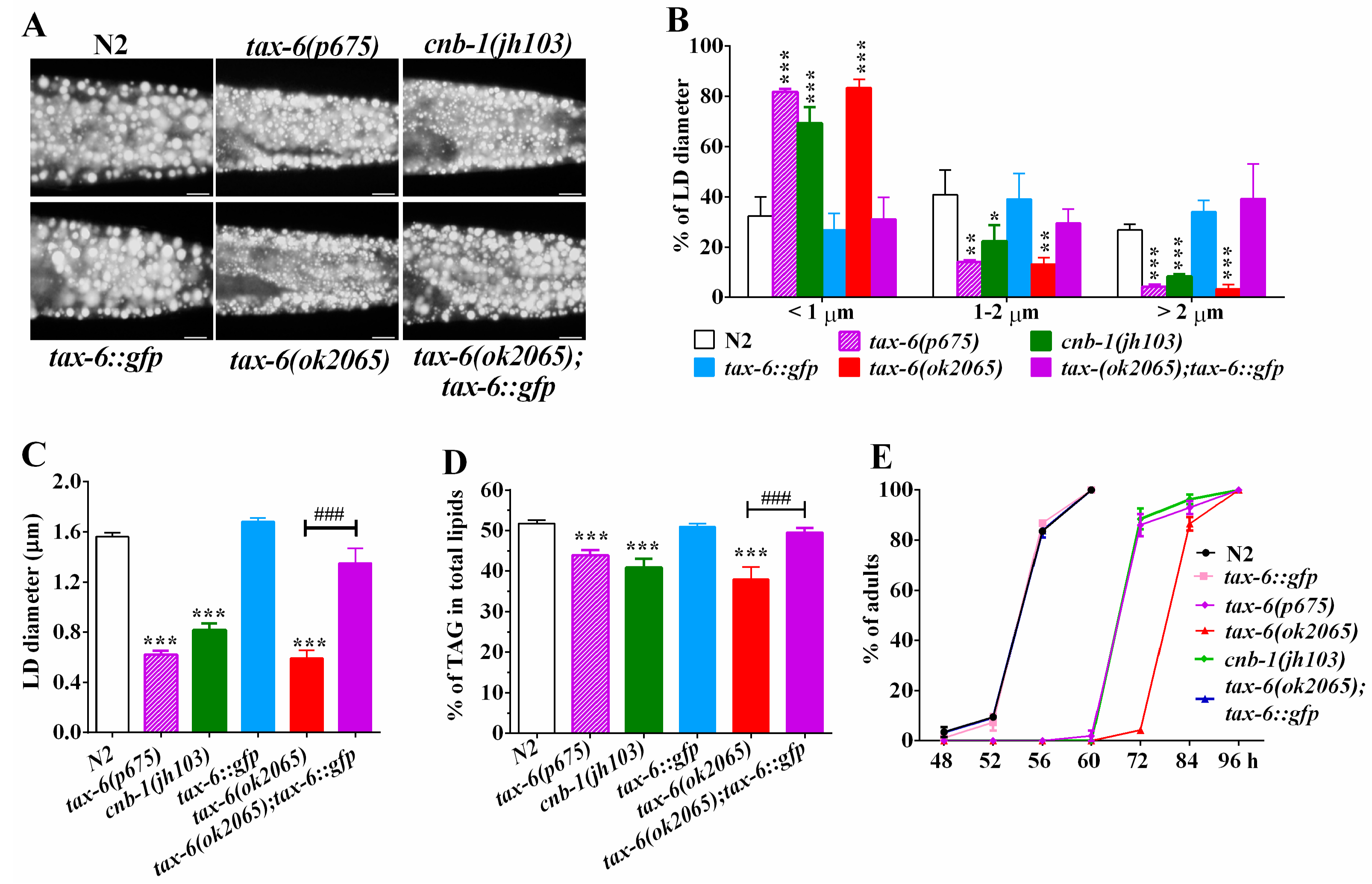
2.1. Genetic Disruption of Calcineurin Reduces Fat Accumulation in C. elegans
2.2. Calcineurin Inhibitor Mimics the Effects of Calcineurin Mutations on Fat Accumulation
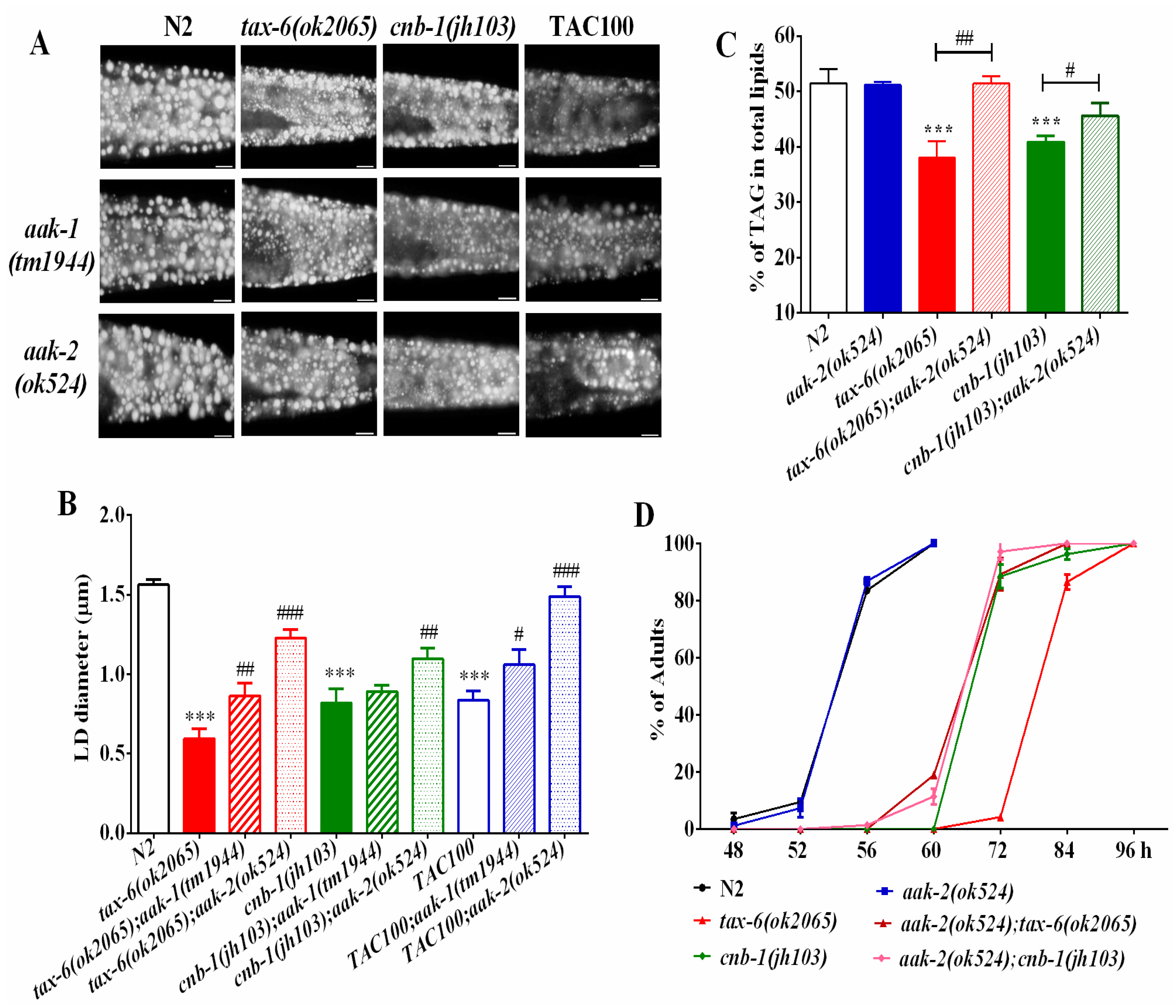
2.3. AMPK Antagonizes Calcineurin to Regulate Fat Metabolism
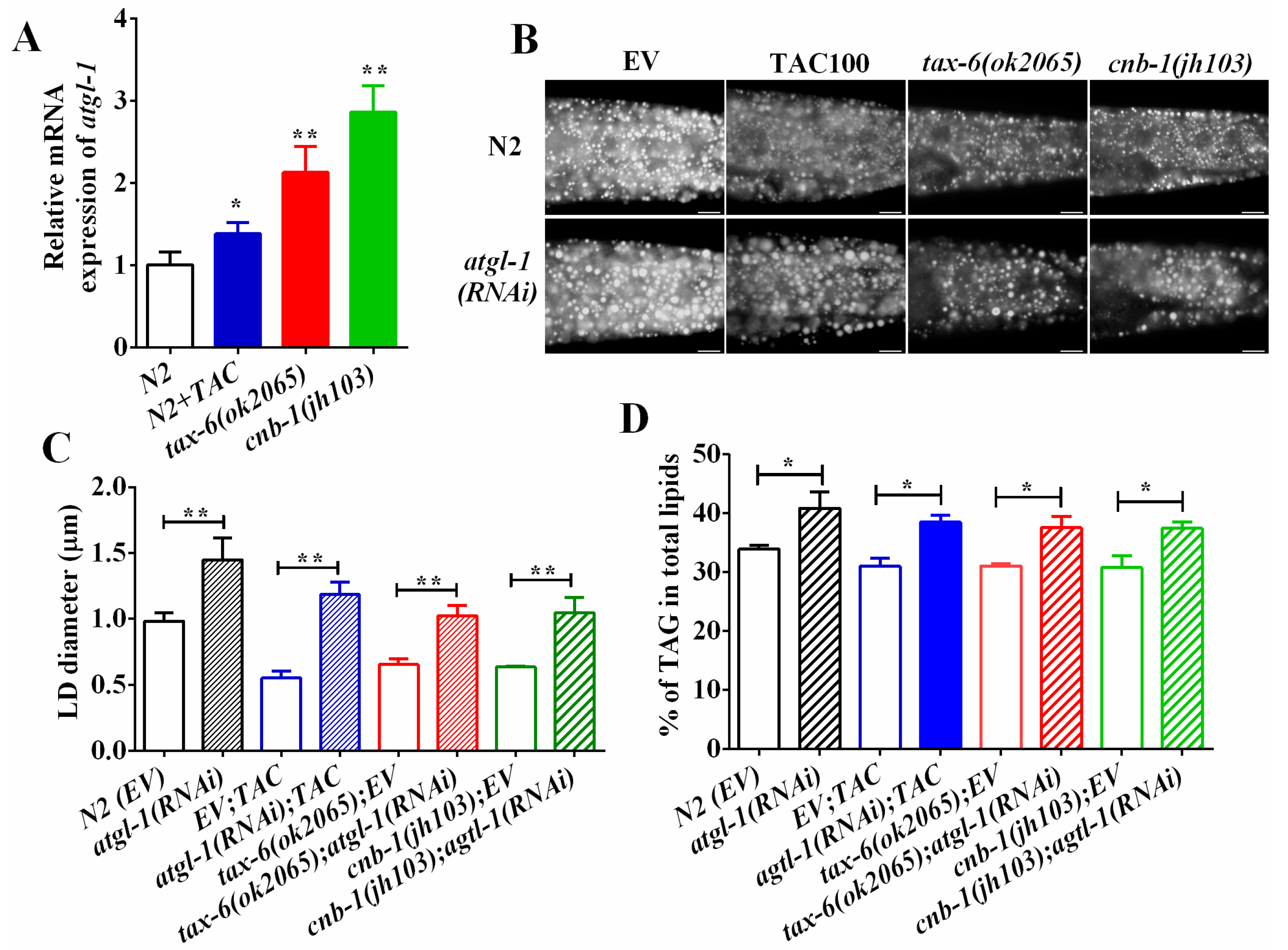
2.4. Calcineurin Promotes Lipolysis via Adipose Triglyceride Lipase (ATGL-1)
3. Discussion
4. Materials and Methods
4.1. Nematode Strains and Growth Conditions
4.2. RNAi Interferance
4.3. Nile Red Staining of Fixed Nematodes
4.4. Lipid Extraction and Analysis
4.5. Growth Rate
4.6. Treatment of Calcineurin Inhibitor
4.7. Quantitative RT-PCR Anaylsis
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klee, C.B.; Krinks, M.H. Purification of cyclic 3′,5′-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry 1978, 17, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [PubMed]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Gooch, J.L.; Pergola, P.E.; Guler, R.L.; Abboud, H.E.; Barnes, J.L. Differential expression of calcineurin A isoforms in the diabetic kidney. J. Am. Soc. Nephrol. 2004, 15, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Kimura, S.; Mori, R.; Kawakubo, T.; Shirotani, K.; Yagishita, S.; Maruyama, K.; Iwata, N. Perturbed calcineurin-NFAT signaling is associated with the development of Alzheimer’s Disease. Biol. Pharm. Bull. 2016, 39, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Leiter, L.M.; Gerber, D.J.; Gainetdinov, R.R.; Sotnikova, T.D.; Zeng, H.; Caron, M.G.; Tonegawa, S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc. Natl. Acad. Sci. USA 2003, 100, 8987–8992. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, J.S.; Rao, V.M.; Meena, A.K.; Anandaraj, M.P. Decreased calcineurin activity in circulation of Duchenne muscular dystrophy. Clin. Biochem. 2007, 40, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, W.R.; Gu, X.; Bottino, R.; Crabtree, G.R.; Kim, S.K. Neonatal beta cell development in mice and humans is regulated by calcineurin/NFAT. Dev. Cell 2012, 23, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Dos Santos, A.X.; Riezman, I.; Aguilera-Romero, M.A.; David, F.; Piccolis, M.; Loewith, R.; Schaad, O.; Riezman, H. Systematic lipidomic analysis of yeast protein kinase and phosphatase mutants reveals novel insights into regulation of lipid homeostasis. Mol. Biol. Cell 2014, 25, 3234–3246. [Google Scholar] [CrossRef] [PubMed]
- Suk, H.Y.; Zhou, C.; Yang, T.T.; Zhu, H.; Yu, R.Y.; Olabisi, O.; Yang, X.; Brancho, D.; Kim, J.Y.; Scherer, P.E.; et al. Ablation of calcineurin Abeta reveals hyperlipidemia and signaling cross-talks with phosphodiesterases. J. Biol. Chem. 2013, 288, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Farmer, J.D.; Lane, J.W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Cohen-Kutner, M.; Yahalom, Y.; Trus, M.; Atlas, D. Calcineurin controls Voltage-Dependent-Inactivation (VDI) of the normal and timothy cardiac channels. Sci. Rep. 2012, 2, 366. [Google Scholar] [CrossRef] [PubMed]
- Sheftic, S.R.; Page, R.; Peti, W. Investigating the human Calcineurin Interaction Network using the piLxVP SLiM. Sci. Rep. 2016, 6, 38920. [Google Scholar] [CrossRef] [PubMed]
- Breuder, T.; Hemenway, C.S.; Movva, N.R.; Cardenas, M.E.; Heitman, J. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc. Natl. Acad. Sci. USA 1994, 91, 5372–5376. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Trence, D.L. Immunosuppressive agents: Effects on glucose and lipid metabolism. Endocrinol. Metab. Clin. N. Am. 2007, 36, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.M.; Popescu, I.; Emre, S.; Schwartz, M.E.; Boccagni, P.; Meneses, P.; Mor, E.; Sheiner, P.; Miller, C.M. Serum lipid changes in liver transplant recipients in a prospective trial of cyclosporine versus FK506. Transplantation 1994, 57, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Maes, B.D.; Vanrenterghem, Y.F. Cyclosporine: Advantages versus disadvantages vis-à-vis tacrolimus. Transplant Proc. 2004, 36 (Suppl. 2), 40S–49S. [Google Scholar] [CrossRef] [PubMed]
- Wheelan, S.J.; Boguski, M.S.; Duret, L.; Makalowski, W. Human and nematode orthologs—Lessons from the analysis of 1800 human genes and the proteome of Caenorhabditis elegans. Gene 1999, 238, 163–170. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Lee, J.; Lee, J.; Lee, J.I.; Yu, J.R.; Jee, C.; Cho, J.H.; Jung, S.; Lee, M.H.; Zannoni, S.; et al. Calcineurin, a Calcium/Calmodulin-dependent Protein Phosphatase, Is Involved in Movement, Fertility, Egg Laying, and Growth in Caenorhabditis elegans. Mol. Biol. Cell 2002, 13, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Singaravelu, G.; Lee, S.; Ahnn, J.; Shim, Y.H. Functional and phenotypic relevance of differentially expressed proteins in calcineurin mutants of Caenorhabditis elegans. Proteomics 2006, 6, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.; Morantte, I.; Rodrigues, A.P.C.; Manning, G.; Montminy, M.; Shaw, R.J.; Dillin, A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 2011, 470, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.K.; Liang, B.; Watts, J.L. The influence of bacterial diet on fat storage in C. elegans. PLoS ONE 2009, 4, e7545. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Ferguson, K.; Kadyk, L.; Watts, J.L. The role of nuclear receptor NHR-64 in fat storage regulation in Caenorhabditis elegans. PLoS ONE 2010, 5, e9869. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, J.; Zou, X.; Greggain, J.; Rodkaer, S.V.; Faergeman, N.J.; Liang, B.; Watts, J.L. Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J. Lipid Res. 2013, 54, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev. Biol. 2006, 292, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.Q.; Venable, J.D.; Au, N.; Xu, T.; Park, S.K.; Cociorva, D.; Johnson, J.R.; Dillin, A.; Yates, J.R., III. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science 2007, 317, 660–663. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, X.; Lv, L.; Liang, H.; Leng, B.; Zhao, D.; Zhang, Y.; Du, Z.; Chen, X.; Li, S.; et al. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death Dis. 2014, 5, e997. [Google Scholar] [CrossRef] [PubMed]
- Apfeld, J.; O’Connor, G.; McDonagh, T.S.; DiStefano, P.S.; Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004, 18, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P.; Roy, R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 2009, 457, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.X.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.L.; van Gilst, M.R. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008, 8, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xie, Q.; Ding, Y.H.; Li, S.T.; Peng, S.; Zhang, Y.P.; Tan, D.; Yuan, Z.; Dong, M.Q. CAMKII and calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. eLife 2013, 2, e00518. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase to Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell. Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rao, A.; Hogan, P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011, 21, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Graef, I.A.; Gastier, J.M.; Francke, U.; Crabtree, G.R. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc. Natl. Acad. Sci. USA 2011, 98, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T. The search for DAF-16/FOXO transcriptional targets: Approaches and discoveries. Exp. Gerontol. 2006, 41, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Palming, J.; Rizell, M.; Aureliano, M.; Carvalho, E.; Svensson, M.K.; Eriksson, J.W. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol. Cell. Endocrinol. 2013, 365, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Kuhara, A.; Inada, H.; Katsura, I.; Mori, I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron 2002, 33, 751–763. [Google Scholar] [CrossRef]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, X.; Ding, Y.; Wang, H.; Wu, X.; Liang, B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zou, X.; Jiang, X.; Wu, J.; Zhang, Y.; Chen, D.; Liang, B. Pu-erh tea down-regulates sterol regulatory element-binding protein and stearyol-CoA desaturase to reduce fat storage in Caenorhaditis elegans. PLoS ONE 2015, 10, e0113815. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the tacrolimus was purchased from Selleck Chemicals (Houston, TX, USA, Code S5003) |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, C.; Diao, Z.; Liang, B. Calcineurin Antagonizes AMPK to Regulate Lipolysis in Caenorhabditis elegans. Molecules 2017, 22, 1062. https://doi.org/10.3390/molecules22071062
Wang Y, Xie C, Diao Z, Liang B. Calcineurin Antagonizes AMPK to Regulate Lipolysis in Caenorhabditis elegans. Molecules. 2017; 22(7):1062. https://doi.org/10.3390/molecules22071062
Chicago/Turabian StyleWang, Yanli, Cangsang Xie, Zhiqing Diao, and Bin Liang. 2017. "Calcineurin Antagonizes AMPK to Regulate Lipolysis in Caenorhabditis elegans" Molecules 22, no. 7: 1062. https://doi.org/10.3390/molecules22071062





